Abstract
Objective
To evaluate the current literature on the impact and potential mechanisms of varicocele repair on male fertility.
Design
Pertinent articles were identified through computer PubMed search on varicocele repair and male infertility. References of selected articles were hand searched for additional citations.
Conclusions
Varicocele repair has been shown to reverse a spectrum of effects contributing to men with impaired fertility. Clinical studies on the intervention have illustrated variable effects on postoperative sperm parameters and pregnancy rates. Studies with conflicting results suffer from a significant number of confounding variables, such variable repair technique or lack of controls. Further studies are warranted on the role of modern microsurgical varicocelectomy given the improvements in assisted reproductive technologies.
Introduction
Varicoceles, or abnormally dilated veins in the pampiniform plexus, have long been associated with male infertility due to the observations that varicoceles are seen more commonly among infertile men and have been associated with abnormalities in semen analyses.1,2 In fact, varicoceles are the most commonly seen and correctable cause of male factor infertility.3 Varicoceles have an incidence of 4.4-22.6% in the general population, 21-41% in men with primary infertility, and 75-81% in men with secondary infertility.4,5 Suspicions that the varicocele should be considered as a possible cause or contributing factor in male infertility have existed for centuries, but Tulloch’s report of his experience with surgical correction and subsequent improved sperm counts and postoperative fertility spawned significant research interest on the topic over the past 55 years.6 While most men with varicoceles are able to father children, an abundance of evidence shows that varicoceles are detrimental to male fertility and surgical correction offers an improvement in a couple’s chances of obtaining a pregnancy, either spontaneously or through assisted reproductive technologies (ART).
Despite numerous reports of pregnancies and restored fertility, few studies exist with an adequate experimental design to fully assess the utility of varicocele repair in the treatment of male infertility. Furthermore, the collection of studies that have been performed are extremely heterogeneous in the parameters of the populations studied, including initial grading of varicocele lesion, presence of infertility, age of patients treated, and age of their partners. This has led to extensive controversy. Based on current evidence, it is the practice guideline of both the American Urological Association (AUA) and the American Society for Reproductive Medicine (ASRM) that correction of a varicocele should be offered to infertile men with palpable lesions and one or more abnormal semen parameters.7,8 However, evidence exists that does not support this statement and has led to the conclusion of the updated Cochrane review in 2009 that treatment of varicocele does not improve the chances of conception when present as the only proven explanation of infertility.9
Because male factor abnormalities continue to be found in up to 50% of couples presenting with infertility10 and because varicocele is the most common finding,3 it would be pertinent to review the existing literature pertaining to the correction, either operative or non-operative, of a varicocele and its impact on fertility. The objective of this article is to provide an overview of the indications and choices for treatment, as well as highlight points of controversy in the literature. Furthermore, this review may help improve counseling of patients prior to pursuing therapy as to anticipated expectations for improvements in fertility.
Diagnosis
Varicoceles can be diagnosed by several means, with physical exam and scrotal ultrasound being the most utilized methods. The condition is graded at the time of initial physical exam from 1-3 (Dubin grading system), with grade 3 being visible while the patient is standing, grade 2 is palpable without Valsalva maneuver, and grade 1 is not able to be visualized and only palpable with Valsalva maneuver. The term ‘clinical varicocele’ refers to those detectable by physical exam, either by palpation or visual inspection. Significant inter-examiner variability exists regarding the diagnosis of varicocele depending largely on level of expertise. As a result, if concern for a varicocele arises in a male, a formal exam by urologist is warranted. For those who utilize scrotal ultrasound as a diagnostic modality, criteria for diagnosing a subclinical varicocele by scrotal ultrasound requires at least the presence of dilated veins with diameter >3.0 mm with concomitant reversal of flow after Valsalva.11 Hirsh et al developed an additional grading system based on Doppler ultrasound, which correlates with increasing degrees of venous reflux, but has been lesser utilized.12 Grading of varicoceles by physical exam seems to have the greatest utility and has been shown to correlate with impact on fertility and response to treatment.
Pathophysiology
The etiology and pathophysiology of varicocele appears complex and multifactorial. Evidence indicates the phenomenon is age-dependent, as the incidence in pre-pubertal boys is extremely rare and increases to about 15% in adolescents.5 Additionally, the effects of a varicocele on semen parameters, testicular size and other indices of testicular function progress with time as men with a varicocele older than 30 years have lower sperm concentrations, impaired Leydig cell function and lower testosterone concentrations.2,13,14 However, significant variability exists in the effect of varicocele on male fertility. Varicoceles have been observed in both fertile and infertile men. As a result, it seems varicoceles may impair spermatogenesis, but with only clinical ramifications in some. Genetic factors and toxins may also serve as potential co-factors in development and implications of a varicocele.15
Most varicoceles are left sided, possibly due to anatomical configuration with a more vertical inlet of the internal spermatic vein to the renal vein as opposed to a more oblique inlet on the right. As a result, the hydrostatic pressure in the left venous system is higher, while the tapering configuration on the right side may protect against venous reflux. Defective or missing venous valves also play an important role in the pathogenesis.16 Other anatomic variants that lead to partial compression of the venous system such the left renal vein between the aorta and the superior mesenteric vein (“nutcracker syndrome”) or extrinsic pressure from retroperitoneal processes on the internal spermatic vein can also contribute to the development of a varicocele secondarily.17
Histological studies of testicular biopsies of varicocele patients indicate varying levels of dysfunction. Abdelrahim et al studied bilateral testicular biopsies from thirty infertile varicocele patients taken both during varicocelectomies, and postoperativly.18 Compared to healthy control subjects, preoperative biopsies showed reduced spermatogenesis with maturation arrest, dead spermatogenic epithelium and an increase in the volume of Leydig cells. After treatment, spermatogenesis improved in twenty-two of the patients, who also showed regeneration of the epithelium. The quantity of Leydig cells was normalized in eighteen patients. Other studies of varicocele patients have found Sertoli cell only syndrome19, spermatogenic arrest20, and hypo- or normo-spermatogenesis.21
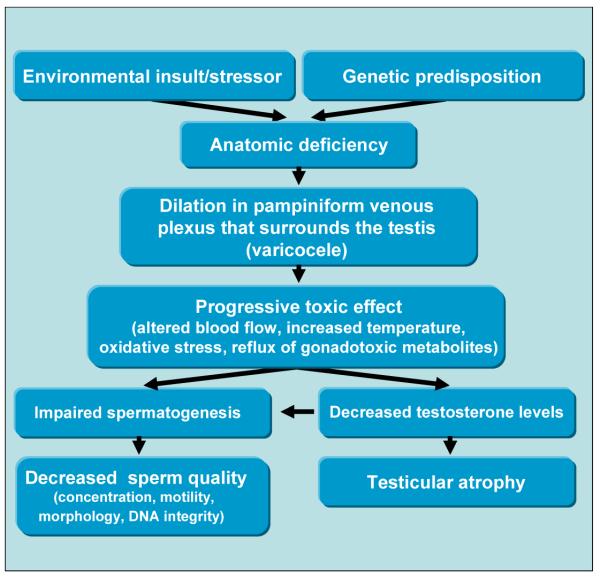
Several studies have focused on determining mechanisms of how varicocele leads to impaired spermatogenesis and infertility. Most of these propose a mechanism of altered or impaired testicular blood flow and include increased scrotal temperature, as well as oxidative stress, (see Figure 1). Additionally, resulting sex hormone changes, reflux of adrenal hormones and autoimmunity have also been cited as possible causal factors.
Figure 1.
Flow chart showing progression of varicocele and spectrum of effects that contribute to impaired fertility.
Normal testicular temperatures are approximately 2°C below core body temperature and increases in scrotal temperature are associated with reductions in both sperm output and quality. It is hypothesized that increased venous reflux caused by varicocele leads to increased testicular temperature. This theory is supported by Naughton et al 22 who found varicoceles are associated with measurable increases in scrotal temperatures. Additionally, Jung et al 23 demonstrated that men with varicocele and reduced sperm quality have significantly higher scrotal temperatures than men with normal sperm quality. Interestingly, these studies found that treatment of varicoceles reduced testicular temperature. Not all studies have been able to directly correlate temperature and infertility in varicocele patients.24 Zorgniotti and MacLeod were able to show a significant difference in the average scrotal temperatures of varicocele patients and controls, however, they also noted a considerable amount of overlap between patients between groups.25 An explanation as to why only some patients become infertile may be that additional temperature increasing factors are required before the sperm quality is reduced significantly. Factors, such as sleeping posture, duration of sedentary posture and exposure to high temperatures, are likely to increase the scrotal temperature further.
Another possible explanation for the negative impact of a varicocele is its association with increased production of reactive oxygen species (ROS). A growing body of literature directly correlates increases in ROS and reduced sperm quality.26 Oxidative stress, from increased testicular temperature, build up of ROS and other gonadotoxic factors associated with varicoceles, may cause reduced sperm function through oxidation of fatty acids in spermatozoa membranes or through direct DNA damage resulting in increased sperm DNA fragmentation.27 Studies of infertile men with varicocele indicate higher levels of DNA damage in sperm compared to fertile controls with no varicocele.28,29 Similar results were found by Blumer et al, who also showed a decrease in sperm mitochondrial activity in men with varicocele.30 In adolescents with bilateral varicocele, Bertolla et al. found significantly higher levels of DNA fragmentation compared to those patients with no varicocele.31 Levels of such biomarkers for oxidative stress have also been found to decrease after varicocele repair.32 Initial reports indicate this sperm DNA damage may be due to increased ROS and reduced total antioxidant capacity (TAC) of semen in men with varicocele.33-36 A meta-analysis by Agarwal et al confirmed that reduced TAC and increased oxidative stress may be a component to the etiology of infertility in men with varicocele.36 Since some men show an increase in ROS while maintaining fertility, it is possible that there is a spectrum of damage or that there is a difference in the amount of protective antioxidant factors. One explanation of this phenomenon could be increased venous pressure, which has been documented in the pampiniform plexus in varicocele patients.37 The increased venous pressure is suspected to reduce testicular blood flow and thus induce testicular hypoxia which could lead to accumulation of toxic metabolites resulting in damage to the testicular tissue. Studies have not yet demonstrated significant changes in testicular blood flow when comparing varicocele patients to healthy controls; however, this may be due to imperfect methods of measuring blood flow38, as it has not yet been possible to measure changes in microcirculation.22
Imbalance in the hypothalamic-pituitary-gonadal axis and reduced testosterone levels in the peripheral blood have been described in some varicocele patients39and may be additional contributors to observed diminished sperm production and quality. The theory is supported by improvements found in sperm quality associated with a normalization of the endocrine axis and an increase in testosterone levels after varicocelectomy.32,39 However, other studies have also shown no difference in sex hormone levels between infertile varicocele patients and healthy controls40 and not all studies on the subject have been able to demonstrate a link between a post-operative increase in testosterone levels and improvement of sperm quality.41 A problem in the cited studies is that testosterone levels are traditionally measured in peripheral blood and testicular testosterone levels may be decreased even though serum levels are normal. Studies examining intratesticular testosterone levels in varicocele patients are lacking. Reduced testosterone levels could be caused by compromised synthesis progressing over time, possibly due to Leydig cell damage. It is also possible that increased genital heat reduces activity of testicular enzymes, which could also explain impaired spermatogenesis in varicocele. An additional explanation for impaired spermatogenesis includes deficient sperm maturation or increased sperm apoptosis as a result of low testosterone.
Some have postulated reflux of catecholamines from the adrenal gland as another hypothesis inferring such metabolites lead to vasoconstriction in the testicular vessels, thereby reducing the testicular function; however definitive support for this theory in animals and humans is lacking.42
Varicoceles have also been associated with possible breaches in the blood-testis barrier and subsequent anti-sperm antibody formation. Studies have shown that infertile men have higher levels of auto-antibodies than fertile men. However, infertile varicocele patients have an auto-antibody level equal to infertile men without varicoceles43 and animal studies44 have shown that the blood-testis barrier is not broken by the induction of a varicocele suggesting no correlation with auto-antibodies. Interestingly, however, some infertile men have both a varicocele and anti-sperm auto antibodies, which could possibly result in an additive effect in these patients.
Regardless of specific mechanisms, it seems likely that the pathophysiology of varicocele is multifactorial and involves additional effects that interrelatedly increase the detrimental effects on spermatogenesis. Differences in the incidence of these factors may, in part, explain the conflicting literature relating to varicocele-associated infertility.
Techniques of Varicocele Repair
Understanding the approach taken for varicocele repair is critical for interpretation of relevant literature regarding subsequent improvements in endpoint parameters, as varying treatments are used depending on severity of the varicocele and may help explain conflicting results. A variety of operative and non-operative approaches have been advocated for varicocele repair, including percutaneous radiological techniques (embolization or sclerotherapy), open surgical (inguinal, subinguinal, retroperitoneal approach), laparoscopic, and microsurgical (inguinal or subinguinal) varicocelectomy. The optimal procedure would be one that ligates both the veins contributing to the varix at the time of repair and those that could lead to a recurrence in the future. However, some veins clearly must be preserved so as to allow drainage of the blood from the testis and prevent vascular engorgement. Additionally, the procedure should also leave intact the testicular arteries, lymphatics and vas deferens.
Unilateral or Bilateral Repair
Though varicoceles most commonly present on the left side, this swelling can occur on the right side as well, individually or in unison. Bilateral varicocele would seemingly be more detrimental than a unilateral defect. Several investigations have examined whether bilateral repair is similar or superior to one-sided repair. Kondoh et al reported a small cases series of 27 men with bilateral varicoceles and 40 unilateral left-sided varicoceles and noted less improvements in sperm density in the group with bilateral when compared to the left-sided only group.45 Four subsequent reports all have demonstrated evidence to support the contrary. Scherr et al prospectively studied 91 men with moderate-to-large left varicocele and small (grade 1) right varicocele and noted significant greater improvements in motile sperm concentrations in those with bilateral repair.46 Fujisawa et al and Libman et al both observed significantly greater improvements in concentration and/or motility after bilateral surgical repair.47,48 Furthermore, Libman et al and Baazeem et al noted significantly higher spontaneous pregnancy rates in those with bilateral repair compared to unilateral repair.48,49 This evidence would support the notion that subfertility in men with abnormal semen analyses and bilateral palpable varicoceles is the result of an additive effect of both and would justify simultaneous repair, even if small.
Additional studies have examined impact of unilateral or bilateral repair of a clinical varicocele in the presence of a subclinical varicocele with conflicting results 50-53 However, grade of varicocele should not dictate treatment. Therefore, in these cases, bilateral repair is likely warranted.
Non-microsurgical Techniques for Spermatic Vein Ligation
In 1948, Palomo described the classic retroperitoneal high ligation. This technique traditionally involves ligating the internal spermatic vein as it exits the inguinal canal and preserves the internal spermatic artery.54 The retroperitoneal approach was one of the first techniques developed and while still a reasonable technique, has been associated with higher rates of recurrence and postoperative hydrocele.55 Two modifications of this technique include the inguinal (Ivanissevich) or subinguinal approaches. Both approaches involve an incision at (subinguinal) or above (inguinal) the external inguinal ring. The subinguinal technique has the benefit of preserving muscle layers and the inguinal canal, however, is also more technically challenging due to the greater number of internal spermatic veins and arteries below the external ring.55 These approaches have also been adapted to include an operating microscope, which allows for greater preservation of other anatomic structures. Furthermore, testicle can be delivered to ligate contributing collateral gubernacular veins. This additional step, described to reduce the risk of recurrence, is less frequently incorporated due to the relatively low recurrence rate with the inguinal and subinguinal approaches and data to support equivalent postoperative outcomes with and without delivery.140,141 The nonmicrosurgical versions of these procedures also have relatively low rates of other complications (e.g., hydrocele). The laparoscopic approach is very similar to the retroperitoneal approach as far as identifying anatomic landmarks. Advantages include higher magnification than other nonmicrosurgical open procedures.55 However, this technique is still considered fairly invasive and can be challenging when attempting to delineate spermatic veins and arteries.
Radiointerventional Techniques
Interventional radiologists offer occlusion procedures (embolization, sclerotherapy) as minimally invasive outpatient options that have the advantage of venography to delineate anatomy more clearly. While less invasive, this is the only approach that offers the potential for failure to ligate the varix. Depending on the skill level of the team performing the procedure, the failure rate can vary from 4-27%.55 Recurrence rates are also noted to be higher with this approach. This approach is also useful in the scenario of recurrent varicoceles as anatomy may be better delineated radiographically.
Microsurgical Approach
The microsurgical approach is now the preferred approach by most urologists, due to the significant reductions noted in recurrence rates or other postoperative complications. An operating microscope is incorporated into either the traditional inguinal or subinguinal approach and allows for the more reliable identification and preservation of the testicular artery or arteries, cremasteric artery or arteries, and lymphatic channels. Enhanced visualization also aids in the identification of all possible routes of venous return contributing to the varix, including external spermatic, cremasteric and gubernacular veins.56,57
Complications
Hydrocele formation was previously the most common complication reported after operative varicocele repair. With the classic nonmicrosurgical approach, the incidence rates were approximately 7%, ranging 3-39%,58 presumably from secondary to the ligation of lymphatic channels of the testicle. When present, approximately half of hydroceles will develop to a size that produce discomfort that warrants surgical hydrocelectomy. However, with the advent of microsurgical and radiointerventional techniques, the incidence has dramatically reduced almost eliminating this as a postoperative complication.56,57
Recurrences after varicocele repair are the most variable complication in incidence and rates depend largely on the technique utilized and the use of magnification. Rates can vary from 0-35%.59 Studies involving venography have demonstrated recurrences to be caused by periarterial, parallel inguinal, midperitoneal, gubernacular and transscrotal collateral veins.58 Again, advances in microsurgery have allowed for greater ability to visualize these contributing vessels.
Testicular artery ligation or injury is also a common complication of nonmicrosurgical varicocelectomy although its true incidence is unknown.60 Because of the presence of other spermatic cord arteries, such as the vasal and cremasteric arteries, injury to the artery does not always result in atrophy. Penn et al reported an incidence of 14% when the testicular artery was purposefully ligated during renal transplantation.61 However, postoperative atrophy can lead to decreases in sperm counts or azoospermia in some cases, a devastating complication to the infertile couple with hopes surgical intervention would improve chances of spontaneous conception.
Comparative Studies
Numerous studies have been published evaluating the various methods of varicocele repair, some evaluating early postoperative courses and others following up for one to two years evaluating for complications or evidence of improved fertility. Most of these studies involve prospective collection of data and randomization of subjects; however, few include a control (no treatment) group.
Reports of operative experiences have noted longer operative times with microsurgical approach in comparison to open and laparoscopic approaches with the exception of one retrospective study.62-65 Published reports of early postoperative courses document only small differences;65,66 however one study did note higher rates of postoperative complications (epididymitis, prolonged pain) when comparing laparoscopy with sclerotherapy.66 Furthermore, microsurgery has also been noted to have higher rates of preserving the testicular artery63 and the lowest rates of recurrence and hydrocele formation. 62-65, 67,68 Embolization and sclerotherapy, while having slightly higher risk of recurrence, have very little to no risk of hydrocele formation.67,69
Improvements in semen parameters have been the main outcome of most studies focusing on men with infertility for at least 12 months as the target population. Yavetz et al noted the greatest overall improvement in measures of sperm quality with subinguinal approach in comparison to transinguinal approach and embolization (significant improvements in concentration, motility and morphology by six months).70 Sayfan et al also noted a more measurable improvement in postoperative sperm counts in open approaches as opposed to embolization.71 Barbalias et al published a trial comparing all three nonmicrosurgical techniques with embolization and reported improvements in sperm concentration for all four groups, but significant increases in motility for only the inguinal and subinguinal approaches.67 Cayan et al, in a large prospective study of 468 infertile men, compared microsurgical approach with high ligation and also noted greater increases in motility with microsurgery.68 Three recent randomized trials comparing open, laparoscopic and microsurgical methods reported improvements in concentration, motility, and/or morphology in comparison to preoperative evaluations, but did not demonstrate significant differences between techniques based on sperm parameters alone.63-65
Regarding pregnancy rates, Cayan et al reported higher rates with microsurgical approach compared to high ligation.68 Al-Kandari et al and Al-Said et al both published randomized controlled trials comparing microsurgical to open and laparoscopic approaches were not able to reproduce this observation, but also did not have as great of power based on size of study population to truly show contradictory evidence.64,65 While these comparative studies provide a significant amount of information on postoperative expectations, it is still difficult to make direct comparisons due to the heterogeneity of study design, follow-up and published statistics without a more standardized method.
Meta-analyses
Given the results of the Cochrane review in 2009 being contrary to the consensus statement by both ASRM and AUA, possibly due to poor patient inclusion criteria, two meta-analyses were recently published in 2009 to provide further insight. Cayan et al specifically evaluated rates of postoperative complications and spontaneous pregnancies and excluded studies involving subclinical varicoceles. After pooling the data from 4473 men undergoing repair by various techniques, pregnancy rates were compared and significant differences were noted depending on the technique. The highest rates were seen with the microsurgical technique, followed by non-microsurgical approaches (see Table 1).72 This method was also noted to have the least documented recurrences, postoperative hydroceles, or other complications. Agarwal et al conducted a meta-analysis that included both randomized controlled trials and observational studies in attempts to evaluate the data with more focused attention on men with documented infertility, abnormal semen analysis and clinical varicoceles. While accepting their study was subject to bias from the variability of semen data in sequential analyses, the authors highlight the significant number of data that document a direct positive relationship between improvements in semen parameters over time and varicocele repair. Agarwal illustrate significant improvements in concentration, motility and morphology in studies evaluating high ligation and microsurgery (see Table 2).73
Table 1.
Rates of pregnancy and complications based on technique. (Modified from data in Cayan et al, 2009).72
| Technique | Artery Preserved |
Hydrocele (p=0.001) |
Recurrence (p=0.001) |
Potential for serious morbidity |
Pregnancy rates (p=0.001) |
|---|---|---|---|---|---|
|
Retroperitoneal
High ligation (Palomo) |
No | 8% | 15% | No | 38% |
|
Macroscopic
inguinal (Ivanissevich) |
No | 7% | 3% | No | 36% |
| Laparoscopic | Yes | 3% | 4% | Yes | 30% |
| Radiologic | Yes | 0% | 12% | No | 33% |
| Microsurgical | Yes | 0.4% | 1% | Yes | 42% |
Table 2.
Differences in postoperative semen analysis. (Modified from data in Agarwal et al, 2007).73
| Technique | Sperm concentration | Average motility |
|---|---|---|
| High ligation | + 12.03 × 106/mL | + 11.72% |
| Microsurgery | + 9.71 × 106/mL | + 9.92% |
Varicocele Repair and Effect on Semen Analysis Profile
Varicoceles have classically been described to induce a “stress pattern” that affects several parameters measurable on semen analyses simultaneously. Most studies focus on abnormalities in concentration, motility and/or morphology. Each of these parameters individually has been examined for relative post-operative improvements after surgery, but also as independent prognostic factors of whether varicocele repair is a successful treatment strategy for male subfertility.
Asthenospermia
Approximately 19% of subfertile men with a clinical varicocele will present with isolated abnormalities in sperm motility.74 In attempts to examine varicocele repair as an intervention for men with varicocele-associated asthenospermia, Boman et al reported a retrospective review of 118 infertile men who met these criteria including 69 who underwent microsurgical varicocelectomy and 49 who chose not to undergo repair. Varicocele repair resulted in significant increases in total motile sperm count (30 million/ejaculate compared to 39 million, p <0.05) and spontaneous pregnancy (65% compared to 32%, p<0.01). Interestingly, those who did not undergo repair were more likely to utilize IVF/ICSI (32% compared to 11%, p<0.05) and no differences in pregnancy rates following assisted reproduction (IUI, IVF, and ICSI) were noted.74 Similarly, Schatte et al prospectively examined 61 subfertile men with clinical varicoceles who underwent microsurgical inguinal varicocelectomy and followed postoperatively for increases in sperm parameters. The study included men with normal sperm concentrations and noted a significant increase in motility (39% preoperatively to 45% postoperatively, p = 0.008).75 This observation supports Schlesinger’s conclusions from his analysis of twelve previous studies. While only five of the studies showed statistically significant improvements in motility, he concluded that varicocelectomy is associated with improvements in motility.76
Teratozoospermia
Several authors have published retrospective data looking at post-operative improvements in sperm morphology as well as teratozoospermia as primary indication for repair. Vazquez-Levin et al published the first report of improvements in terms of Kruger morphology in postoperative semen analyses after microsurgical varicocelectomy. Of the 33 men they studied, 13 had oligozoospermia, 31 had asthenospermia and 29 had teratozoospermia (based on morphology less than 14% strict normal forms). The authors noted significant improvements in concentration and morphology. Twelve of the 26 (46%) with morphology ranging 4-14% noted significant improvements to normal values by three months postoperatively. The three patients with severe teratozoospermia (less than 4% normal forms) also showed improvements.77 Seftel et al and Kibar et al subsequently published studies also examining postoperative improvements in morphology and observed mixed results.78,79 Seftel et al reviewed 30 infertile men with teratozoospermia who underwent microsurgical varicocelectomy and noted improvements in concentration and motility, but not in morphology.78 Kibar et al, however, reviewed 90 men with infertility and teratozoospermia and stratified results based on concentration (less than 5 million/mL, 5-20 million/mL, greater than 20 million/mL) and noted improvements in morphology for the whole group, but significant improvements in all sperm parameters in the oligospermic (5-20 million/mL) group.79
Results from studies examining men with teratozoospermia but otherwise normal sperm counts are also conflicting. Okeke et al published a retrospective analysis of 167 men with infertility and bilateral varicoceles who underwent high ligation and stratified them according to concentration.80 Postoperative semen analyses for the whole cohort showed improvements in concentration and motility, but not in morphology. After stratification, men with normal sperm counts had no significant improvements in sperm parameters and 21% (10/46) became oligospermic afterwards.80 A separate report of subfertile men with isolated teratozoospermia and clinical varicocele by Cakan et al showed significant improvement in all sperm parameters and spontaneous pregnancy rate of 18% (5/29) after subinguinal repair in comparison to those who decided against surgery who had no improvements in semen analysis and no pregnancies (0/23) in the follow up period (12 months).81 These results only further underline the need for controlled studies that have less confounding variables that may influence the significance of the data.
Oligozoospermia
A multitude of studies have been published illustrating the improvement of sperm counts after varicocele repair. By 1994, Schlesinger identified 16 studies involving 1077 treated patients. Twelve of the 16 studies showed statistically significant improvements in sperm concentration postoperatively.76 The majority of these studies, however, included a varying fraction of men with normal sperm concentrations. Data from early observational trials have shown an effect of preoperative sperm concentration as a determining factor on chances of improving sperm parameters and pregnancy. Dubin and Amelar noted lesser percentage of improved semen quality in those with sperm concentration < 10 million/mL.82 Subsequent studies from Matkov et al, Kamal et al, and Fujisawa et al published evidence to support that men with severe oligozoospermia (less than 5 million/mL) are less likely to see improvements in semen parameters.83-85 Kamal et al were also able to display a direct relationship between preoperative sperm count and postoperative pregnancy rates, most significantly that men with severe oligozoospermia have much lower chance of spontaneous pregnancy (8% compared to 61% in those with greater than 5 million/mL).84
Studies that examined men with low sperm counts in the less severe range show greater postoperative improvements. Madgar et al restricted their prospective study to men with concentration 5-20 million/mL, limiting the number of confounding variables and were able to demonstrate a significant improvement in sperm concentration, motility and morphology (by six months postoperatively) and higher pregnancy rates than the control group, as previously later discussed (see section on Clinical Varicocele).85 Baazeem et al noted similar improvements in semen parameters in their recent review of 360 patients with concentrations ranging 1-20 million/mL.49
Severe oligozoospermia/nonobstructive azoospermia
Historically, varicocele repair has been as a primary treatment strategy of male infertility with the goal of improving spontaneous pregnancy rates. Severe oligozoospermia (SO) and nonobstructive azoospermia (NOA) are both conditions that significantly reduce a couple’s chances at spontaneous pregnancy. Early reports of varicocele repair demonstrate the potential, in some, to induce spermatogenesis and regain the potential for otherwise unassisted fertility.6 Additionally, the introduction of IVF and ICSI has allowed for further research on the role of varicocele repair as an adjunct to ART.
Approximately 4-13% of men with a palpable varicocele will present with azoospermia or severe oligoasthenospermia. Matthews et al first published a study looking specifically at this population of men. The authors prospectively evaluated patients after microsurgical varicocelectomy and followed for improvements in semen parameters as well as pregnancies. The majority of the cohort was observed to have return of motile sperm to the ejaculate postoperatively, 55% (12/22) of azoospermic men and 69% (35/51) of those with severe oligoasthenospermia. Mean total motile sperm count increased from 0.08 +/− 0.02 × 106 to 7.2 +/− 2.3 × 106 illustrating the potential for men to conceive a subsequent spontaneous pregnancy. Twenty-four (31%) conceived pregnancies, fifteen of which were unassisted.87 Additionally, testicular atrophy on initial exam had no prognostic value. A subsequent study by Kim et al noted return of motile sperm as well, but no spontaneous pregnancies.19
Several other small cohort studies have reported varying experiences with varicocele repair in men with SO or NOA. Rates at which motile sperm are noted in post-operative ejaculates range from 21-69%.19,87-95 Additionally, rates of subsequent spontaneous pregnancy range from 5.3-19%. 19,87,88,90-95 Alternatively, some authors examined number of motile sperm as an indirect measure of whether varicocele repair served to help avoid ICSI or TESE. Kadioglu et al noted in their study 21% maintained total motile sperm counts greater than 5 million (possibly avoiding IVF).88 Additionally, Schlegel et al noted 10% demonstrated at least enough motile sperm in the ejaculate to avoid testicular aspiration when pursuing ICSI.89 A limitation of these studies is the lack of a control group, as a previous study showed up to 35% of patients labeled as having NOA may have spermatozoa in the ejaculate, demonstrating the possibility of some low level variability in sperm production without intervention.96
Investigators have also looked at diagnosis on testicular biopsy (hypospermatogenesis, maturation arrest, Sertoli cell only) to identify which patients would be most likely to benefit. Based on the studies by Kim et al, Kadioglu et al, Esteves et al, and Lee et al, those with hypospermatogenesis and maturation arrest at later stages are more likely to see return of motile sperm and pregnancies postoperatively.19,88,90,92 Pasqualotto et al and Lee et al showed that patients are at risk for relapse to azoospermia in the follow-up period and recommend cryopreservation of post-operative samples containing motile sperm.91,92 Other variables investigated such as grade of varicocele, FSH level, testicular volume and laterality have not been associated statistically significant prognostic relationships.
Sperm DNA Damage
Though not a routine parameter measured in a standard semen analysis, sperm DNA integrity and the impact of varicocele and its treatment on sperm DNA damage is a growing area of interest, especially as more convenient methods of assessing sperm DNA integrity become available. Conflicting results exist regarding the impact of varicocelectomy on restoring two indirect markers of DNA integrity, total antioxidant capacity (TAC) and reactive oxygen species (ROS) levels. Mancini et al found no absolute change in semen TAC following surgical repair of varicocele.97 However, Mostafa et al showed that varicocelectomy can improve semen TAC and reduce ROS.98 Differences in findings may result from variances in grade of varicocele, as this can impact amount of ROS,99 surgical approach, as well as choice of measurement to assess TAC. With the advent of new laboratory assessment tools to aid in selection of higher quality sperm with less DNA fragmentation for use with ICSI100-102it will be interesting to see if varicocelectomy will be required in the future for specific patients, depending on subsequent treatment plans.
Classification of Varicocele and Effect of Repair on Fertility
Subclinical Varicocele
A subclinical varicocele is diagnosed when a non-palpable reflux or dilation in the internal spermatic vein is observed by radiological imaging study, most commonly by scrotal Doppler ultrasonography, but also venography or thermography. Attention to nomenclature is important in interpreting conclusions of studies reporting success or lack thereof after varicocele repair. Critics of varicocele repair as a treatment of male infertility often cite studies that included heterogeneous collection of men with subclinical and clinical varicoceles. Several investigators have thus questioned the impact of treating subclinical lesions and it continues to be a topic of clinical research.
Several studies in the mid-1980s and early 1990s looked at intervening in populations diagnosed with a subclinical varicocele and compared results with those diagnosed with clinical varicoceles. Marsman et al and Comhaire et al performed retrospective analyses of patients who underwent embolization as treatment. Marsman et al noted significant improvements in postoperative sperm quality (density, motility and morphology).103 Additionally, Comhaire et al noted similar postoperative pregnancy rates when compared to men with a clinical (or palpable) varicocele.104 Similar post-operative improvements in semen parameters105,106and pregnancy rates106 were noted in studies comparing men with subclinical and clinical varicoceles after surgical repair. These studies seemed to agree with previous observational findings of Dubin and Amelar in their series of 111 men who underwent varicocele repair, that size of varicocele did not determine the likelihood for improvement after intervention.107
Three subsequent prospective randomized controlled trials examining varicocele repair for subclinical varicoceles showed modest post-operative improvements. Yamamoto et al randomized 85 infertile men with subclinical varicocele to either high ligation or observation and followed for one year postoperatively. In comparison to the control group, those who underwent ligation had significant improvements in sperm density and total motile counts; however, no significant difference was noted in pregnancy rates.108 Unal et al randomized 42 infertile men with isolated left subclinical varicocele to either high ligation or clomiphene and similarly noted improvements in certain sperm parameters (sperm density and motility), but no significant difference in pregnancy rates.109 Grasso et al, in their randomized controlled trial of 68 infertile men with abnormal semen analysis and ultrasound-detected left varicocele (Hirsh classification), observed no differences when comparing post-operative semen analyses or pregnancy rates between the group who underwent high ligation (Palomo technique) versus observation for 12 months.110,12 Thus, conclusive evidence in favor of repair of subclinical lesions is lacking.
Clinical Varicocele
An abundance of studies have been published on clinical varicocele and despite some with large numbers and some with adequate control groups, the debate continues on whether or not treatment of varicocele offers a true improvement in fertility. Of all the studies on the topic, several inherently carry more weight due to better study design. The emphasis of this review is to focus on the articles that minimize confounding variables.
No Benefit
Nilsson et al published the first randomized controlled trial on varicocele repair in 1979. Ninety-six men with infertility and visible varicoceles were randomized to Palomo high ligation or no treatment. Men were followed at 6 month intervals and assessed with semen analyses and whether or not pregnancy occurred. No difference between pre- and postoperative semen analyses were noted when comparing treatment group to the control group, nor was a difference in pregnancy rates detected.111 Critics of this study cite that the authors had a suboptimal study population as a significant proportion of men with normal semen analyses were included in the study. Furthermore, pre and postoperative semen analyses are reported as only “before” and “after” and not stratified based on postoperative month at which the sperm sample was obtained. Similar concerns regarding the selected study population have been voiced regarding the study performed by Breznik et al.112 Breznik et al presented a prospective study of 96 infertile men randomized to either no treatment or treatment by various methods (surgical, sclerotherapy, or embolization) which again included a significant proportion with either normal preoperative semen analysis or subclinical varicocele. No significant differences in semen parameters or pregnancy rates were noted between treatment and control groups.112 An additional critique of this study would be the heterogeneous nature of the intervention assigning the treatment group to three various and not equal methods of repair, thus introducing another extraneous variable. Krause et al provides the most recent evidence of no benefit in their multi-center randomized controlled trial of 65 infertile men who were randomized to sclerotherapy or no treatment. No significant difference was noted in rates of spontaneous conception at 12 months; however, the study suffered from poor recruitment and poor follow-up. From the initial report of the intention to treat analysis, 34 patients were lost to follow-up and assumed that no conception occurred.113
Based on the results of these studies and the modest evidence from others, the Cochrane systematic review on varicocele repair calculated an odds ratio of postoperative spontaneous pregnancy as 1.10 (95% CI, 0.73-1.68), indicating no benefit of varicocele repair over expectant management in subfertile couples that have no other abnormal findings in their workup.9
Benefit
Studies reporting the benefit of varicocele repair date back to even before Tulloch’s report that popularized repair to the modern medical community. The studies included in this review represent those with larger study populations and/or controlled study design within the past 25 years.
Okuyama et al published a case-control study of 224 subfertile men and a clinical varicocele who either underwent repair via Palomo technique (n=141) or chose no treatment (n=83). Men were followed up for 12 months with semen analyses and monitored for incidence of pregnancy postoperatively for two years. The authors noted a significant improvement in sperm density and percentage of progressive motile sperm. Pregnancy rates were also noted to be significantly higher in the corrected group (30% compared to 18%). The majority of the pregnancies (85%) occurred within the first year after repair.114 The study published by Madgar et al, by far, demonstrates the greatest correlation with repair and improvements in subsequent fertility potential. Madgar selected infertile men with visible or palpable left-sided varicoceles and oligozoospermia (concentration ranging between 5-20 million/mL) and randomized to immediate high ligation (Palomo technique) or observation followed by ligation at 12 months if not pregnant. This crossover study demonstrated significant improvements in sperm concentration, motility and morphology and a significantly higher pregnancy rate within 12 months after surgery (60% compared to 10% with the control group). Pregnancy rates were also significantly higher within the delayed-treatment group when comparing 12 months of observation to first 12 months after surgical correction (44% compared with 10%).86 As heterogeneity of the study population or treatment technique increase, the effects of varicocele repair become less demonstrable by studies. Onozawa et al noted more modest improvements in sperm parameters in their case-control study of 64 men115 and Nieschlag et al noted no statistical difference in pregnancy rates when comparing treatment to no treatment.116 Schlegel, in his review of twelve controlled studies of varicocelectomy, estimated pregnancy rates for those who undergo repair are significantly greater than those who choose to defer (33% and 16%, respectively).117
The conclusions of Evers and Collins in their systematic review spurred Ficarra et al and Marmar et al both to re-evaluate the existing data with meta-analyses as well.118,119 Ficarra et al published a meta-analysis including only three of the randomized controlled trials 113,86,116 and excluded those where subjects with normal semen analyses or subclinical varicoceles were included. The authors formally concluded that the heterogeneity of the data and poor quality of study design does not allow for formal analysis. At the same time, the authors used this same argument to refute the conclusions by Evers and Collins.118 Marmar et al published a meta-analysis of five studies on surgical repair only on infertile men with clinical varicocele and abnormal semen analysis looking at spontaneous pregnancy rates. The authors included randomized controlled trials and also observational studies. While accepting that this is not standard for the meta-analysis format, the authors state that their inclusion and exclusion criteria allow for less heterogeneity in the population studied and intervention being studied. Odds ratio of spontaneous pregnancy after varicocelectomy was calculated to be 2.87 (95%CI, 1.33-6.20, P=0.007). Pregnancy rates also were significantly higher in those treated than in those not treated (33% vs. 15.5%, respectively).119
The current published studies have focused on the now dated non-microsurgical approaches that are associated with higher rates of recurrence and hydrocele formation. To date, no randomized controlled trial (RCT) has examined the effect of modern microsurgical varicocelectomy on fertility in men with abnormal semen analysis and a clinical varicocele. A modern RCT focusing on the microsurgical technique in appropriate patients is needed to resolve the controversy over the benefit or lack of benefit of this technique.
Grade of Clinical Varicocele
Given the observations that not all men benefit from varicocele repair, researchers have sought out markers that would identify those who benefit the most (e.g., greatest improvements in sperm parameters and/or improvement in fertility). Dubin and Amelar first looked at grade of varicocele as a prognostic factor in 1970 and noted no difference in degree of improvement when comparing grade of varicocele and differences between pre-operative and post-operative semen quality.107 This finding was supported by the report published by Marks et al.120 Findings from these studies have been interpreted by some as supporting evidence to advocate for repair of subclinical varicoceles. However, three subsequent studies 121-123 noted either significantly greater percentage of men with improvements or greater absolute improvements in postoperative sperm parameters (density and/or motility) in men with larger varicoceles. The study carried out by the World Health Organization in 1992 examined 9038 men with infertility, 921 of which had a varicocele noted on exam, and demonstrated lower sperm concentrations in men with varicoceles of increasing grade.2 Jarow et al noted in their prospective study no correlation in postoperative improvements based on grading, but did note significantly greater improvements in total motile sperm counts in those with clinical varicoceles when compared to subclinical, suggesting a modest but still present effect of size.124 This finding was more recently supported by the findings Ishikawa and Fujisawa published in 2005.125 Regardless, if associated with abnormal semen parameters and infertility, grade of varicocele should not be deterrent of varicocele repair.
Recurrent Varicocele
Recurrent or persistent varicoceles seen after attempted repair are due to the numerous collaterals (retroperitoneal, inguinal, or scrotal) of the internal spermatic vein. Given the risk of recurrence, it is routine for surgeons to follow-up patients to identify and treat any evidence of recurrences or persistent varicocele postoperatively. However, with the overall low incidence of recurrence seen after microsurgical approach, few studies have examined best route of repair if a recurrence is noted and if patients will experience similar improvements in sperm parameters demonstrated with primary correction. Madjar et al and Grober et al both noted good surgical outcomes from subinguinal approach.126,127 Madjar et al utilized nonmicrosurgical technique and noted marked improvement in size in 91% (21/23) of those undergoing secondary repair.126 Grober et al, with the addition of microscopy, noted no recurrences in the 35/54 patients that completed 6 months of follow-up.127 Both demonstrated significant improvements in semen parameters (concentration and motility) with repeat procedure.
Interestingly, Flati et al report a unique approach with microsurgical creation of a shunt from the internal spermatic vein to the inferior epigastric vein stating 20-30% of varicoceles result from iliospermatic or mixed ilio- and renospermatic reflux. The authors noted disappearance of varicosity in 97% and partial reduction in the remaining 3% as well as significant improvement in semen parameters with their approach.128 No studies have directly compared techniques utilized in the secondary repair of a persistent or recurrent varicocele; however, these studies do illustrate potential benefit to those patients looking for further improvements in semen parameters.
Surgical Treatment of Varicocele and Success of Assisted Reproductive Technologies
For those men with varicocele-associated infertility who have persistent infertility, ART has significantly contributed to their treatment. Sperm processing with intrauterine insemination (IUI), in vitro fertilization (IVF), and intracytoplasmic sperm injection (ICSI) can either serve patients as an alternative to surgery or as adjuvant therapy to achieve a pregnancy.
Intrauterine insemination
Marmar et al initially reported intrauterine insemination as a possible treatment option for men with history of a varicocele and refractory infertility. Of the 71 couples who underwent 187 inseminations, only six achieved a pregnancy.129 Pregnancy rates were observed to be much higher in a subsequent analysis by Daitch et al.130 To investigate if varicocele repair improved chance of success with intrauterine insemination, Daitch et al studied 58 couples with varicocele-associated infertility, 34 who previously underwent inguinal or subinguinal microsurgical repair and 24 who chose not to undergo repair. Pregnancy rates per cycle were noted to be 6.3% in the untreated group compared to 11.8% in those who underwent surgical repair (p=0.04). Odds of pregnancy were 4.4-fold higher in the surgically treated group favoring varicocelectomy as a strategy to improve chances of pregnancy with assisted means.130
IVF & ICSI
Several studies have examined effect of varicocele repair on various semen analysis parameters in attempts to correlate varicocelectomy with improved fertility. Unfortunately, these endpoint measurements are limited and more functional endpoint measurements are difficult to assess. At least one small observational study examining infertile men with a history of previous failed fertilization with IVF indicates that varicocelectomy could improve fertilization potential of sperm in a subsequent IVF cycle131 Another study by Ashkenazi et al utilized patients who previously failed to achieve pregnancy following IVF/ICSI, and attempted to attribute subsequent pregnancy success with IVF/ICSI following varicocele repair to the corrective surgery itself 132 However, it is impossible to attribute this success to the repair procedure and potential improved sperm quality without a more well-controlled study design. Additionally, other studies indicate varicocele repair has no impact on rates of pregnancy following IVF/ICSI,133,134 though it may decrease their pursuit of additional ART procedures.134 Whether this is attributed to improved fertility or simply due to cost, ethical concerns related to ART, or other factors is unknown. Interestingly, there is debate on whether varicocele is correlated with antisperm antibodies, and whether surgical correction has an impact135,136 that may influence subsequent ART treatment.
While some studies have indirectly attempted to answer this question by looking at postoperative motile sperm counts137,134, none have directly answered the question of whether varicocele repair in men with NOA or SO allows for less subsequent need for ART to be applied (e.g., what percentage of patients who do need IVF after repair would still be able to avoid testicular biopsy or ICSI). This question is worth answering considering its implications involve more than simply cost (chance of success, slight increase in risk of birth defects). Further, more well-designed studies are warranted to investigate if surgery and ART have an additive relationship.
Three cost analyses have been published that both favor varicocele repair as a more cost effective strategy. Schlegel et al and Meng et al both reported decision analyses that favor varicocele repair over ART.117,138 Schlegel estimated cost per live delivery after varicocelectomy and after ICSI to be $26,268 and $89,091, respectively.117 However, a subsequent analysis including only men with nonobstructive azoospermia who would require microsurgical testicular sperm extraction (TESE) favored microTESE as the more cost effective strategy to varicocelectomy in this subpopulation.139
Conclusion and Upcoming Research Directions
Varicocele repair is a reasonable consideration as the primary treatment option when a couple with documented infertility involves a male with a palpable varicocele and suboptimal semen quality and female partner has a normal evaluation. Bilateral repair is warranted when varicoceles are noted on both sides, regardless of grade. Persistent or recurrent varicoceles may be treated by either surgical ligation or percutaneous embolization. Comparative studies favor microsurgical approach as the technique with the highest rates of success and lowest rates of complications. However, approach to varicocele treatment should be based on the physician’s experience and the additional options available. Assisted reproductive technologies may serve as a viable adjunct or alternative to surgery to improve chances of pregnancy. Currently, two clinical trials investigating the contemporary role of varicocele repair in the treatment of male infertility are registered with ClinicalTrials.gov. A multicenter randomized study based in Mount Sinai Hospital, Canada (NCT00961558) is evaluating the effect of surgical repair versus observation alone on spontaneous pregnancy rates in infertile couples. Additionally, another multicenter randomized study sponsored by The Reproductive Medicine Network (NCT00767338) is evaluating the effect of microsurgical varicocelectomy versus intrauterine insemination on live birth rates in couples affected by male infertility. With improvements in ART lab technology, future research efforts are warranted to delineate the benefit of varicocele repair in patients who will require subsequent IVF/ICSI.
Table 3.
Summary of postoperative measurements after varicocele repair. Data is limited to statistically significant differences from randomized controlled trials
| Type of Varicocele |
Improvements in Semen Parameters | Improvements in spontaneous PR |
||
|---|---|---|---|---|
| Concentration | Motility | Morphology | ||
|
Subclinical Yamamoto108 Unal109 Grasso110 |
+ + + |
− + − |
− − − |
− − − |
|
Clinical Nilsson111 Breznik112 * Madgar86 Nieschlag116 Krause113 |
− − + + − |
− − + − − |
− Not assessed + − − |
− − + − − |
Note: Study included subclinical and clinical varicoceles.
Acknowledgements
The authors graciously acknowledge the support and input from all of the members of the Reproductive Medicine Network and supporting NICHD staff.
Funding/Support: This work is supported in part by NIH/NICHD grant U10 HD055936 (GMC and DAO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Russell JK. Varicocele in groups of fertile and subfertile males. Br Med J. 1954;1:1231–1233. doi: 10.1136/bmj.1.4873.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. Fertil Steril. 1992;57(6):1289–93. [PubMed] [Google Scholar]
- 3.Dubin L, Amelar RD. Etiologic factors in 1294 consecutive cases of male infertility. Fertil Steril. 1971;22:469–74. doi: 10.1016/s0015-0282(16)38400-x. [DOI] [PubMed] [Google Scholar]
- 4.Saypol DC. Varicocele. J Androl. 1981;2:61–71. [Google Scholar]
- 5.Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59(3):613–616. [PubMed] [Google Scholar]
- 6.Tulloch WS. Varicocele in subfertility: results of treatment. Br Med J. 1955;2:356–8. doi: 10.1136/bmj.2.4935.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarow JP, Sharlip ID, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. J Urol. 2002;167:2138–44. [PubMed] [Google Scholar]
- 8.Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. Fertil Steril. 2002;77:873–82. doi: 10.1016/s0015-0282(02)03105-9. [DOI] [PubMed] [Google Scholar]
- 9.Evers JH, Collins J, Clarke J. Surgery or embolisation for varicocele in subfertile men. Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD000479.pub2. CD000479. [DOI] [PubMed] [Google Scholar]
- 10.Macleod J. Semen quality in 1000 men of known fertility and 800 cases of infertile marriages. Fertil Steril. 1994;62:862–8. doi: 10.1016/s0015-0282(16)30482-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Binsaleh S, Lo K, Jarvi K. Varicoceles: The Diagnostic Dilemma. J Androl. 2008;29(2):143–146. doi: 10.2164/jandrol.107.003467. [DOI] [PubMed] [Google Scholar]
- 12.Hirsh AV, Cameron KM, Tyler JP, Simpson J, Pryor JP. The Doppler assessment of varicoceles and internal spermatic vein reflux in infertile men. Br J Urol. 1980;52(1):50–6. doi: 10.1111/j.1464-410x.1980.tb02919.x. [DOI] [PubMed] [Google Scholar]
- 13.Zini A, Buckspan M, Berardinucci D, Jarvi K. The influence of clinical and Subclinical varicocele on testicular volume. Fertil Steril. 1997;68(4):671–4. doi: 10.1016/s0015-0282(97)00311-7. [DOI] [PubMed] [Google Scholar]
- 14.Chehval MJ, Purcell MH. Deterioration of semen parameters over time in men with untreated varicocele: evidence of progressive testicular damage. Fertil Steril. 1992;57(1):174–7. doi: 10.1016/s0015-0282(16)54796-7. [DOI] [PubMed] [Google Scholar]
- 15.Marmar JL. The pathophysiology of varicoceles in the light of current molecular and genetic information. Hum Reprod Update. 2001;7(5):461–72. doi: 10.1093/humupd/7.5.461. [DOI] [PubMed] [Google Scholar]
- 16.Braedel HU, Steffens J, Ziegler M, et al. A possible ontogenic etiology for idiopathic left varicocele. J Urol. 1994;151:62–6. doi: 10.1016/s0022-5347(17)34872-3. [DOI] [PubMed] [Google Scholar]
- 17.Graif M, Hauser R, Hirshebein A, et al. Varicocele and the testicular-renal venous route: hemodynamic Doppler sonographic investigation. J Ultrasound Med. 2000;19:627–31. doi: 10.7863/jum.2000.19.9.627. [DOI] [PubMed] [Google Scholar]
- 18.Abdelrahim F, Mostafa A, Hamdy A, et al. Testicular morphology and function in varicocele patients: pre-operative and post-operative histopathology. Br J Urol. 1993;72:643–7. doi: 10.1111/j.1464-410x.1993.tb16225.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim ED, Leibman BB, Grinblat DM, et al. Varicocele repair improves semen parameters in azoospermic men with spermatogenic failure. J Urol. 1999;162:737–40. doi: 10.1097/00005392-199909010-00031. [DOI] [PubMed] [Google Scholar]
- 20.Etriby A, Girgis SM, Hefnawy H, Ibrahim AA. Testicular changes in subfertile males with varicocele. Fertil Steril. 1967;18(5):666–71. doi: 10.1016/s0015-0282(16)36428-7. [DOI] [PubMed] [Google Scholar]
- 21.Dubin L, Hotchkiss RS. Testis biopsy in subfertile men with varicocele. Fertil Steril. 1969;20(1):51–7. [PubMed] [Google Scholar]
- 22.Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Update. 2001;7(5):473–81. doi: 10.1093/humupd/7.5.473. [DOI] [PubMed] [Google Scholar]
- 23.Jung A, Schuppe HC. Influence of genital heat stress on semen quality in humans. Andrologia. 2007;39:203–15. doi: 10.1111/j.1439-0272.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 24.Lund L, Nielsen KT. Varicocele testis and testicular temperature. BJU Int. 1996;78:113–5. doi: 10.1046/j.1464-410x.1996.05122.x. [DOI] [PubMed] [Google Scholar]
- 25.Zorgniotti AW, MacLeod J. Studies in temperature, human semen quality, and varicocele. Fertil Steril. 1973;24:854–863. [PubMed] [Google Scholar]
- 26.Gomez E, Buckingham DW, Brindle J, Lanzafame F, Irvine DS, Aitken RJ. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J Androl. 1996;17(3):276–87. [PubMed] [Google Scholar]
- 27.Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod. 1998;13(6):1429–36. doi: 10.1093/humrep/13.6.1429. [DOI] [PubMed] [Google Scholar]
- 28.Saleh RA, Agarwal A, Sharma RK, Said TM, Sikka SC, Thomas AJ., Jr. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003;80(6):1431–6. doi: 10.1016/s0015-0282(03)02211-8. [DOI] [PubMed] [Google Scholar]
- 29.Smith R, Kaune H, Parodi D, Madariaga M, Rios R, Morales I, Castro A. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress. Hum Reprod. 2006;21(4):986–93. doi: 10.1093/humrep/dei429. [DOI] [PubMed] [Google Scholar]
- 30.Blumer CG, Fariello RM, Restelli AE, Spaine DM, Bertolla RP, Cedenho AP. Sperm nuclear DNA fragmentation and mitochondrial activity in men with varicocele. Fertil Steril. 2008;90(5):1716–22. doi: 10.1016/j.fertnstert.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Bertolla RP, Cedenho AP, Hassun Filho PA, Lima SB, Ortiz V, Srougi M. Sperm nuclear DNA fragmentation in adolescents with varicocele. Fertil Steril. 2006;85(3):625–8. doi: 10.1016/j.fertnstert.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 32.Hurtado de Catalfo GE, Ranieri-Casilla A, Marra FA, de Alaniz MJ, Marra CA. Oxidative stress biomarkers and hormonal profile in human patients undergoing varicocelectomy. Int J Androl. 2007;30(6):519–30. doi: 10.1111/j.1365-2605.2007.00753.x. [DOI] [PubMed] [Google Scholar]
- 33.Hendin BN, Kolettis PN, Sharma RK, Thomas AJ, Jr, Agarwal A. Varicocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. J Urol. 1999;161(6):1831–4. [PubMed] [Google Scholar]
- 34.Pasqualotto FF, Sundaram A, Sharma RK, Borges E, Jr, Pasqualotto EB, Agarwal A. Semen quality and oxidative stress scores in fertile and infertile patients with varicocele. Fertil Steril. 2008;89(3):602–7. doi: 10.1016/j.fertnstert.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 35.Mostafa T, Anis T, Imam H, El-Nashar AR, Osman IA. Seminal reactive oxygen species-antioxidant relationship in fertile males with and without varicocele. Andrologia. 2009;41(2):125–9. doi: 10.1111/j.1439-0272.2008.00900.x. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal A, Prabakaran S, Allamaneni SS. Relationship between oxidative stress, varicocele and infertility: a meta-analysis. Reprod Biomed Online. 2006;12(5):630–3. doi: 10.1016/s1472-6483(10)61190-x. [DOI] [PubMed] [Google Scholar]
- 37.Shafik A, Bedeir GA. Venous tension patterns in cord veins. I. In normal and varicocele individuals. J Urol. 1980;123:383–5. doi: 10.1016/s0022-5347(17)55945-5. [DOI] [PubMed] [Google Scholar]
- 38.Ross JA, Watson NE, Jr, Jarow JP. The effect of varicoceles on testicular blood flow in man. Urology. 1994;44:535–9. doi: 10.1016/s0090-4295(94)80053-7. [DOI] [PubMed] [Google Scholar]
- 39.Cayan S, Kadioglu A, Orhan I, et al. The effect of microsurgical varicocelectomy on serum follicle stimulating hormone, testosterone and free testosterone levels in infertile men with varicocele. BJU Int. 1999;84:1046–9. doi: 10.1046/j.1464-410x.1999.00353.x. [DOI] [PubMed] [Google Scholar]
- 40.Swerdloff RS, Walsh PC. Pituitary and gonadal hormones in patients with varicocele. Fertil Steril. 1975;26:1006–12. doi: 10.1016/s0015-0282(16)41416-0. [DOI] [PubMed] [Google Scholar]
- 41.Su LM, Goldstein M, Schlegel PN. The effect of varicocelectomy on serum testosterone levels in infertile men with varicoceles. J Urol. 1995;154:1752–5. [PubMed] [Google Scholar]
- 42.Sofikitis N, Miyagawa I. Left adrenalectomy in varicocelized rats does not inhibit the development of varicocele-related physiologic alterations. Int J Fertil Menopausal Stud. 1993;38:250–5. [PubMed] [Google Scholar]
- 43.Oshinsky GS, Rodriguez MV, Mellinger BC. Varicocele-related infertility is not associated with increased sperm-bound antibody. J Urol. 1993;150(3):871–3. doi: 10.1016/s0022-5347(17)35636-7. [DOI] [PubMed] [Google Scholar]
- 44.Turner TT, Caplis LA, Rhoades CP. Testicular vascular permeability: effects of experimental lesions associated with impaired testis function. J Urol. 1996;155(3):1078–82. doi: 10.1016/s0022-5347(01)66395-x. [DOI] [PubMed] [Google Scholar]
- 45.Kondoh N, Koh E, Matsui T, Takeyama M, Nakamura M, Namiki M, Fujioka H, Kiyohara H, Okuyama A. Improvement of semen characteristics after surgical repair of bilateral testicular varicocele as compared to unilateral varicocele patients. Arch Androl. 1990;24(1):61–7. doi: 10.3109/01485019008986859. [DOI] [PubMed] [Google Scholar]
- 46.Scherr D, Goldstein M. Comparison of bilateral versus unilateral varicocelectomy in men with palpable bilateral varicoceles. J Urol. 1999;162(1):85–8. doi: 10.1097/00005392-199907000-00021. [DOI] [PubMed] [Google Scholar]
- 47.Fujisawa M, Ishikawa T, Takenaka A. The efficacy of bilateral varicocelectomy in patients with palpable bilateral varicoceles: comparative study with unilateral varicocele. Urol Res. 2003;31(6):407–9. doi: 10.1007/s00240-003-0361-y. [DOI] [PubMed] [Google Scholar]
- 48.Libman J, Jarvi K, Lo K, Zini A. Beneficial effect of microsurgical varicocelectomy is superior for men with bilateral versus unilateral repair. J Urol. 2006;176(6 Pt 1):2602–5. doi: 10.1016/j.juro.2006.07.161. [DOI] [PubMed] [Google Scholar]
- 49.Baazeem A, Boman JM, Libman J, Jarvi K, Zini A. Microsurgical varicocelectomy for infertile men with oligospermia: differential effect of bilateral and unilateral varicocele on pregnancy outcomes. BJU Int. 2009;104(4):524–8. doi: 10.1111/j.1464-410X.2009.08431.x. [DOI] [PubMed] [Google Scholar]
- 50.Kondoh N, Meguro N, Matsumiya K, Namiki M, Kiyohara H, Okuyama A. Significance of subclinical varicocele detected by scrotal sonography in male infertility: a preliminary report. J Urol. 1993;150(4):1158–60. doi: 10.1016/s0022-5347(17)35713-0. [DOI] [PubMed] [Google Scholar]
- 51.Elbendary MA, Elbadry AM. Right subclinical varicocele: how to manage in infertile patients with clinical left varicocele? Fertil Steril. 2009;92(6):2050–3. doi: 10.1016/j.fertnstert.2009.05.069. [DOI] [PubMed] [Google Scholar]
- 52.Grasso M, Lania C, Castelli M, Galli L, Rigatti P. Bilateral Varicocele: Impact of Right Spermatic Vein Ligation on Fertility. J Urol. 1995;153:1847–8. doi: 10.1016/s0022-5347(01)67327-0. [DOI] [PubMed] [Google Scholar]
- 53.Zheng YQ, Gao X, Li ZJ, Yu YL, Zhang ZG, Li W. Efficacy of Bilateral and Left Varicocelectomy in Infertile Men with Left Clinical and Right Subclinical Varicoceles: A Comparative Study. J Urol. 2009;73(6):1236–40. doi: 10.1016/j.urology.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 54.Palomo A. Radical cure of varicocele by a new technique: Preliminary report. J Urol. 1948;61:604. doi: 10.1016/S0022-5347(17)69113-4. [DOI] [PubMed] [Google Scholar]
- 55.Lipshultz L, Thomas AJ, Jr., Khera M. Wein: Campbell-Walsh Urology. 9th ed. W.B. Saunders; 2007. Chapter 20: Surgical Management of Male Infertility. [Google Scholar]
- 56.Goldstein M, Gilbert BR, Dicker AP, Dwosh J, Gnecco C. Microsurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing technique. J Urol. 1992;148(6):1808–11. doi: 10.1016/s0022-5347(17)37035-0. [DOI] [PubMed] [Google Scholar]
- 57.Marmar JL, Kim Y. Subinguinal microsurgical varicocelectomy: a technical critique and statistical analysis of semen and pregnancy data. J Urol. 1994;152(4):1127–32. doi: 10.1016/s0022-5347(17)32521-1. [DOI] [PubMed] [Google Scholar]
- 58.Szabo R, Kessler R. Hydrocele following internal spermatic vein ligation: a retrospective study and review of the literature. J Urol. 1984;132(5):924–5. doi: 10.1016/s0022-5347(17)49950-2. [DOI] [PubMed] [Google Scholar]
- 59.Cayan S, Shavakhabov S, Kadioğlu A. Treatment of palpable varicocele in infertile men: a meta-analysis to define the best technique. J Androl. 2009;30(1):33–40. doi: 10.2164/jandrol.108.005967. [DOI] [PubMed] [Google Scholar]
- 60.Wosnitzer M, Roth JA. Optical magnification and Doppler ultrasound probe for varicocelectomy. Urology. 1983;22(1):24–6. doi: 10.1016/0090-4295(83)90339-4. [DOI] [PubMed] [Google Scholar]
- 61.Penn I, Mackie G, Halgrimson CG, et al. Testicular complications following renal transplantation. Ann Surg. 1972;176:697. doi: 10.1097/00000658-197212000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghanem H, Anis T, El-Nashar A, Shamloul R. Subinguinal microvaricocelectomy versus retroperitoneal varicocelectomy: comparative study of complications and surgical outcome. Urology. 2004;64(5):1005–9. doi: 10.1016/j.urology.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe M, Nagai A, Kusumi N, Tsuboi H, Nasu Y, Kumon H. Minimal invasiveness and effectivity of subinguinal microscopic varicocelectomy: a comparative study with retroperitoneal high and laparoscopic approaches. Int J Urol. 2005;12(10):892–8. doi: 10.1111/j.1442-2042.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- 64.Al-Kandari AM, Shabaan H, Ibrahim HM, Elshebiny YH, Shokeir AA. Comparison of outcomes of different varicocelectomy techniques: open inguinal, laparoscopic, and subinguinal microscopic varicocelectomy: a randomized clinical trial. Urology. 2007;69(3):417–20. doi: 10.1016/j.urology.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 65.Al-Said S, Al-Naimi A, Al-Ansari A, Younis N, Shamsodini A, A-sadiq K, Shokeir AA. Varicocelectomy for male infertility: a comparative study of open, laparoscopic and microsurgical approaches. J Urol. 2008;180(1):266–70. doi: 10.1016/j.juro.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 66.Sautter T, Sulser T, Suter S, Gretener H, Hauri D. Treatment of varicocele: a prospective randomized comparison of laparoscopy versus antegrade sclerotherapy. Eur Urol. 2002;41(4):398–400. doi: 10.1016/s0302-2838(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 67.Barbalias GA, Liatsikos EN, Nikiforidis G, Siablis D. Treatment of varicocele for male infertility: a comparative study evaluating currently used approaches. Eur Urol. 1998;34(5):393–8. doi: 10.1159/000019772. [DOI] [PubMed] [Google Scholar]
- 68.Cayan S, Kadioglu TC, Tefekli A, Kadioglu A, Tellaloglu S. Comparison of results and complications of high ligation surgery and microsurgical high inguinal varicocelectomy in the treatment of varicocele. Urology. 2000;55(5):750–4. doi: 10.1016/s0090-4295(99)00603-2. [DOI] [PubMed] [Google Scholar]
- 69.Zucchi A, Mearini L, Mearini E, Costantini E, Bini V, Porena M. Treatment of varicocele: randomized prospective study on open surgery versus Tauber antegrade sclerotherapy. J Androl. 2005;26(3):328–32. doi: 10.2164/jandrol.04143. [DOI] [PubMed] [Google Scholar]
- 70.Yavetz H, Levy R, Papo J, Yogev L, Paz G, Jaffa AJ, Homonnai ZT. Efficacy of varicocele embolization versus ligation of the left internal spermatic vein for improvement of sperm quality. Int J Androl. 1992;15(4):338–44. doi: 10.1111/j.1365-2605.1992.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 71.Sayfan J, Soffer Y, Orda R. Varicocele treatment: prospective randomized trial of 3 methods. J Urol. 1992;148(5):1447–9. doi: 10.1016/s0022-5347(17)36934-3. [DOI] [PubMed] [Google Scholar]
- 72.Cayan S, Shavakhabov S, Kadioğlu A. Treatment of palpable varicocele in infertile men: a meta-analysis to define the best technique. J Androl. 2009;30(1):33–40. doi: 10.2164/jandrol.108.005967. [DOI] [PubMed] [Google Scholar]
- 73.Agarwal A, Deepinder F, Cocuzza M, Agarwal R, Short RA, Sabanegh E, Marmar JL. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology. 2007;70(3):532–8. doi: 10.1016/j.urology.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 74.Boman JM, Libman J, Zini A. Microsurgical varicocelectomy for isolated asthenospermia. J Urol. 2008;180(5):2129–32. doi: 10.1016/j.juro.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 75.Schatte EC, Hirshberg SJ, Fallick ML, Lipschultz LI, Kim ED. Varicocelectomy improves sperm strict morphology and motility. J Urol. 1998;160(4):1338–40. [PubMed] [Google Scholar]
- 76.Schlesinger MH, Wilets IF, Nagler HM. Treatment outcome after varicocelectomy. A critical analysis. Urol Clin North Am. 1994;21(3):517–29. [PubMed] [Google Scholar]
- 77.Vazquez-Levin MH, Friedmann P, Goldberg SI, Medley NE, Nagler HM. Response of routine semen analysis and critical assessment of sperm morphology by Kruger classification to therapeutic varicocelectomy. J Urol. 1997;158(5):1804–7. doi: 10.1016/s0022-5347(01)64134-x. [DOI] [PubMed] [Google Scholar]
- 78.Seftel AD, Rutchik SD, Chen H, Stovsky M, Goldfarb J, Desai N. Effects of subinguinal varicocele ligation on sperm concentration, motility and Kruger morphology. J Urol. 1997;158(5):1800–3. doi: 10.1016/s0022-5347(01)64133-8. [DOI] [PubMed] [Google Scholar]
- 79.Kibar Y, Seckin B, Erduran D. The effects of subinguinal varicocelectomy on Kruger morphology and semen parameters. J Urol. 2002;168(3):1071–4. doi: 10.1016/S0022-5347(05)64577-6. [DOI] [PubMed] [Google Scholar]
- 80.Okeke L, Ikuerowo O, Chiekwe I, Etukakpan B, Shittu O, Olapade-Olaopa O. Is varicocelectomy indicated in subfertile men with clinical varicoceles who have asthenospermia or teratospermia and normal sperm density? Int J Urol. 2007;14(8):729–32. doi: 10.1111/j.1442-2042.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 81.Cakan M, Bakirtas H, Aldemir M, Demirel F, Altug U. Results of varicocelectomy in patients with isolated teratozoospermia. Urol Int. 2008;80(2):172–6. doi: 10.1159/000112609. [DOI] [PubMed] [Google Scholar]
- 82.Dubin L, Amelar RD. Varicocelectomy: 986 cases in a twelve-year study. Urology. 1977;10(5):446–9. doi: 10.1016/0090-4295(77)90132-7. [DOI] [PubMed] [Google Scholar]
- 83.Matkov TG, Zenni M, Sandlow J, Levine LA. Preoperative semen analysis as a predictor of seminal improvement following varicocelectomy. Fertil Steril. 2001;75(1):63–8. doi: 10.1016/s0015-0282(00)01644-7. [DOI] [PubMed] [Google Scholar]
- 84.Kamal KM, Jarvi K, Zini A. Microsurgical varicocelectomy in the era of assisted reproductive technology: influence of initial semen quality on pregnancy rates. Fertil Steril. 2001;75(5):1013–6. doi: 10.1016/s0015-0282(01)01698-3. [DOI] [PubMed] [Google Scholar]
- 85.Fujisawa M, Dobashi M, Yamasaki T, Okada H, Arakawa S, Kamidono S. Therapeutic strategy after microsurgical varicocelectomy in the modern assisted reproductive technology era. Urol Res. 2002;30(3):195–8. doi: 10.1007/s00240-002-0253-6. [DOI] [PubMed] [Google Scholar]
- 86.Madgar I, Weissenberg R, Lunenfeld B, Karasik A, Goldwasser B. Controlled trial of high spermatic vein ligation for varicocele in infertile men. Fertil Steril. 1995;63(1):120–4. doi: 10.1016/s0015-0282(16)57306-3. [DOI] [PubMed] [Google Scholar]
- 87.Matthews GJ, Matthews ED, Goldstein M. Induction of spermatogenesis and achievement of pregnancy after microsurgical varicocelectomy in men with azoospermia and severe oligoasthenospermia. Fertil Steril. 1998;70(1):71–5. doi: 10.1016/s0015-0282(98)00108-3. [DOI] [PubMed] [Google Scholar]
- 88.Kadioglu A, Tefekli A, Cayan S, Kandirali E, Erdemir F, Tellaloglu S. Microsurgical inguinal varicocele repair in azoospermic men. Urology. 2001;57(2):328–33. doi: 10.1016/s0090-4295(00)00908-0. [DOI] [PubMed] [Google Scholar]
- 89.Schlegel PN, Kaufmann J. Role of varicocelectomy in men with nonobstructive azoospermia. Fertil Steril. 2004;81(6):1585–8. doi: 10.1016/j.fertnstert.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 90.Esteves SC, Glina S. Recovery of spermatogenesis after microsurgical subinguinal varicocele repair in azoospermic men based on testicular histology. Int Braz J Urol. 2005;31(6):541–8. doi: 10.1590/s1677-55382005000600005. [DOI] [PubMed] [Google Scholar]
- 91.Pasqualotto FF, Sobreiro BP, Hallak J, Pasqualotto EB, Lucon AM. Induction of spermatogenesis in azoospermic men after varicocelectomy repair: an update. Fertil Steril. 2006;85(3):635–9. doi: 10.1016/j.fertnstert.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 92.Lee JS, Park HJ, Seo JT. What is the indication of varicocelectomy in men with nonobstructive azoospermia? Urology. 2007;69(2):352–5. doi: 10.1016/j.urology.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 93.Ishikawa T, Kondo Y, Yamaguchi K, Sakamoto Y, Fujisawa M. Effect of varicocelectomy on patients with unobstructive azoospermia and severe oligospermia. BJU Int. 2008;101(2):216–8. doi: 10.1111/j.1464-410X.2007.07279.x. [DOI] [PubMed] [Google Scholar]
- 94.Haydardedeoglu B, Turunc T, Kilicdag EB, Gul U, Bagis T. The Effect of Prior Varicocelectomy in Patients With Nonobstructive Azoospermia on Intracytoplasmic Sperm Injection Outcomes: A Retrospective Pilot Study. Urology. 2010;75(1):83–86. doi: 10.1016/j.urology.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 95.Youssef T, Abd-Elaal E, Gaballah G, Elhanbly S, Eldosoky E. Varicocelectomy in men with nonobstructive azoospermia: is it beneficial? Int J Surg. 2009;7(4):356–60. doi: 10.1016/j.ijsu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 96.Ron-El R, Strassburger D, Friedler S, et al. Extended sperm preparation: an alternative to testicular sperm extraction in non-obstructive azoospermia. Hum Reprod. 1997;12:1222–6. doi: 10.1093/humrep/12.6.1222. [DOI] [PubMed] [Google Scholar]
- 97.Mancini A, Meucci E, Milardi D, Giacchi E, Bianchi A, Pantano AL, Mordente A, Martorana GE, de Marinis L. Seminal antioxidant capacity in pre- and postoperative varicocele. J Androl. 2004;25(1):44–9. doi: 10.1002/j.1939-4640.2004.tb02757.x. [DOI] [PubMed] [Google Scholar]
- 98.Mostafa T, Anis TH, El-Nashar A, Imam H, Othman IA. Varicocelectomy reduces reactive oxygen species levels and increases antioxidant activity of seminal plasma from infertile men with varicocele. Int J Androl. 2001;24(5):261–5. doi: 10.1046/j.1365-2605.2001.00296.x. [DOI] [PubMed] [Google Scholar]
- 99.Allamaneni SS, Naughton CK, Sharma RK, Thomas AJ, Jr, Agarwal A. Increased seminal reactive oxygen species levels in patients with varicoceles correlate with varicocele grade but not with testis size. Fertil Steril. 2004;82(6):1684–6. doi: 10.1016/j.fertnstert.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 100.Yagci A, Murk W, Stronk J, Huszar G. Spermatozoa Bound to Solid State Hyaluronic Acid Show Chromatin Structure with High DNA Chain Integrity: An Acridine Orange Fluorescence Study. J Androl. 2010 Feb 4; doi: 10.2164/jandrol.109.008912. [DOI] [PubMed] [Google Scholar]
- 101.Razavi SH, Nasr-Esfahani MH, Deemeh MR, Shayesteh M, Tavalaee M. Evaluation of zeta and HA-binding methods for selection of spermatozoa with normal morphology, protamine content and DNA integrity. Andrologia. 2010 Feb;42(1):13–9. doi: 10.1111/j.1439-0272.2009.00948.x. [DOI] [PubMed] [Google Scholar]
- 102.Nasr-Esfahani MH, Razavi S, Vahdati AA, Fathi F, Tavalaee M. Evaluation of sperm selection procedure based on hyaluronic acid binding ability on ICSI outcome. J Assist Reprod Genet. 2008 May;25(5):197–203. doi: 10.1007/s10815-008-9223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marsman JWP. Clinical versus Subclinical Varicocele: Venographic Findings and Improvement of Fertility after Embolization. Radiology. 1985;155:635–8. doi: 10.1148/radiology.155.3.4001363. [DOI] [PubMed] [Google Scholar]
- 104.Comhaire FH, Kunnen M. Factors affecting the probability of conception after treatment of subfertile men with varicocele by transcatheter embolization with Bucrylate. Fertil Steril. 1985;43(5):781–6. doi: 10.1016/s0015-0282(16)48566-3. [DOI] [PubMed] [Google Scholar]
- 105.McClure RD, Khoo D, Jarvi K, Hricak H. Subclinical varicocele: the effectiveness of varicocelectomy. J Urol. 1991;145(4):789–91. doi: 10.1016/s0022-5347(17)38452-5. [DOI] [PubMed] [Google Scholar]
- 106.Dhabuwala CB, Hamid S, Moghissi KS. Clinical versus subclinical varicocele: improvement in fertility after varicocelectomy. Fertil Steril. 1992;57(4):854–7. doi: 10.1016/s0015-0282(16)54970-x. [DOI] [PubMed] [Google Scholar]
- 107.Dubin L, Amelar RD. Varicocele size and the results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21:606–9. doi: 10.1016/s0015-0282(16)37684-1. [DOI] [PubMed] [Google Scholar]
- 108.Yamamoto M, Hibi H, Hirata Y, Miyake K, Ishigaki T. Effect of Varicocelectomy on Sperm Parameters and Pregnancy Rate in Patients with Subclinical Varicocele: A Randomized Prospective Controlled Study. J Urol. 1996;155:1636–8. [PubMed] [Google Scholar]
- 109.Unal D, Yeni E, Verit A, Karatas OF. Clomiphene citrate versus varicocelectomy in treatment of subclinical varicocele: a prospective randomized study. Int J Urol. 2001;8:227–30. doi: 10.1046/j.1442-2042.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 110.Grasso M, Lania C, Castelli M, Galli L, Franzoso F, Rigatti P. Low-grade left varicocele in patients over 30 years old: the effect of spermatic vein ligation on fertility. BJU International. 2000;85:305–7. doi: 10.1046/j.1464-410x.2000.00437.x. [DOI] [PubMed] [Google Scholar]
- 111.Nilsson S, Edvinsson A, Nilsson B. Improvement of semen and pregnancy rate after ligation and division of the internal spermatic vein: fact or fiction? Br J Urol. 1979;51(6):591–6. doi: 10.1111/j.1464-410x.1979.tb03609.x. [DOI] [PubMed] [Google Scholar]
- 112.Breznik R, Vlaisavljević V, Borko E. Treatment of varicocele and male fertility. Arch Androl. 1993;30(3):157–60. doi: 10.3109/01485019308987750. [DOI] [PubMed] [Google Scholar]
- 113.Krause W, Müller HH, Schäfer H, Weidner W. Does treatment of varicocele improve male fertility? results of the ‘Deutsche Varikozelenstudie’, a multicentre study of 14 collaborating centres. Andrologia. 2002;34(3):164–71. doi: 10.1046/j.1439-0272.2002.00494.x. [DOI] [PubMed] [Google Scholar]
- 114.Okuyama A, Fujisue H, Matsui T, Doi Y, Takeyama M, Nakamura N, Namiki M, Fuijoka H, Matsuda M. Surgical repair of varicocele: effective treatment for subfertile men in a controlled study. Eur Urol. 1988;14(4):298–300. doi: 10.1159/000472964. [DOI] [PubMed] [Google Scholar]
- 115.Onozawa M, Endo F, Suetomi T, Takeshima H, Akaza H. Clinical study of varicocele: statistical analysis and the results of long-term follow-up. nt J Urol. 2002;9(8):455–61. doi: 10.1046/j.1442-2042.2002.00501.x. [DOI] [PubMed] [Google Scholar]
- 116.Nieschlag E, Hertle L, Fischedick A, Abshagen K, Behre HM. Update on treatment of varicocele: counselling as effective as occlusion of the vena spermatica. Hum Reprod. 1998;13(8):2147–50. doi: 10.1093/humrep/13.8.2147. [DOI] [PubMed] [Google Scholar]
- 117.Schlegel P. Is assisted reproduction the optimal treatment for varicocele-associated male infertility? A cost effective analysis. Urol. 1997;49(1):83–90. doi: 10.1016/S0090-4295(96)00379-2. [DOI] [PubMed] [Google Scholar]
- 118.Ficarra V, Cerruto MA, Liguori G, Mazzoni G, Minucci S, Tracia A, Gentile V. Treatment of varicocele in subfertile men: The Cochrane Review--a contrary opinion. Eur Urol. 2006;49(2):258–63. doi: 10.1016/j.eururo.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 119.Marmar JL, Agarwal A, Prabakaran S, Agarwal R, Short RA, Benoff S, Thomas AJ., Jr. Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis. Fertil Steril. 2007;88(3):639–48. doi: 10.1016/j.fertnstert.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 120.Marks JL, McMahon R, Lipshultz LI. Predictive parameters of successful varicocele repair. J Urol. 1986;136(3):609–12. doi: 10.1016/s0022-5347(17)44990-1. [DOI] [PubMed] [Google Scholar]
- 121.Okuyama A, Fujisue H, Matsui T, Doi Y, Koh E, Kondoh N, Takeyama M, Nakamura M, Namiki M, Fujioka H, et al. Preoperative parameters related to the improvement of semen characteristics after surgical repair of varicocele in subfertile men. Eur Urol. 1988;14(6):442–6. doi: 10.1159/000473005. [DOI] [PubMed] [Google Scholar]
- 122.Steckel J, Dicker AP, Goldstein M. Relationship between varicocele size and response to varicocelectomy. J Urol. 1993;149(4):769–71. doi: 10.1016/s0022-5347(17)36203-1. [DOI] [PubMed] [Google Scholar]
- 123.Takahara M, Ichikawa T, Shiseki Y, Nakamura T, Shimazaki J. Relationship between grade of varicocele and the response to varicocelectomy. Int J Urol. 1996;3(4):282–5. doi: 10.1111/j.1442-2042.1996.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 124.Jarow JP, Ogle SR, Eskew LA. Seminal Improvement Following Repair of Ultrasound Detected Subclinical Varicoceles. J Urol. 1996;155:1287–90. [PubMed] [Google Scholar]
- 125.Ishikawa T, Fujisawa M. Effect of age and grade on surgery for patients with varicocele. Urology. 2005;65(4):768–72. doi: 10.1016/j.urology.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 126.Madjar S, Moskovitz B, Issaq E, Weinberger M, Nativ O. Low inguinal approach for correction of recurrent varicocele. Int Urol Nephrol. 1998;30(1):69–73. doi: 10.1007/BF02550281. [DOI] [PubMed] [Google Scholar]
- 127.Grober ED, Chan PT, Zini A, Goldstein M. Microsurgical treatment of persistent or recurrent varicocele. Fertil Steril. 2004;82(3):718–22. doi: 10.1016/j.fertnstert.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 128.Flati G, Porowska B, Flati D, Veltri S, Sportelli G, Carboni M. Improvement in the fertility rate after placement of microsurgical shunts in men with recurrent varicocele. Fertil Steril. 2004;82(6):1527–31. doi: 10.1016/j.fertnstert.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 129.Marmar JL, Corson SL, Batzer FR, Gocial B. Insemination data on men with varicoceles. Fertil Steril. 1992;57(5):1084–90. [PubMed] [Google Scholar]
- 130.Daitch JA, Bedaiwy MA, Pasqualotto EB, Hendin BN, Hallak J, Falcone T, Thomas AJ, Jr, Nelson DR, Agarwal A. Varicocelectomy improves intrauterine insemination success rates in men with varicocele. J Urol. 2001;165(5):1510–3. [PubMed] [Google Scholar]
- 131.Yamamoto M, Hibi H, Tsuji Y, Miyake K. The effect of varicocele ligation on oocyte fertilization and pregnancy after failure of fertilization in in vitro fertilization-embryo transfer. Hinyokika Kiyo. 1994;40(8):683–7. [PubMed] [Google Scholar]
- 132.Ashkenazi J, Dicker D, Feldberg D, Shelef M, Goldman GA, Goldman J. The impact of spermatic vein ligation on the male factor in in vitro fertilization-embryo transfer and its relation to testosterone levels before and after operation. Fertil Steril. 1989;51(3):471–4. doi: 10.1016/s0015-0282(16)60556-3. [DOI] [PubMed] [Google Scholar]
- 133.O’Brien JH, Bowles B, Kamal KM, Jarvi K, Zini A. Microsurgical varicocelectomy for infertile couples with advanced female age: natural history in the era of ART. J Androl. 2004;25(6):939–43. doi: 10.1002/j.1939-4640.2004.tb03165.x. [DOI] [PubMed] [Google Scholar]
- 134.Zini A, Boman J, Baazeem A, Jarvi K, Libman J. Natural history of varicocele management in the era of intracytoplasmic sperm injection. Fertil Steril. 2008;90(6):2251–6. doi: 10.1016/j.fertnstert.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 135.Knudson G, Ross L, Stuhldreher D, Houlihan D, Bruns E, Prins G. Prevalence of sperm bound antibodies in infertile men with varicocele: the effect of varicocele ligation on antibody levels and semen response. J Urol. 1994;151(5):1260–2. doi: 10.1016/s0022-5347(17)35226-6. [DOI] [PubMed] [Google Scholar]
- 136.Djaladat H, Mehrsai A, Rezazade M, Djaladat Y, Pourmand G. Varicocele and antisperm antibody: fact or fiction? South Med J. 2006;99(1):44–7. doi: 10.1097/01.smj.0000197036.08282.70. [DOI] [PubMed] [Google Scholar]
- 137.Cayan S, Erdemir F, Ozbey I, Turek PJ, Kadioğlu A, Tellaloğlu S. Can varicocelectomy significantly change the way couples use assisted reproductive technologies? J Urol. 2002;167(4):1749–52. doi: 10.1016/s0022-5347(05)65192-0. [DOI] [PubMed] [Google Scholar]
- 138.Meng MV, Greene KL, Turek PJ. Surgery or assisted reproduction? A decision analysis of treatment costs in male infertility. J Urol. 2005;174(5):1926–31. doi: 10.1097/01.ju.0000176736.74328.1a. [DOI] [PubMed] [Google Scholar]
- 139.Lee R, Li PS, Goldstein M, Schattman G, Schlegel PN. A decision analysis of treatments for nonobstructive azoospermia associated with varicocele. Fertil Steril. 2009;92(1):188–96. doi: 10.1016/j.fertnstert.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 140.Goldstein M, Gilbert BR, Dicker AP, Dwosh J, Gnecco C. Microsurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing technique. J Urol. 1992;148(6):1808–11. doi: 10.1016/s0022-5347(17)37035-0. [DOI] [PubMed] [Google Scholar]
- 141.Ramasamy R, Schlegel PN. Microsurgical inguinal varicocelectomy with and without testicular delivery. Urology. 2006;68(6):1323–6. doi: 10.1016/j.urology.2006.08.1113. [DOI] [PubMed] [Google Scholar]