Abstract
Background
Survey findings report that 48% of people with type 2 diabetes use complementary and alternative medicine (CAM) practice. Publications suggest a high incidence of health promotion counseling in naturopathic practice, yet clinical data on risk factor changes are not available in the literature.
Objectives
The primary aim of this study was to describe clinical risk factor changes during the utilization of naturopathic CAM services in patients with type 2 diabetes.
Design
A retrospective, observational study design was used to describe naturopathic care.
Setting
Abstracted medical charts were from patients of the Bastyr Center for Natural Health in Seattle, WA.
Participants
The patients in this study had type 2 diabetes and received naturopathic care between 2001 and 2006.
Outcomes
Abstracted data included patient demographics, duration of care, number of visits, laboratory values for hemoglobin A1c (HbA1c), low density lipoprotein (LDL) and high density lipoprotein (HDL) cholesterol, triglycerides (TAG); and systolic/diastolic blood pressure (SPB, DBP).
Results
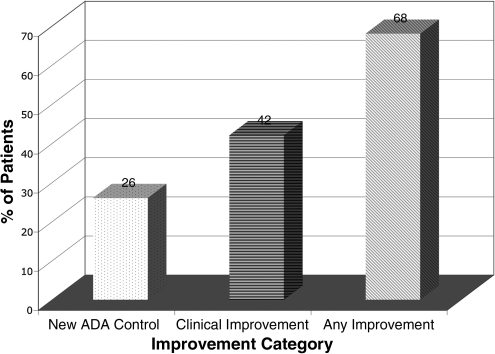
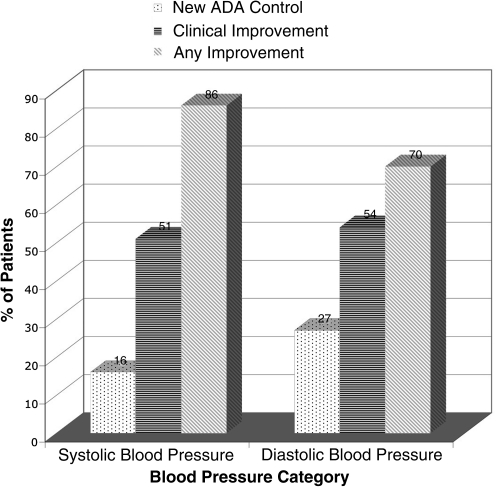
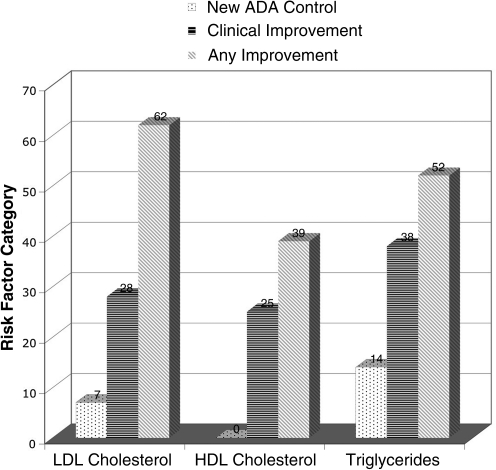
Thirty-seven (37) patient records met inclusion criteria and were abstracted in detail. Mean and median duration of care were 27 and 20 months, respectively. The mean number of visits was 11. Significant mean changes in clinical laboratory risk factors over the duration of care were: −0.65% for HbA1c (p = 0.046), −45mg/dL for TAG (p = 0.037), −7 mm Hg in SBP (p = 0.02), and −5 mm Hg in DBP (p = 0.003). Mean changes for cholesterol did not reach statistical significance. The percentage of patients who reached new control, had clinically significant risk factor improvements, or had any improvement was: 26%, 42%, and 68% for HbA1c, 7%, 28%, and 62% for LDL, 0%, 25%, and 39% for HDL, 14%, 38%, and 52% for TAG, 16%, 51%, and 86% for SBP, and 27%, 54%, and 70% for DBP.
Comments/conclusions
These preliminary outcomes suggest that risk factor improvements occur during naturopathic care for diabetes, although the contribution of naturopathic care to these changes cannot be determined. Effectiveness and generalizability of naturopathic approaches in treating type 2 diabetes should be evaluated in controlled prospective studies in representative populations or randomized trials.
Introduction
Type 2 diabetes (T2DM) is a costly, complex, chronic disease that is expected to increase in prevalence in the coming decades. Estimated costs of health care for treating diabetes reached $174 billion dollars in 2007, with approximately 8%–17% of the total expenditures accumulated through prescription medication costs.1 The U.S. Preventative Services Task Force recommends “intensive behavior dietary counseling for patients with hyperlipidemia and other known risk factors for cardiovascular and diet-related chronic disease,” as do most major clinical guidelines for chronic cardiometabolic disease; however, studies based in physician offices suggest that the delivery of these recommendations in conventional medical care remains infrequent, even when risk factors are present.2–9 Lifestyle change has been shown to be as effective in patients to prevent diabetes as well as to assist control in those with poorly controlled diabetes, suggesting a role for lifestyle improvement at all stages of the disease.10,11
A large survey of complementary and alternative medicine (CAM) utilization reported that 48% of adults with T2DM use some form of CAM practice, and CAM utilization was associated with increased receipt of preventive services.12 In Washington State, 17% of insured patients with diabetes have visited a licensed CAM provider.13
Naturopathic medicine is a unique, whole system of medicine that emphasizes patient wellness through the delivery of health-promotion counseling in clinical practice. In Washington State, naturopathic doctors (N.D.s) are physician-level providers who diagnose, treat, and manage chronic disease, including T2DM. Common clinical recommendations include diet counseling, exercise prescription, stress management recommendation, nutritional/botanical supplementation, and prescription medications as necessary to control risk factors and improve health.14,15 Naturopathic care provides an interesting model to study the efficacy of health-promotion counseling in clinical practice.
There are few descriptions of naturopathic practice in the published literature. As a result, health care providers in other disciplines have little knowledge of what to expect if their patients pursue N.D. care; this limitation in experience, and thus hesitation, with N.D. care may be eased if better practice descriptions, including changes in clinical laboratory benchmarks, were available. Previous studies by the investigation team have suggested that improvement occurs during N.D. care for T2DM; however, these improvements were not described in detail due to limited availability of laboratory data.14
In order to provide a detailed, semiquantitative view of naturopathic practice in T2DM, the aims of the current study were to (1) quantify the delivery of health-promotion counseling in N.D. care for T2DM; (2) quantify change in clinical risk factors hemoglobin A1c (HbA1c), low density cholesterol (LDL) and high density cholesterol (HDL), and systolic and diastolic blood pressure (SBP and DBP) during N.D. clinical care; and (3) evaluate degree of clinical improvement from N.D. care using three a priori-specified definitions of “improvement,” including the percentage of patients who reach new control per the American Diabetes Association (ADA) definition.
Materials and Methods
A retrospective, observational study was conducted based on data abstracted from medical charts between December 2006 and June 2007. The study was approved by the Bastyr University Institutional Review Board. Medical charts were identified through clinic scheduling software, searchable by International Classification of Diseases, 9th Revision (ICD-9) codes. This study included patients meeting three inclusion criteria: (1) an ICD-9 assessment of T2DM was made, (2) evidence of at least 6 months of naturopathic care between 2001 and 2006 was available, and (3) N.D. care was provided specifically for diabetes (versus accompanying symptoms). Using either too short or too long a time period as an inclusion criterion results in inherent bias. Including only those patients who have maintained care for too long a period leads to selection bias (e.g., oversampling of the uniquely motivated); allowing too short a time period and we may underestimate the effects of a care process, recognizing that especially the lifestyle elements of care may take some time for patients to adopt. A 6-month duration of care was specified in an attempt to balance bias between either extreme.
Collected data included patient demographics, dates and duration of care, frequency of clinical visits, clinical service utilization, characteristics of care including treatment recommendations, physical examination findings including blood pressure and results of clinical laboratory risk factors including HbA1c, LDL/HDL cholesterol, and triglycerides.
To describe any changes in the distribution of clinical risk factors in the sample, two-tailed, paired t tests for homogeneity were applied to laboratory values and blood pressure from the patient's first visit compared to the most recently available value at the time of chart abstraction. Laboratory values within 3 months of the baseline visit were included as acceptable baseline values.
In addition to calculating average changes in risk factors, three a priori-specified definitions were applied to the data to further quantify the degree of clinical change. The percentages of patients achieving (1) “new ADA control,” (2) “clinically significant improvement,” and (3) “any improvement” were calculated. “New ADA control” equates to control of risk factors per the American Diabetes Association definition during the period of N.D. clinical care, if the patient was “uncontrolled” at the beginning of care. The ADA definitions for risk factor control are: <7% for hemoglobin A1c, <130 mm Hg for systolic blood pressure, <80 mm Hg for diastolic blood pressure, <100mg/dL for LDL cholesterol, >40mg/dL for HDL cholesterol, and <150mg/dL for triglycerides.8 “Clinically significant improvement” was defined for each risk factor based on the minimum change deemed clinically important; for our purposes, “clinically significant improvement” equates to the following: a minimum 0.5% reduction in HbA1c, a minimum 10% decrease in LDL cholesterol, a minimum 10% increase in HDL cholesterol, a minimum 30% reduction in triglycerides or a 5 mm Hg reduction in SBP or diastolic DBP blood pressure from the beginning of care to the most recent measure. “Any improvement” was the least strict definition and equates to any improvement in the measured risk factor from the beginning of care and the most recent measure available in the chart.
Results
There were 123 candidate patients assessed with T2DM during their naturopathic care between 2001 and 2006; 37 of 123 (30%) met inclusion criteria for detailed abstraction in this study. The most common reason for chart exclusion was that the duration of care did not meet the 6-month minimum; 76 of 123 (62%) charts were excluded for this reason. For these 37 patients, data from 418 total visits was abstracted and included in analyses; on average, patients attended 11 naturopathic visits over a 27-month duration of care. The average age for those patients meeting inclusion criteria was 62 years. Female gender was more common (58% female), and considerable racial homogeneity (80% white) was observed in the patient base receiving N.D. care for T2DM at the study clinic. Care for T2DM was predominantly adjunctive care (80%) versus primary care (20%) during our observation period.
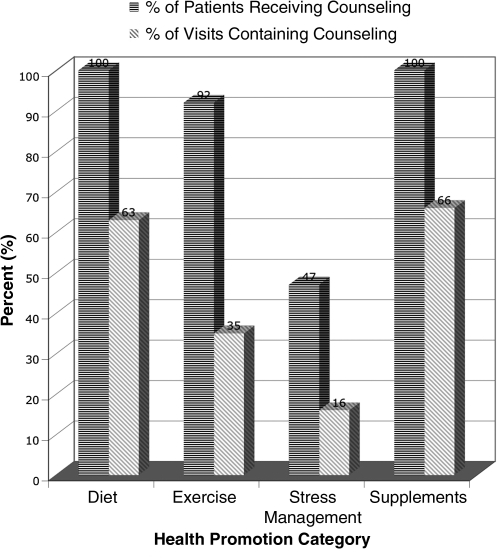
A high frequency of health-promotion counseling was observed during N.D. clinical practice. Health-promotion advice was given to 100% (diet), 92% (exercise), and 47% (stress reduction) of patients. Health-promotion recommendations were reiterated or modified in subsequent clinical encounters; 63%, 35%, and 16% of total visits included advice on diet, exercise, and stress management, respectively. Figure 1 summarizes these data. Recommendations for utilization of available integrated services (nutrition, psychological counseling, and other CAM services) were common: 32% (nutritionist), 11% (Ph.D. psychologist or N.D. counselor), 26% (acupuncturist), 18% (homeopath), and 18% (N.D. manual therapy provider).
FIG. 1.
Health-promotion counseling and nutritional supplementation in naturopathic practice.
Repeat clinical laboratory or physical examination data were available for HbA1c, LDL/HDL/triglycerides, and blood pressure in 31/37 (84%), 29/37 (78%), and 37/37 (100%) of patients, respectively. On average, significant improvements in risk factor distributions were achieved for HbA1c, and SBP and DBP. The average changes in LDL and HDL cholesterol over the course of the observation period did not reach statistical significance. Table 1 reports the average baseline values of select clinical risk factors and reports average changes.
Table 1.
Mean Changes in Clinical Risk Factors During the Observation Period
| Risk factor | Baseline-mean (SD) | Last available-mean (SD) | Mean Δ | p-valuea |
|---|---|---|---|---|
| HbA1c (%) | 7.4 (1.7) | 6.7 (0.90) | −0.65 | 0.046 |
| LDL (mg/dL) | 108.6 (44.9) | 106.2 (41) | −2.4 | NS |
| HDL (mg/dL) | 51.5 (14.4) | 53.8 (21.7) | +1.7 | NS |
| Triglycerides (mg/dL) | 225.2 (140.9) | 179.9 (105.3) | −45 | 0.037 |
| SBP (mm Hg) | 140.4 (21.2) | 133.6 (16.4) | −7.3 | 0.02 |
| DBP (mm Hg) | 82.4 (12.2) | 76.9 (12.6) | −5.6 | 0.003 |
Two-sided, t-tests for homogeneity.
SD, standard deviation; NS, not significant; HbA1c, hemoglobin A1c; LDL, low density lipoprotein; HDL, high density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Figures 2–4 summarize the frequency of clinical improvement achieved by patients during the observation period. Patients appear to make graded improvement on all measured risk factors over the course of N.D. care for diabetes. As shown, the majority of patients show improvement during their course of N.D. care, with considerable proportions of patients achieving clinically important risk factor improvements. Of note, “new control” for HbA1c, SBP and DBP (per definition 1) was achieved in an additional 26%, 16%, and 27% of patients, respectively, despite a considerable percentage of patients being “under control” at baseline for these measures (HbA1c: 55%, SBP: 30%, and DBP: 30%). Baseline control was rather high for HDL (93%), which may explain why few additional patients achieved “new control” for HDL; baseline control was less common for LDL cholesterol (41%). Regardless of baseline control, approximately 25% still achieved a clinically significant improvement in cholesterol risk factors, per definition 2.
FIG. 2.
Percent of patients with changes in hemoglobin A1c (HbA1c) per specified definitions. “New ADA Control” = HbA1c <7% at last observation (when >7% at baseline); “Clinical Improvement” = a 0.5% minimum reduction between baseline and last observation; “Any Improvement” = any reduction in HbA1c from baseline at last observation. ADA, American Diabetes Association.
FIG. 4.
Percent of patients with changes in blood pressure per specified definitions; “New ADA Control” = systolic blood pressure (SBP) <130 mm Hg and diastolic blood pressure (DBP) <80 mm Hg at last observation (when uncontrolled at baseline); “Clinical Improvement” = a 5 mm Hg reduction in either SBP or DBP; “Any Improvement” = any blood pressure reduction from baseline at last observation. ADA, American Diabetes Association.
FIG. 3.
Percent of patients with changes in lipid measures per specified definitions; “New ADA Control” = low density lipoprotein (LDL) <100mg/dL, high density lipoprotein (HDL) >35 mg/dL, and triglycerides <150mg/dL at last observation (when uncontrolled at baseline); “Clinical Improvement” = a 10% minimum improvement in LDL/HDL and a 30% reduction in triglycerides between baseline and last observation; “Any Improvement” = any improvement in measure from baseline at last observation. ADA, American Diabetes Association.
Discussion
This report suggests that clinically important risk factors reductions are achieved during N.D. care for T2DM. Health-promotion counseling, including dietary change, exercise, and stress reduction, appear to be fundamental elements of N.D. practice. These high rates of health-promotion counseling contrast with those estimates reported from conventional, allopathic primary care.2–5 It is possible that this self-selecting patient cohort is more receptive to individual health-promotion counseling, yet it is also likely that the N.D. standard is to recommend lifestyle modification in practice in an effort to affect the patient's readiness to change. As lifestyle change has a considerable impact on mortality in patients with cardiovascular disease (CVD), and because T2DM is considered a CVD equivalent, optimizing delivery and receptivity of health-promotion messages in clinical practice remains a critical and fundamental challenge to reducing chronic disease risk.16 N.D. practice appears to be an existing model of health-promotion counseling in physician practice, though controlled evaluation is necessary.
Nutritional and botanical supplementation is also recommended commonly. Numerous nutritional supplements were prescribed, and many have clinical trial evidence for effect in T2DM17; a review of the level of evidence of nutritional supplementation used in naturopathic practice has been reported elsewhere.14,18 Unfortunately, the relative contribution of each supplement or combination of supplements to the observed changes in clinical risk factors cannot be determined from this study design due to its limited statistical power; logistic regression analyses in larger, controlled samples would be necessary to determine promising supplements and/or supplement combinations.
Objective clinical risk factors were moderately well controlled for most patients at baseline; however, additional improvements were observed during the course of N.D. care. Observed changes in clinical risk factors in this cohort are clinically meaningful. An additional average reduction in HbA1c of 0.65% corresponds to an approximate 14% risk reduction for microvascular complications.19 In addition, the observed SBP and DBP reductions are clinically meaningful; a 5 mm Hg reduction in SBP and DBP are comparable to estimates of blood pressure reduction achieved through lifestyle modification and correspond to approximately 25% and 50% reductions in relative risk for cardiovascular event, respectively.7 Although changes in the distribution of cholesterol measures did not meet statistical significance, 25%–28% of patients had at least 10% improvements in HDL and LDL, respectively. A near perfect linear relationship exists between LDL reduction and risk reduction for coronary event; a 1% reduction in LDL corresponding to a 1% reduction in risk.20,21 Additive risk reduction is achieved through HDL elevations, with a 1% increase in HDL corresponding to a 1% reduction in risk.20,22
Although this report suggests that clinical risk factor improvements occur during the course of N.D. care for T2DM, this study has several important limitations. Most notably, since many patients are utilizing naturopathic services as adjunctive care, and patients self-select N.D. services, the generalizability of these findings in more diverse populations is unknown. As evidenced by the mean levels of clinical risk factors at the beginning of care, patients continuing with N.D. care for T2DM appear to be moderately well controlled (i.e., a relatively healthy cohort despite diabetes). This observation, plus the lack of a natural history control or conventional care control, suggests the possibility of bias in the reported estimates of clinical change. These findings are biased, by design, in favor of those patients who continue with N.D. care for at least 6 months, who may be exceptionally motivated regardless of their exposure to N.D. care. In addition, the risk factor reductions reported here are unadjusted estimates of change over the course of care, comparing baseline values to the most recent values in the medical chart. Therefore, they do not describe the longitudinal time course of change. Also, we performed analyses on limited risk factors understanding that additional risk factors may be altered throughout the course of N.D. care and remain unquantifiable by this analysis; similarly, this description does not include any measures of patient experience with N.D. care.
A further limitation is that the contributions of medication modifications to the observed changes cannot be determined using these descriptive analyses. A high prevalence of use of pharmaceutical medications for glucose (54%) and blood pressure (70%) was observed at baseline. Medication change, including either new medications or increases in medication dosage, was evident for 10/37 (27%) and 7/27 (26%) of observed cases for glucose and blood pressure medications, respectively. Evidence of medication discontinuation or dosage reduction was available for 4/37 (11%) of patients. Interestingly, in several circumstances, a recommendation to improve adherence to already prescribed medications, or a recommendation for the patient to return to their primary care physician for medication management, was recommended in the N.D. treatment plan, suggesting a recognition of a need for additional, or optimal, medication management. It is nearly impossible to quantify the effect of this type of naturopathic advice on patient self-management.
Ongoing studies are in progress to evaluate risk factor changes longitudinally over time to determine the relationship, if any, between duration of care and the observed changes in risk factors and to determine the average rate of change. In addition, controlled, observational studies have just begun to evaluate the promise of N.D. care in a more generalized, managed care patient population.
Conclusions
This study provides a description of risk-factor changes that occur during long-term naturopathic care for T2DM. Health-promotion counseling was ubiquitous in naturopathic practice, although the relative contribution of counseling to the observed changes could not be determined. Traditional clinical biomarkers HbA1c, SBP, and DBP were improved, on average, during the course of naturopathic care, with notable percentages of patients achieving improvements in all measures. Controlled, observational studies are currently under way in a managed care patient population to determine whether the changes observed in this descriptive study will be generalizable to community-based, naturopathic practice.
Acknowledgments
The authors would like to acknowledge Jamey Wallace, N.D., Medical Director of the Bastyr Center for Natural Health, for supporting the conduct of this study. This publication was made possible by grant 1KL2RR025015-01 from the National Center for Research Resources (NCRR) and by the National Center for Complementary and Alternative Medicine (NCCAM) via grant 5T32 AT00815-03.
Disclosure Statement
No competing financial interests exist for any of the authors of this publication.
References
- 1.American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:597. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 2.Wee CC, et al. Physician counseling about exercise. JAMA. 1999;282:1583–1588. doi: 10.1001/jama.282.16.1583. [DOI] [PubMed] [Google Scholar]
- 3.Dailey R, et al. Challenges in making therapeutic lifestyle changes among hypercholesterolemic African-American patients and their physicians. J Natl Med Assoc. 2006;98:1895–1903. [PMC free article] [PubMed] [Google Scholar]
- 4.Heaton PC. Frede SM. Patients' need for more counseling on diet, exercise, and smoking cessation: Results from the National Ambulatory Medical Care Survey (2003) J Am Pharm Assoc. 2006;46:364–369. doi: 10.1331/154434506777069516. [DOI] [PubMed] [Google Scholar]
- 5.Mellen PB, et al. Prevalence of nutrition and exercise counseling for patients with hypertension. United States, 1999 to 2000. J Gen Intern Med. 2004;19:917–924. doi: 10.1111/j.1525-1497.2004.30355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipsy RJ. The National Cholesterol Education Program Adult Treatment Panel III guidelines. J Manag Care Pharm. 2003;9(1 suppl):2–5. doi: 10.18553/jmcp.2003.9.s1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikhail N. Cope D. The JNC-7 guidelines and the optimal target for systolic blood pressure. Hypertension. 2004;43:e31. doi: 10.1161/01.HYP.000012525.15835.f1. author reply e31. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2008;31(s1):s12–s54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 9.Agency for Healthcare Research and Quality (AHRQ) Rockville, MD: AHRQ; 2006. The Guide to Clinical Preventive Services: Recommendations of the U.S. Preventive Services Task Force; p. 125. [Google Scholar]
- 10.Aas AM, et al. An intensified lifestyle intervention programme may be superior to insulin treatment in poorly controlled type 2 diabetic patients on oral hypoglycaemic agents: Results of a feasibility study. Diabet Med. 2005;22:316–322. doi: 10.1111/j.1464-5491.2005.01421.x. [DOI] [PubMed] [Google Scholar]
- 11.Williamson DF. Vinicor F. Bowman BA. Primary prevention of type 2 diabetes mellitus by lifestyle intervention: Implications for health policy. Ann Intern Med. 2004;140:951–957. doi: 10.7326/0003-4819-140-11-200406010-00036. [DOI] [PubMed] [Google Scholar]
- 12.Garrow D. Egede LR. Association between complementary and alternative medicine use, preventive care practices, and use of conventional medical services among adults with diabetes. Diabetes Care. 2006;29:15–19. doi: 10.2337/diacare.29.01.06.dc05-1448. [DOI] [PubMed] [Google Scholar]
- 13.Lind BK. Grembowski DE. Diehr PK. Complementary and alternative provider use by insured patients with diabetes in Washington State. J Altern Complement Med. 2006;12:71–77. doi: 10.1089/acm.2006.12.71. [DOI] [PubMed] [Google Scholar]
- 14.Bradley R, et al. Naturopathic medicine and type 2 diabetes: A retrospective analysis from an academic clinic. Alt Med Rev. 2006;11:30–39. [PMC free article] [PubMed] [Google Scholar]
- 15.Chinnock J. Tippens K. Calabrese C. Survey of Naturopathic Treatment of Diabetes. Presented at the American Association of Naturopathic Physicians Annual Convention; 2007. [Google Scholar]
- 16.Iestra JA, et al. Effect size estimates of lifestyle and dietary changes on all-cause mortality in coronary artery disease patients: A systematic review. Circulation. 2005;112:924–934. doi: 10.1161/CIRCULATIONAHA.104.503995. [DOI] [PubMed] [Google Scholar]
- 17.Bradley R, et al. Algorithm for complementary and alternative medicine practice and research in type 2 diabetes. J Altern Complement Med. 2007;13:159–175. doi: 10.1089/acm.2006.6207. [DOI] [PubMed] [Google Scholar]
- 18.Yeh G. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;36:1277–1294. doi: 10.2337/diacare.26.4.1277. [DOI] [PubMed] [Google Scholar]
- 19.Stratton IM. Neil HA. Matthews DR, et al. Association of glycemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown BG. Stukovsky KH. Zhao XQ. Simultaneous low-density lipoprotein-C lowering and high-density lipoprotein-C elevation for optimum cardiovascular disease prevention with various drug classes, and their combinations: A meta-analysis of 23 randomized lipid trials. Curr Opin Lipidol. 2006;17:631–636. doi: 10.1097/MOL.0b013e32800ff750. [DOI] [PubMed] [Google Scholar]
- 21.Wilson PW. Anderson KM. Castelli WP. Twelve-year incidence of coronary heart disease in middle-aged adults during the era of hypertensive therapy: The Framingham offspring study. Am J Med. 1991;90:11–16. doi: 10.1016/0002-9343(91)90500-w. [DOI] [PubMed] [Google Scholar]
- 22.Gordon DJ. Garrison RJ. Neaton JD, et al. High-density lipoprotein cholesterol and cardiovascular disease: Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]