Abstract
Recent evidence suggests that putting feelings into words activates the prefrontal cortex (PFC) and suppresses the response of the amygdala, potentially helping to alleviate emotional distress. To further elucidate the relationship between brain structure and function in these regions, structural and functional magnetic resonance imaging (MRI) data were collected from a sample of 20 healthy human subjects. Structural MRI data were processed using cortical pattern-matching algorithms to produce spatially normalized maps of cortical thickness. During functional scanning, subjects cognitively assessed an emotional target face by choosing one of two linguistic labels (label emotion condition) or matched geometric forms (control condition). Manually prescribed regions of interest for the left amygdala were used to extract percentage signal change in this region occurring during the contrast of label emotion versus match forms. A correlation analysis between left amygdala activation and cortical thickness was then performed along each point of the cortical surface, resulting in a color-coded r value at each cortical point. Correlation analyses revealed that gray matter thickness in left ventromedial PFC was inversely correlated with task-related activation in the amygdala. These data add support to a general role of the ventromedial PFC in regulating activity of the amygdala.
Introduction
The amygdala influences a broad range of physiological and behavioral responses associated with emotion and is anatomically connected with several brain regions including the thalamus, anterior temporal lobes, prefrontal cortex (PFC), anterior cingulate cortex (ACC), and ventral striatum (Aggleton et al., 1980). Functional magnetic resonance imaging (fMRI) studies of healthy adults have further shown that the amygdala, particularly the left amygdala, is responsive to negative emotional stimuli, with a preference for faces depicting negative emotional expressions (Sergerie et al., 2008). During higher-order cognitive evaluation of these stimuli, such as when emotions are consciously suppressed (Ochsner et al., 2002; Beer et al., 2006; Urry et al., 2006; Delgado et al., 2008) or when labeling negative emotional facial expressions (Hariri et al., 2000, 2003; Lieberman et al., 2007; Foland et al., 2008), the ventral PFC also becomes active and correlations, typically negative, are observed between activation in these two regions (Hariri et al., 2000, 2003; Ochsner et al., 2004; Urry et al., 2006; Lieberman et al., 2007; Foland et al., 2008). It has been posited that this inverse relationship reflects a neural network whereby ventral PFC suppresses activation in the amygdala (Urry et al., 2006; Lieberman et al., 2007), thereby helping to potentially alleviate emotional distress (Ochsner et al., 2004; Urry et al., 2006; Berkman and Lieberman, 2009).
Here we aimed to assess whether activation level in the left amygdala, present during the higher-order cognitive evaluation of negative emotional facial expressions, would be associated with prefrontal cortical structure, as measured by gray matter thickness. Both human (Phelps et al., 2004; Milad et al., 2005, 2007; Kalisch et al., 2006; Urry et al., 2006; Delgado et al., 2008; Sergerie et al., 2008) and animal (Amaral, 1992; Milad and Quirk, 2002; Rosenkranz and Grace, 2002; Quirk et al., 2003; Sotres-Bayon et al., 2004; Amat et al., 2005; Likhtik et al., 2005) studies provide extensive evidence for the suppression of amygdala response by ventral PFC. Given this, we hypothesized that these measures would be inversely correlated in healthy human subjects, such that individuals with lower gray matter thickness in ventral prefrontal cortex would demonstrate greater activation level in the left amygdala.
Materials and Methods
Subjects.
A total of 20 healthy subjects (12 males, 8 females; mean age, 35.1 ± 12.7 years) were scanned. Exclusion criteria included left-handedness, hypertension, neurological illness, metal implants, history of skull fracture or head trauma with loss of consciousness >5 min, current medication use, and current or past psychiatric diagnosis (including history of substance abuse). The UCLA Institutional Review Board approved the study protocol and each subject provided written informed consent.
Imaging.
Structural MRI data were collected using a 1.5 T Siemens Sonata MRI scanner. High-resolution, three-dimensional (3D) MP-RAGE, volumetric T1-weighted images acquired for each subject [160 slices; field-of-view (FOV) = 256 mm; slice thickness = 1 mm; repetition time (TR)/echo time (TE) = 1900/4.38 ms; flip angle (FA) = 15°]. Functional MRI data were collected using a 3.0 T Siemens Allegra MRI scanner using an asymmetric spin echo sequence (28 slices; FOV = 200 mm; slice thickness = 3 mm; TR/TE = 2500/25 ms; FA = 90°) during the performance of an affect-labeling task (described below). A structural T2-weighted volume (28 slices; FOV = 200 mm; slice thickness = 3 mm; TR/TE = 5000/34 ms; FA = 90°) was also acquired on the 3.0 T scanner, coplanar to functional scans.
Task paradigm used during fMRI.
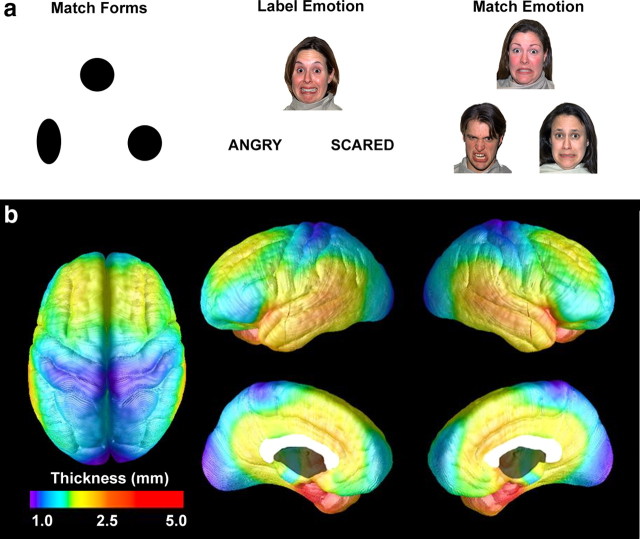
The affect-labeling paradigm used during fMRI scanning has been detailed previously (Hariri et al., 2000; Lieberman et al., 2007; Foland et al., 2008). Briefly, during the label emotion condition, subjects chose one of two words at the bottom of the screen that best described the emotional face at the top of the screen (Fig. 1a). In the match forms (control) condition, subjects matched one of two geometric shapes at the bottom of the screen to a target shape at the top of the screen. A third match emotion condition involved subjects choosing one of two emotional faces on the bottom of the screen that best matched the emotional expression of a face at the top of the screen. And a fourth condition, label gender, required subjects choose one of two names on the bottom of the screen that best matched the gender of the emotional face at the top of the screen. The entire behavioral paradigm included two label emotion blocks, two match emotion blocks, two label gender blocks, and six match forms blocks. Each block lasted 35 s and began with a 3 s instruction cue, followed by eight 4 s trials. Importantly, the label emotion versus match forms contrast was used in the correlation of amygdala activation with cortical thickness because of our a priori interest in emotion regulatory frontolimbic networks and because several studies (Hariri et al., 2000, 2003; Lieberman et al., 2007; Foland et al., 2008) show that labeling facial emotions, in contrast to matching facial emotions, evokes activation in ventral PFC and attenuates activation in the left amygdala. To determine whether a suppression of amygdala response occurs during affect labeling compared with affect matching, as has been found in previous studies (Hariri et al., 2000, 2003; Lieberman et al., 2007; Foland et al., 2008), we also examined amygdala activation occurring during the contrasts of match emotion versus match forms and match emotion versus label emotion. The order of task presentation was balanced across subjects and subjects responded by pressing one of two buttons with their right hand.
Figure 1.
a, Sample stimuli from the affect labeling task. b, Mean cortical thickness across subjects; this pattern is highly consistent with thickness measurements in postmortem samples (Von Economo, 1929).
Functional MRI data analysis.
Functional imaging data were analyzed using FSL (www.fmrib.ox.ac.uk/fsl). All images were examined closely for severe motion or spike artifacts. Image data were motion corrected and smoothed using a full-width half-maximum 5 mm Gaussian kernel. A time-series statistical analysis was performed using local autocorrelation correction (Woolrich et al., 2001) to assess activation differences between different experimental conditions within subjects. All functional images were aligned to subjects' coplanar T2-weighted structural scan using a six-parameter, rigid-body transform in FLIRT (Jenkinson and Smith, 2001).
To measure amygdala activation from fMR images, manually prescribed regions of interest (ROIs) for the left amygdala were delineated on each subject's 1.5T scan by a trained neuroanatomist (C.P.) using a previously validated protocol (Bartzokis et al., 1993). ROIs were spatially registered to fMRI data using a six-parameter linear transformation of the 1.5T structural scan to the 3T structural scan that was acquired coplanar to functional images (Jenkinson and Smith, 2001). As the match emotion condition has previously been shown to elicit a robust response of the left amygdala (Hariri et al., 2000, 2003; Lieberman et al., 2007; Foland et al., 2008), the match emotion condition, compared with the match forms (control) condition was used as a localizer to identify the peak voxel in the left amygdala. Activity at this voxel was then computed for the contrast of label emotion versus match forms. We used the peak, rather than the entire cluster, since the primary aim of the current study was to examine whether activation in the amygdala relates to an external measure (prefrontal cortical thickness) and because some voxels within the amygdala may represent a blurring of activity elsewhere in the ROI that may be caused either by the vascular structure in the region or by spatial smoothing, signal loss, or registration error. Nevertheless, since this peak voxel approach may be biased toward the selection of a voxel showing a decreased response to the match forms or label emotion conditions, follow-up exploratory analyses were conducted to assess whether a similar pattern of activation was present across the entire anatomic region during this contrast, as well as to assess whether a similar pattern of activation was present both at this voxel and across the entire anatomic region using the direct comparison of match emotion versus label emotion. Percentage signal change values visible at a height threshold of z > 1.7 and a cluster probability of p < 0.05, corrected, were extracted using featquery (part of FSL; www.fmrib.ox.ac.uk/fsl) as an average percentage signal change occurring across all TRs during the respective task conditions.
Structural MRI data analysis.
For the analysis of cortical thickness, T1-weighted images from the 1.5T scan session were processed using the following steps: (1) correction for magnetic field inhomogeneities (Zijdenbos and Dawant, 1994); (2) automated and manual removal of nonbrain tissue (Shattuck and Leahy, 2002); (3) adjustment for head position and transformation into a common stereotaxic coordinate system without scaling using a three-translation and three-rotation rigid-body transformation (Jenkinson and Smith, 2001); (4) automatic classification of voxels into gray matter, white matter, and CSF using a partial volume correction method (Shattuck and Leahy, 2002); (5) image resampling to create 0.33 mm cubic voxels to compute cortical thickness at subvoxel accuracy; and (6) calculation of cortical gray matter thickness. Specifically, thickness was defined as the shortest three-dimensional distance from the cortical white-gray matter boundary to the hemispheric surface, without crossing voxels classified as CSF. This measurement was computed at thousands of points along the brain surface using the Eikonal equation (Sapiro, 2001). Cortical thickness maps for each individual were then projected onto subject-specific three-dimensional cortical surface models, created using a spherical mesh surface that was deformed to fit surface tissue using a threshold intensity value that differentiated extra cortical CSF from gray and white matter.
To relate homologous cortical regions across subjects, cortical pattern-matching methods were applied (Thompson et al., 2004). This registration procedure uses sulcal landmarks as anchors in a warping process that drives cortical anatomy into direct correspondence between subjects. Thirty-one separate sulci were manually delineated on each hemisphere using subjects' 3D cortical surface models. Sulcal tracing was performed according to a previously validated anatomical protocol (Sowell et al., 2004) by a trained neuroanatomist (J.S.) blind to subject characteristics. Intertracer and intratracer reliability was measured using the three-dimensional root mean square difference (in millimeters) between sulci in a set of six test brains and those of a gold standard set. Disparities between the test and gold standard brains were computed to be <2 mm for all landmarks.
The amount of shift in the x, y, and z directions needed to explicitly match each sulcus from an individual subject to that of the average anatomical template was computed using cortical pattern-matching algorithms (Thompson et al., 2004). Importantly, the same parameter space coordinates were associated across subjects, without actually deforming subjects' cortical surface models. This way, individual subject maps were reparameterized, such that the corresponding anatomy bore the same coordinate locations in each subject. The result is a very high-resolution spatial association between subjects that accounts for variations in cortical patterning, but which avoids distortions that may be otherwise caused by directly warping surface maps (Thompson et al., 2004).
Relating structural and functional MRI data.
The primary aim of this study was to determine whether thickness of ventral prefrontal cortical gray matter shared significant correlations with activation level of the amygdala during the labeling of emotional stimuli. As such, associations between gray matter thickness and percentage signal change in the left amygdala occurring during the contrast of label emotion versus match forms were examined using correlation analyses at each point along the prefrontal cortex, with activity level in the peak voxel of the amygdala modeled as a covariate for linkage with local gray matter distribution.
To confirm that these patterns of correlation were unique to the processes of affect labeling (i.e., to determine whether any activation of the amygdala would elicit similar structure–function correlations within the PFC), additional analyses were conducted to examine the relationship between prefrontal cortical thickness and percentage signal change occurring in the left amygdala during the match emotion versus the match forms contrast.
Given our a priori hypotheses regarding the ventral prefrontal cortical brain region, correlations between thickness and amygdala response were identified within the PFC using permutation testing to correct for multiple comparisons across voxels contained within prefrontal Brodmann areas, adapted to each subject's anatomy (Rasser et al., 2005). Other cortical regions detected at this threshold for which we did not have a priori hypotheses (i.e., regions outside the PFC) are treated here as exploratory and require replication to ensure their validity. A two-tailed alpha level of p = 0.05, corrected, was determined as the threshold for statistical significance within our prefrontal cortical region of interest.
Results
Behavioral data
All subjects performed with high accuracy and sufficient reaction time across the label emotion (97.4 ± 4.3% correct and 1.7 ± 0.30 s, respectively), match emotion (92.6 ± 7.0% correct and 1.9 ± 0.30 s, respectively), and match forms conditions (92.4 ± 1.9% correct and 0.98 ± 0.50 s, respectively).
Imaging data
Consistent with previous studies using this task (Hariri et al., 2000, 2003; Lieberman et al., 2007; Foland et al., 2008), there was robust activation in the left amygdala during affect matching compared with shape matching (0.45 ± 0.24% signal change; mean cluster size = 12.9 ± 76.8 voxels). Activation at this cluster was highly correlated with that of the peak voxel (r = 0.89; p < 0.01; 1.19 ± 0.73% signal change). Activation occurring during the contrast of affect labeling compared with shape matching at this voxel (0.40 ± 0.51% signal change) was also correlated with that of the cluster (r = 0.51; p = 0.02; 0.39 ± 0.49% signal change). Paired one-sample t tests showed activation at the voxel identified by the localizer was significantly greater during affect matching compared with affect labeling (0.40 ± 0.51% signal change; p = 0.002, t = 3.56, df = 19). This difference remained significant using the direct contrast of match emotion versus label emotion, both at the peak voxel identified by the localizer (p < 0.005; t = 4.21; df = 19) and across the entire ROI (p < 0.001; t = 11.71; df = 19; mean cluster size = 18.1 ± 7.9 voxels).
Average cortical gray matter thickness (across subjects) for each cortical surface point is shown in Figure 1b. Visual inspection of cortical thickness maps demonstrated consistency with thickness values obtained from postmortem samples (Von Economo, 1929).
Results from the point-wise correlation analyses between percentage signal change in the left amygdala occurring during the contrast of label emotion versus match forms and cortical gray matter thickness are shown in Figure 2a. Within our hypothesized region of interest, the PFC, significant inverse correlations were present in the left ventromedial subregion (BA11; p = 0.04, corrected) (Fig. 2b). Other, smaller clusters of correlations were observed outside this area; however, these clusters did not survive correction for multiple comparisons.
Figure 2.
a, c, Correlation maps showing associations between cortical gray matter thickness and left amygdala activation during affect labeling (a) and affect matching (c). b, d, Percentage signal change in the amygdala plotted against mean thickness of left ventromedial PFC (BA11) during affect labeling (b) reveals negative correlations with left ventromedial prefrontal cortical thickness that are absent during affect matching (d). The black outlines in a and c indicate the BA11 region, projected onto the average anatomical template using the deformable Brodmann Area Atlas (Rasser et al., 2005). The yellow arrows indicate the left ventromedial subregion of PFC where significant negative correlations with amygdala response were found during affect labeling (but not affect matching).
Examination of the structure–function correlations using amygdala activity measured during the contrast of match emotion versus match forms confirmed that the negative correlations that we observed between thickness of ventromedial PFC (vmPFC) and amygdala activation measured during the label emotion task were specific to the processes of affect labeling (Fig. 2c,d). Additionally, during the match emotion condition, positive correlations with thickness were present in left temporal cortices (BA40, p = 0.01, corrected; BA22, p = 0.02, corrected).
Discussion
We found that greater ventromedial prefrontal cortical gray matter thickness was associated with greater reduction of activation in the left amygdala during affect labeling, a cognitive task that has previously been shown to dampen amygdala response (Hariri et al., 2000, 2003; Lieberman et al., 2007; Foland et al., 2008) and suppress negative emotional states (Berkman and Lieberman, 2009) (M. D. Lieberman, T. Inagaki, G. Tabibnia, and M. J. Crockett, unpublished observations). Our findings add support to previous suggestions (Milad et al., 2005) that the architecture of the vmPFC, a region to which the amygdala is both structurally (Amaral, 1992; Stefanacci and Amaral, 2002) and functionally (Quirk et al., 2003; Likhtik et al., 2005; Urry et al., 2006) connected, may influence activity level in the amygdala.
Numerous studies of animals involving extinction learning paradigms and extracellular recordings have previously highlighted a role for the vmPFC in the suppression of amygdala output (Milad and Quirk, 2002; Rosenkranz and Grace, 2002; Quirk et al., 2003; Sotres-Bayon et al., 2004; Likhtik et al., 2005). These findings are corroborated by studies of human subjects that show that both vmPFC structure (Milad et al., 2005) and vmPFC function (Kalisch et al., 2006; Milad et al., 2007) are positively correlated with extinction learning rate and negatively correlated with activity level in the amygdala (Phelps et al., 2004). Similar inverse patterns of activation between the vmPFC and amygdala have been observed in functional neuroimaging studies of humans scanned during the performance of tasks involving more complex cognition-based control of negative emotional states (Beer et al., 2006; Urry et al., 2006; Delgado et al., 2008). Thus, regulation of amygdala output, whether through extinction learning or higher-order cognitive processes, appears associated with evolutionarily conserved mechanisms of the vmPFC. Our data add to the existing literature to support a general role of the vmPFC in diminishing amygdala response.
Several factors may drive this structure–function correlation. First, thinner cortex may contain fewer neurons; thus, subjects with thinner cortical gray matter in ventromedial PFC may have fewer neurons available in this region to make inhibitory network connections with the amygdala. Second, prior studies have linked cortical structure to activation at the same location (Lu et al., 2009). Decreases in vmPFC gray matter thickness may therefore lead to a reduction in activation of ventromedial PFC (Kalisch et al., 2006; Milad et al., 2005) and thus to secondary decreases in the regulation of activation level in the left amygdala. Third, cortical gray matter may appear thinner as a result of increased myelination (Sowell et al., 2003; Bartzokis et al., 2009). This may tend to favor an increased, rather than decreased, level of prefrontal-amygdala structural connectivity. However, future studies are needed to address this specific possibility.
The ventrolateral PFC is a region that, in addition to ventromedial PFC, has been implicated in functional neuroimaging studies of emotion regulation and affect labeling (Hariri et al., 2000, 2003; Ochsner et al., 2002; Beer et al., 2006; Lieberman et al., 2007; Delgado et al., 2008; Foland et al., 2008). However, associations between thickness of the ventrolateral PFC and activity of the left amygdala were not observed here. Direct anatomical connections between these regions are sparse, and it has been posited that communication between the ventrolateral PFC and amygdala is more likely mediated via intermediary ventromedial regions of PFC (McDonald et al., 1996; Groenewegen et al., 1997; Milad and Quirk, 2002; Stefanacci and Amaral, 2002; Lieberman et al., 2007; Delgado et al., 2008). In this regard, it is interesting that our group has observed significant functional deficiencies in ventrolateral PFC of patients with mood disorders (Altshuler et al., 2005; Foland et al., 2008) (L. C. Foland-Ross, S. Y. Bookheimer, M. D. Lieberman, J. D. Townsend, J. Fischer, S. Torrisi, C. Penfold, S. K. Madsen, and P. M. Thompson, unpublished observations) and that these functional deficits do not correspond with thinning of the same overlapping ventrolateral regions, but with thinning in separate ventromedial areas of PFC (L. C. Foland-Ross, P. M. Thompson, C. A. Sugar, S. K. Madsen, J. K. Shen, C. Penfold, K. Ahlf, P. E. Rasser, J. Fischer, Y. Yang, J. Townsend, S. Y. Bookheimer, and L. L. Altshuler, unpublished observations). Future investigations that more rigorously investigate network-based dysfunction of the PFC and the impact of this dysfunction on amygdala response in mood disordered populations would be of interest.
Associations between prefrontal cortical gray matter thickness and activation of the amygdala measured during affect labeling were the primary interest of this study. However, several other cortical regions were found to demonstrate thickness correlations with the left amygdala during this labeling task. Nevertheless, because these correlations did not survive correction for multiple comparisons, an interpretation of these findings is not included here. Future studies involving larger samples may be better powered to be able to assess whether other structure–function relationships exist outside the ventromedial PFC.
The structure–function correlations that we observed between ventromedial PFC thickness and amygdala activation were unique to the cognitive process of emotion labeling. The specificity of this finding, along with findings from earlier investigations, which have reported a lack of a functional relationship between PFC and amygdala during emotion matching compared with emotion labeling (Hariri et al., 2000, 2003; Lieberman et al., 2007; Foland et al., 2008), highlights the importance of prefrontal cortical networks in regulating amygdala response during the process of putting feelings into words. Nevertheless, robust positive correlations were observed between amygdala response during emotion matching and thickness of temporal cortex. Positive correlations in this region may point to the involvement of this cortical area in emotion perception. Gray matter reductions in the temporal cortex, for example, have been linked with impaired performance on emotion matching tasks for negative facial expressions (Rosen et al., 2004). Thus, structure and function in this brain area may be critical to the more basic processes underlying the perception and recognition of facial emotion.
Our study has several limitations. First, we interpreted the negative correlation between left amygdala and ventromedial PFC to be involved in a top-down regulatory network whereby PFC directly suppresses amygdala output. However, the methods used here cannot resolve causality. Therefore, it may be argued that heightened amygdala response could result from deficient modulation by cortical regions outside our a priori ROI. This seems unlikely, given research showing functional coupling between the left ventromedial PFC and left amygdala during emotion regulation tasks (Urry et al., 2006), as well data from animal studies that show direct anatomical connections between these two regions (Stefanacci and Amaral, 2002). Moreover, permutation testing revealed no other areas of cortex shared significant associations with amygdala response during affect labeling.
Second, cortical gray matter may appear thinner in some subjects due to an increased myelination of neurons (Sowell et al., 2003), which would imply an increased, rather than decreased, structural connectivity between ventromedial PFC and left amygdala. As we do not have diffusion tensor imaging data on these subjects, it is not possible to address this possibility here. However, future studies that investigate this issue would be of interest.
Third, the labeling paradigm used in the current study has been suggested to represent a type of emotion regulation strategy; performance of this task has been previously associated with both a reduction in self-reported affect and a reduction in the physiological responses associated with negative emotions (M. D. Lieberman, T. Inagaki, G. Tabibnia, and M. J. Crockett, unpublished observations). Moreover, affect labeling has been associated with patterns of neural activity that are similar to intentional emotion regulation (Berkman and Lieberman, 2009). Given this, it is tempting to speculate that the negative correlations we observed between amygdala response and vmPFC thickness may relate to one's ability to successfully suppress amygdala response and therefore also to one's ability to successfully suppress negative emotional states. But, because no behavioral or physiological data on emotion arousal were collected from subjects in this study, this interpretation remains tentative.
Fourth, activation of the amygdala was significantly decreased during affect labeling compared with affect matching. It remains possible that this difference may be due to a difference in the number of affective stimuli (three faces vs one). However, our group has shown in separate studies that emotion matching produces amygdala activity of a magnitude that is similar to that found during passive observation of a single negative emotional image (Lieberman et al., 2007). Thus, we consider this possibility unlikely.
Fifth, the match forms condition was used as an experimental control in the examination of amygdala activity during the matching and labeling of emotionally evocative stimuli. It remains possible that something peculiar about matching forms could be responsible for the amygdala activation we observed during these contrasts (i.e., there could have been a decrease in activation during the match forms condition, rather than an increase in activation during the match emotion and label emotion conditions). Since we do not have a separate, independent baseline, we cannot evaluate this possibility directly. However, given the extensive literature on amygdala activity in response to this task (Hariri et al., 2000, 2003; Lieberman et al., 2007; Foland et al., 2008), this possibility seems unlikely. In addition, if the associations between amygdala activity and cortical thickness were due to a decrease in activation during the match forms task, then we would expect the correlation maps in Figure 2 to be highly similar, which they are not. Future studies involving affect labeling that use an independent baseline would be of interest.
To conclude, prior studies have linked cortical structure to activation at the same location (Rasser et al., 2005; Lu et al., 2009), but this is the first report, to our knowledge, to demonstrate that the structural features of one brain region correlate with activity in another brain region to which it is both structurally (Amaral, 1992; Stefanacci an Amaral, 2002) and functionally (Quirk et al., 2003; Likhtik et al., 2005; Urry et al., 2006) connected. Future studies that examine vmPFC structure–function relationships in psychiatric populations would be of interest.
Footnotes
This study was supported by grants from the National Institute of Mental Health (F31MH078556 to L.C.F.R. and K24 MH001848, R21 MH075944, and MH01848 to L.L.A.), the National Institute of Biomedical Imaging and Bioengineering (EB01651 to P.M.T.), and the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) (RR12169, RR13642, and RR00865). This study was also supported by the National Association for Research on Schizophrenia and Affective Disorders, the Brain Mapping Medical Research Organization, the Brain Mapping Support Foundation, the Pierson-Lovelace Foundation, the Ahmanson Foundation, the William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, the Tamkin Foundation, the Jennifer Jones-Simon Foundation, the Capital Group Companies Charitable Foundation, the Robson Family, and the Northstar Fund. We thank Dr. Katherine L. Narr for methodological consultations.
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bookheimer SY, Townsend J, Proenza MA, Eisenberger N, Sabb F, Mintz J, Cohen MS. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The amygdala: neurobiological aspects of emotion. New York: Wiley; 1992. [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Mintz J, Marx P, Osborn D, Gutkind D, Chiang F, Phelan CK, Marder SR. Reliability of in vivo volume measures of hippocampus and other brain structures using MRI. Magn Reson Imaging. 1993;11:993–1006. doi: 10.1016/0730-725x(93)90218-3. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Stewart SB, Oluwadara B, Lucas AJ, Pantages J, Pratt E, Sherin JE, Altshuler LL, Mintz J, Gitlin MJ, Subotnik KL, Nuechterlein KH. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113:322–331. doi: 10.1016/j.schres.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer JS, Knight RT, D'Esposito M. Controlling the integration of emotion and cognition: the role of frontal cortex in distinguishing helpful from hurtful emotional information. Psychol Sci. 2006;17:448–453. doi: 10.1111/j.1467-9280.2006.01726.x. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD. Using neuroscience to broaden emotion regulation: theoretical and methodological considerations. Soc Person Psych Comp. 2009;3:475–493. doi: 10.1111/j.1751-9004.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Uylings HB. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J Psychopharmacol. 1997;11:99–106. doi: 10.1177/026988119701100202. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LH, Dapretto M, O'Hare ED, Kan E, McCourt ST, Thompson PM, Toga AW, Bookheimer SY, Sowell ER. Relationships between brain activation and brain structure in normally developing children. Cereb Cortex. 2009;19:2595–2604. doi: 10.1093/cercor/bhp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasser PE, Johnston P, Lagopoulos J, Ward PB, Schall U, Thienel R, Bender S, Toga AW, Thompson PM. Functional MRI BOLD response to Tower of London performance of first-episode schizophrenia patients using cortical pattern matching. Neuroimage. 2005;26:941–951. doi: 10.1016/j.neuroimage.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Pace-Savitsky K, Perry RJ, Kramer JH, Miller BL, Levenson RW. Recognition of emotion in the frontal and temporal variants of frontotemporal dementia. Dement Geriatr Cogn Disord. 2004;17:277–281. doi: 10.1159/000077154. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapiro G. Partial differential equations and image analysis. Cambridge: Cambridge UP; 2001. [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Leahy RM. Brainsuite: an automated cortical surface identification tool. Med Image Anal. 2002;6:129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human lifespan. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, Doddrell DM, Wang Y, van Erp TG, Cannon TD, Toga AW. Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia. Neuroimage. 2004;23(Suppl 1):S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Economo C. The cytoarchitectonics of the human cerebral cortex. London: Oxford Medical Publications; 1929. [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Dawant BM. Brain segmentation and white matter lesion detection in MRimages. Crit Rev Biomed Eng. 1994;22:401–465. [PubMed] [Google Scholar]