Abstract
The role of IL-17 in atherogenesis remains controversial. We previously reported that the TLR/MyD88 signaling pathway plays an important role in high–fat diet, as well as Chlamydophila pneumoniae (Cpn) infection-mediated acceleration of atherosclerosis in ApoE deficient mice. Here we investigated the role of the IL-17A in high-fat diet and Cpn-induced acceleration of atherosclerosis. The aortic sinus plaque and aortic lesion size and lipid composition as well as macrophage accumulation in the lesions were significantly diminished in IL-17A−/− mice fed high-fat diet compared to wild-type C57Bl/6 control mice. As expected, Cpn infection led to a significant increase in size and lipid content of the atherosclerotic lesions in wild-type mice. However, IL-17A−/− mice developed significantly less acceleration of lesion size following Cpn infection compared to wild-type control despite similar levels of blood cholesterol levels. Furthermore, Cpn infection in wild-type but not in IL-17A−/− mice was associated with significant increases in serum concentrations of IL-12p40, CCL2, INFγ and numbers of macrophages in their plaques. Additionally, in vitro studies suggest that IL-17A activates vascular endothelial cells, which secrete cytokines that in turn enhance foam cell formation in bone marrow macrophages. Taken together, our data suggest that IL-17A is pro-atherogenic and that it plays an important role in both diet-induced atherosclerotic lesion development, and Cpn infection-mediated acceleration of atherosclerotic lesions in the presence of high fat diet.
Introduction
Atherosclerosis is a lipid-driven, chronic inflammatory disease of the vessel wall in which both innate and adaptive immune responses play a role (1). Immune cells and their mediators directly cause the chronic arterial inflammation that is a hallmark of atherosclerosis. Numerous genetic loss- or gain-of-function studies in animal models and other evidence shows that immune cell types are generally neither bystanders nor a consequence of plaque development, but instead directly participate in the disease. T-cells are known to play a critical role during lesion development. Both Th1 and Th2 cells are involved in the process in a delicate interplay between the two cell types (2). The recent addition of Th17 cells to the mix has further complicated the roles each cell type might play during lesion development. IL-17 and its related proteins have been cloned, grouped and designated as the IL-17 cytokine family (IL-17A-F) (3, 4). Interleukin 17A, which is primarily produced by Th17 cells, co-ordinates local tissue inflammation via induced release of pro-inflammatory cytokines and neutrophil-mobilizing chemokines from various cell types including epithelial cells (5). IL-17A is involved in the pathogenesis of several autoimmune diseases such as rheumatoid arthritis (6-8), inflammatory bowel disease (9-13), and asthma(14). This cytokine also contributes to host defense during microbial infections of the lung (15). Recent studies have revealed that IL-17A can drive Th1 responses (16), which at present are regarded as important proinflammatory regulators during atherogenesis. Given these findings, one would expect that IL-17 would have a proatherogenic function. However, the effects of IL-17 are very complex, including both protective and pro-inflammatory roles during immune responses (17). Thus it is not surprising that the role of IL-17A in atherogenesis remains controversial.
Previously, only indirect clues existed for the potential role of IL-17 in atherogenesis. LDLR/IL-6 double KO mice, which exhibit a decrease IL-17 levels (18) were found to have a modest reduction in atherosclerotic lesion development, suggesting a potential role for Th17 in the promotion of atherogenesis. However, IL-6 is a pleiotropic cytokine, making interpretation of the results very difficult. A recent human study demonstrated the concomitant presence of IL-17A and IFN-γ producing T cells in clinical specimens of coronary atherosclerosis, and a synergistic effect of IL-17 and IFN-γ on elicitation of pro-inflammatory cytokine and chemokine production by cultured human vascular smooth muscle cells (VSMCs)(19). Another clinical study reported that patients with acute coronary syndrome have significantly increased peripheral Th17 cell numbers, circulating Th17 related cytokine levels (IL-17, IL-6 and IL-23), increased expression of the transcription factor (RORγt), and a decrease in Treg numbers (20). Additionally, Treg related cytokines (IL-10 and TGF-β1), and the transcription factor Foxp3, were all reduced as compared with patients with stable angina and controls (20).
Several recent studies have attempted to address the role of Il-17 more directly in mice. Kuiper et al transplanted IL-17R-deficient bone marrow into irradiated LDLR deficient recipient mice and observed that Western-type diet-induced atherosclerotic lesions were reduced in the aortic root and the plaque in the recipient mice, suggesting a pro-atherogenic role of IL-17 in this model (21). Most recently, Erbel et al. administered in-vivo IL-17A blocking antibody in apolipoprotein E (ApoE) −/− mice, and found that functional blockade of IL-17A reduced atherosclerotic lesion development and decreased plaque vulnerability, cellular infiltration, and tissue activation in ApoE-deficient mice (22, 23). They conclude that the involvement of IL-17 in proatherogenesis appears to be via proinflammatory changes at multiple levels such as cell adhesion, extravasation, cell activation, T cell (co)stimulation/proliferation, and antigen presentation in the inflammatory cascade of atherosclerosis (22). In contrast to these studies, Taleb et al demonstrated that loss of suppressor of cytokine signaling-3 (SOCS-3) in mouse T-cells increases both IL-17A and IL-10 production, inducing an anti-inflammatory macrophage phenotype which results in a reduction in lesion development and vascular inflammation, suggesting that IL-17 may have a protective role in atherogenesis (24). Collectively, these studies have shown conflicting data, one study suggesting a protective function for IL-17 in atherogenesis (24), while several others showing proatherogenic properties of IL-17 and Th17 T-cells (21, 22). However, all these studies used indirect methods to assess the role of IL-17 in the development of atherosclerosis.
In order to more directly investigate the role of IL-17 in high-fat diet-induced atherosclerotic lesion development, we investigated IL-17A−/− mice in our studies. Under high fat diet, IL-17A−/− mice had reduced atherosclerotic lesion development as measured by aortic sinus plaque and aortic lesion development. Macrophage accumulation in the lesions was also significantly diminished in IL-17A−/− mice compared to wild-type controls. Additionally, we investigated the role IL-17A may play during Cpn infection-mediated acceleration of atherosclerosis. As expected, Cpn infection led to significantly larger atherosclerotic lesions and lipid composition in wild-type mice compared to uninfected controls. However, Cpn infection-induced acceleration of lesion development was significantly reduced in IL-17A−/− mice compared to wild-type controls despite similar levels of blood cholesterol levels. Furthermore, as we reported earlier (25), Cpn infection in wild-type mice was associated with significant increases in serum concentrations of IL-12p40 and CCL2, which was not observed in IL-17A−/− mice. Additionally, in vitro studies suggest that mouse aortic endothelial cells respond to IL-17A and the resultant cytokine responses can in turn enhance bone marrow macrophages to become foam cells. Taken together, we show that IL-17A plays an important role in both high-fat diet-induced atherosclerotic lesion development, and Cpn-infection-mediated acceleration of atherosclerotic lesions. To our knowledge, this is the first direct evidence that IL-17A plays a proatherogenic role in mice.
Materials and Methods
Animals
C57BL/6 wild-type (WT), mice were purchased from Jackson laboratories. IL-17A−/− mice (26) were kindly provided by Dr. Richard Flavell (Yale University, CT, US), A homogenous population of these mice was established by backcrossing onto the C57BL/6 background for at least 8 generations as previously described (26, 27). Only male mice were used for this study. Mice were fed for 12 weeks with high fat diet (HFD) containing 15.8% fat (approx. half from cocoa butter), 1.25% cholesterol and 0.5% sodium cholate (Paigen diet) (TD88051, Harlan Teklad, Madison, WI) as described by several investigators (28-31). See result section for detail methods. All animal experiments were performed under protocols that had been approved by the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center.
C. pneumoniae Infection
C. pneumoniae strain CM-1 (ATCC, Manassas, VA) was propagated in Hep-2 cells as previously described. Hep-2 cells and the C. pneumoniae stocks were determined to be free of Mycoplasma contamination by PCR. Mice were anesthetized with isofluorane prior to intranasal (i.n.) application of 5 × 104 IFU/mouse of C. pneumoniae suspended in sucrose-phosphate-glutamate buffer (20μl per nostril). The intranasal administration of the buffer alone was performed as a negative control (mock infection, data not shown). Mice were inoculated a total of three times one week apart, and after the last inoculation, a high fat diet was initiated and continued for 12 weeks, at which point mice were sacrificed and dependent variables measured as we published earlier (25, 27).
Lipid Profiles
Mice were sacrificed and Sera from mice were obtained at the end of experiments, after an overnight fast. Total cholesterol concentrations were determined in duplicate by using a colorimetric assay (infinity cholesterol reagent, Sigma Diagnostics, St. Louis) as we described earlier (27).
Assessment of Atherosclerotic Lesions in the Aorta and Aortic Sinus
Mice were anesthetized with isofluorane before the aorta and the heart were excised. Aortas were excised, from the aortic arch to the iliac bifurcation. Adherent (advential) connective fat was removed, and specimens were then fixed in Histo-CHOICE (Amrecso, Solon, OH). Whole aortas were opened longitudinally and mounted en face, then stained for lipids with Oil red O. Hearts were embedded in OCT compound (Tissue Tek, Sakura, Torrance, CA), and cross sections of the aortic sinus were stained with Oil red O. Lesions areas were quantified with Image-Pro Plus (Media Cybernetics, Silver Spring, MD). Image analysis was performed by a trained observer blinded to the genotypes of mice as previously described (27, 32). The lesion area and lipid-stained areas in the aortic sinus were measured. Lipid content in aortic root plaques was expressed as aortic sinus lesion area or as percent of plaque area. The lesion area in the aorta en face preparations was expressed as a percent of the aortic surface area as previously reported (27).
Immunohistochemical Staining and Quantification of Macrophages in the Aortic Sinus
Frozen heart sections (aortic sinuses) were analyzed for infiltration of macrophages using rat anti-mouse MOMA-2 Ab (Serotec) as we described previously (27). Secondary biotinylated Ab was used followed by streptavidin-biotin-peroxidase complex. An isotype control (IgG2a; Serotec) was used to demonstrate specificity of staining.
COX-2 Staining
Sections were blocked in 0.1 M Tris·HCl/0.15 M NaCl/0.5% blocking reagent (TNB blocking buffer) and incubated with primary COX-2 or control IgG antibody (Santa Cruz Biotechnology) diluted in TNB blocking buffer at 4°C overnight (1:5,000 dilution), followed by incubation with streptavidin-horseradish peroxidase complex. The signal was enhanced by using the tyramide signal amplification kit (NEN Life Science Products, Boston) according to the manufacturer’s recommendations and sections were counterstained for nuclei with 100 nM SYTOX green (Molecular Probes) as we described earlier (27).
Assessment of T cells in the in the Aortic Sinus
Frozen heart sections were analyzed for T cell infiltration using rat anti-mouse CD3 (eBioscience), a T cell marker, as described earlier (17). Rat IgG2b was used as isotype control (Serotec).
Computer-assisted image analysis for Immunohistrochemistry
Three consecutive sections of the aortic root were photographed at a magnification of ×100 with a charge-coupled device camera (Nikon DXM 1200). Macrophages, COX-2 expression, and T cells in the lesions were quantified by computer-assisted histomorphometry (Image-Pro Plus; Media Cybernetics). For each analysis, the color threshold for immunostained cells was manually adjusted in the images until the computerized detection matched visual interpretation. The numbers of immunostained cells were digitally counted in the lesion area of the aortic root section. For each cell type, the mean cell number was calculated out of the corresponding three consecutive sections for each animal (5-7 animals/group). Microscopic analyses were performed independently by two different investigators, and intra- and interobserver coefficients of variabilities were <10%.
Serum Levels of Cytokines and Chemokines
IL-12p40, CCL2 and IFNγ concentrations in the sera of mice were measured by ELISAs according to the manufacturer’s instructions (BD Biosciences).
Flow Cytometry
Primary murine aortic ECs were isolated and purified to >95% purity from WT mice as we previously described (27). Cell surface staining was performed MAEC using PE-anti-mouse IL-17RA mAb (Clone : PAJ-17R. eBioscience, San Diego, CA, USA) with appropriate IgG isotype controls (eBioscience). Flow cytometric analysis was performed by FACScan flow cytometer (BD Biosciences) and the data was analyzed by Summit (Dako, Carpinteria, CA, USA).
IL-6 and CCL2 induction by IL-17A stimulated murine endothelial cells (ECs)
Primary murine aortic ECs were grown to 80% confluency and stimulated overnight with 100 ng/ml rIL-17A. IL-6 and CCL2 release into the cell-free supernatant were determined using ELISA (eBioscience) after 24 h of treatment.
Preparation of Peritoneal Macrophages and Assessment of Foam Cell Formation
Peritoneal macrophages were isolated by injecting Hanks’ balanced salt solution (HBSS, GIBCO, USA) into the peritoneal cavity of mice immediately after euthanization. Peritoneal cells were washed and seeded in 24-well plates (2.5×105 cells/well) in RPMI 1640 medium (Cellgro, Los Angeles, CA) with 10% FBS, incubated at 37 °C, 5% CO2 for 3h, washed twice with HBSS to remove non-adherent cells, and cultured for 24 hours prior to treatment. For assesment of foam cells, Cells were stained with Oil Red O as we described previously (33).
Statistical Analysis
Data are reported as mean values ± SEM. Statistical differences were assessed by Student’s t test, and p values less than 0.05 were considered significant. Where appropriate, two-way ANOVA was used followed by a Bonferonni post-test to determine significance.
RESULTS
IL-17A Plays a Proatherogenic Role in High-fat Diet-Induced Acceleration of Atherosclerosis in C57BL/6 Mice
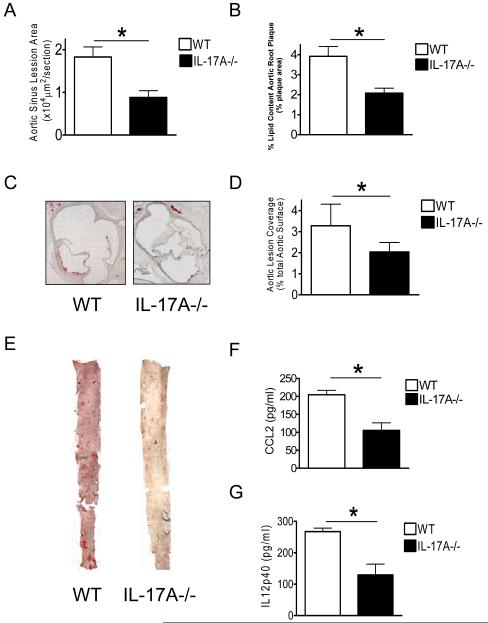
Similar to the findings of Smith et al (23), we found that IL-17A was upregulated in the plaques of LDLR−/− mice fed high fat diet (data not shown). Based on published data and our finding of increased IL-17A expression in the aortic root lesions of LDLR−/− mice, we next investigated directly the role of IL-17A in atherogenesis using IL-17A−/− mice. IL-17A−/− mice were fed on a high fat diet for 12 weeks as described in the materials and methods. Quantification of the lesion area of aortic sinus plaques revealed a significant reduction in lesion size in IL-17A−/− mice compared to WT mice (p<0.05) (Fig. 1A and 1D) after 12 weeks of high cholesterol diet. IL-17A−/− mice also showed a significant reduction in lipid accumulation in both the aortic sinus plaque lesions (Fig. 1B) and the total lesion area in the en face aorta (p<0.05) (Fig. 1C and 1E) compared to WT mice.
Figure 1. Lack of IL-17A reduced High cholesterol diet-induced acceleration of atherosclerosis.
A, B, D, Quantification of lipid content in the aortic sinus and aorta lesions en face from WT and IL17A−/− mice. C, E, Representative oil red O staining of aortic sinus plaque and aorta en face from WT and IL-17A−/− mice are shown. Data are presented as mean value ± SEM. N=12 for WT, N=15 for IL-17A−/− group. F, G, Serum concentration of CCL2 and IL-12p40 are reduced in IL-17A−/− mice compared with C57BL/6 mice after fed with a high-cholesterol diet for 12 weeks. (n = 10 in each group). Means and SD are shown (*p < 0.05).
IL-17A Deficient mice fed High-fat Diet Have Reduced Circulating Levels of the CCL2, IL-12p40
Although the results above indicate that IL-17A plays a direct role in atherogenesis, we do not know if its effects are in the arterial wall, a general proinflammatory response, or both. We therefore determined how IL-17A deficiency altered circulating concentrations of a key chemokine and proinflammatory cytokine (CCL2, IL-12p40 respectively) in WT mice and IL-17A deficient mice at the end of the experiment. IL-17A−/− mice had significantly reduced serum levels of CCL2, IL-12p40 compared to WT mice after 12 weeks high cholesterol diet (p<0.05) (Fig. 1F and 1G), suggesting that IL-17A can act as a general proinflammatory inducer during atherogenesis.
IL-17A Plays a Role in C. pneumoniae-Infection-Induced Acceleration of Atherosclerosis
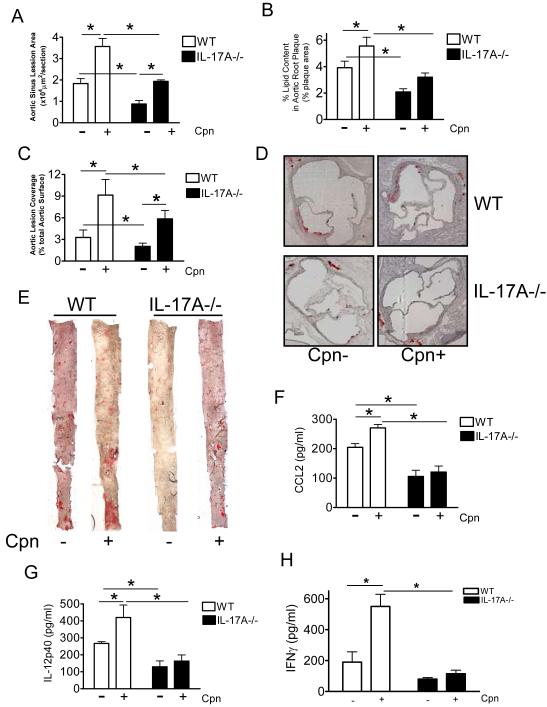
We and others have previously shown that Chlamydophila pneumoniae infection can significantly accelerate the development of atherosclerotic lesions in hypercholesterolemic mice (25, 34) . While there has been no direct study investigating the role of IL-17A during Cpn infection, IL-17 was shown to be an important factor during Chlamydia muridarum pulmonary infections in mice (35-37). Therefore we investigated the ability of Cpn to accelerate atherogenesis in IL-17A−/− mice. We administered C. pneumoniae or an equivalent volume of buffer intranasally to WT and IL-17A−/− mice. Mice were infected once a week for 3 consecutive weeks then started on a high fat diet which was maintained until the time of sacrifice 3 months later as previously described (25). Cpn infection led to significantly enlarged lesion size in both WT and IL-17A−/− mice compared to uninfected WT and IL-17A−/− mice (p<0.005) (Fig 2A and 2C), while IL-17A−/− mice displayed a trend toward increased aortic root lipid content with infection (Fig. 2B). Importantly, IL-17A deficient mice developed significantly less C. pneumoniae-induced acceleration of atherosclerosis compared to WT infected mice (p<0.005) (Fig.2A-2E) despite similar levels of blood cholesterol and similar lipoprotein profiles between groups (Table 1, and data not shown). We showed above that uninfected IL-17A−/− mice demonstrated a reduction in lipid content in aortic sinus plaques and a reduction in the size of atherosclerotic lesions in the aorta compared to WT controls (Fig. 1A, 1B, and 1C). However, following C. pneumoniae infection both Lipid content in aortic sinus plaques (expressed as percentage of plaque area; Fig. 2B) and Aortic lesion coverage size (aorta en face) (Fig. 2C) were significantly reduced in IL-17A−/− mice compared to infected WT controls mice (p<0.05). These observations suggest that while IL-17A−/− mice still develops accelerated lesion size following Cpn infection, the overall lesion size and the increases are significantly smaller compared to WT mice and thus IL-17A partially contributes to Cpn-infection-mediated acceleration of atherosclerosis. All infected mice developed similar levels of specific Ab titers against C. pneumoniae, whereas all uninfected controls were Ab negative (data not shown). We did not observe any effect of IL-17A gene deficiency on bacterial replication and clearance in lung post-infection with Cpn (unpblished data).
Figure 2. Lack of IL-17A reduced Chlamydia pneumoniae infection-induced acceleration of atherosclerosis in C57BL/6 mice.
A, B, C, Quantification of lipid content in the aortic sinus, and aorta en face from WT and IL-17A−/− mice with and without infection. D, E, Representative Oil red O staining of aortic sinus and aorta en face lesions from infected and uninfected WT and IL-17A−/− mice are shown. Data are shown as mean values ± SEM, n=12-15 for the aortic sinus experiments, and n=12-15 for the en face aorta experiments. F, G, H, CCL2, IL-12p40 and IFN-γ serum concentrations of C57BL/6 mice and IL-17A−/− mice fed with a high-cholesterol diet for 12 weeks with and without C. pneumoniae infection (n = 10 in each group). Means and SD are shown (*p < 0.05).
Table 1.
Total Cholesterol level in Serum (mg/dl)
| WT | IL-17−/− | |
|---|---|---|
| Reg Chow | 165.1±22.4 | 146.8±25.6 |
|
High Fat Diet
Cpn− |
412.5±41.2* | 377.9±89.6* |
|
High Fat Diet
Cpn+ |
358.5±61.2* | 478.9±103.6* |
IL-17A-Deficient Mice Infected with Cpn have Lower serum CCL2, IL-12p40 and IFNγ levels Compared to Wild type Mice
Cpn infection was associated with significant increases in serum concentrations of CCL2, IL-12p40 and IFNγ in WT mice but not in IL-17−/− mice (p<0.05) (Fig 2F ,2G, 2H). These results appear most consistent with the interpretation that at least part of the acceleration of atherosclerosis observed in Cpn-infected WT mice may be mediated by this general increase in circulating levels of proatherogenic inflammatory cytokines and chemokines, which were not observed in IL-17A−/− mice.
IL-17A- Deficient mice Infected with Cpn Display Reduced Macrophage Infiltration, COX-2 Immunoreactivity and T cell infiltration in Aortic Sinus Plaques
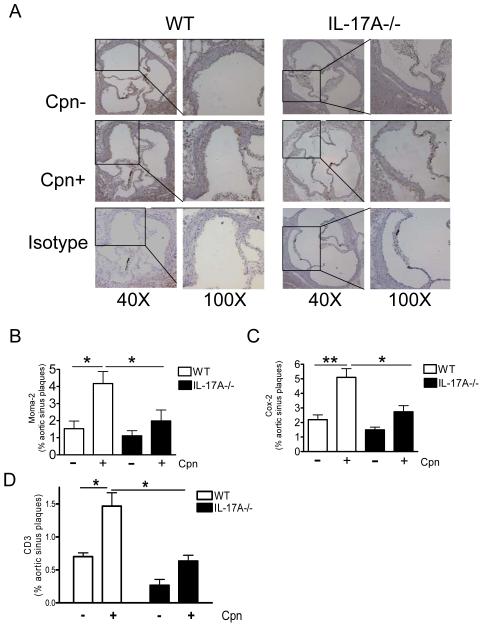
To examine the effect of IL-17A deficiency on the cellular composition of atherosclerotic plaques and on the expression of the proinflammatory enzyme COX-2 in the aortic lesions, we examined the extent of macrophage infiltration with MOMA-2 immunostaining and COX-2 immunostaining in the aortic sinus plaques of WT mice and IL-17A−/− mice. Aortic sinus plaques of uninfected WT and IL-17A−/− mice on high-fat diet exhibited similar levels macrophage accumulation and COX-2 immunostaining (Fig. 3A and 3B). However, as we have previously published, Cpn infection significantly increased the macrophage accumulation (Fig. 3B) and COX2 expression (Fig. 3C) (p<0.05) in the aortic root plaques of WT mice. However, Cpn-infected IL-17A−/− mice exhibited significantly less macrophage accumulation (Fig 3A, 3B) and COX-2 immunoreactivity (Fig 3C) in the lesions compared to infected WT mice (p<0.05). To assess the presence of T cells infiltrating the aortic root lesions in this mouse model, we performed IHC staining for CD3+ cells in WT mice and IL-17A−/− mice with and without Cpn infection. As shown in Fig. 3D, Cpn infected WT mice showed a significantly increased accumulation of CD3+ cells in the aortic sinus lesions (p<0.05). Interestingly, there were significantly reduced numbers of CD3+ T cells were observed in infected IL-17A−/− mice aortic lesion compared to the infected WT mice (Fig3D, p<0.05).
Figure 3. C. pneumoniae infection leads to increasing of macrophage infiltration, Cox-2 expression, and CD3 T cell infiltration in the aortic sinus of C57BL/6 mice compared with IL-17A−/− mice.
A, Representative MOMA-2-positive staining in uninfected and infected IL-17A−/− and WT mice. Infection with C. pneumoniae (5 × 104 IFU/mouse) led to greater accumulation of MOMA-2 -positive and (brown nuclear) in the aortic sinus from C57BL/6 mice, but not in IL-17A−/− mice (only blue nuclears). B, Quantitative analysis of macrophage immunoreactivity in aortic sinus plaques of WT and IL-17A−/− mice, expressed as a proportion of the total plaque areas (n=7 in each group). Means and SD are shown (*p < 0.05). C, Quantitative analysis of COX-2 immunofluorescent staining in sclerotic plaques of WT and IL-17A−/− mice (n = 6 in each group). Means and SD are shown (*p < 0.05). D, Quantification of CD3+ cells and quantitative analysis of CD3 immunoreactivity in the aortic sinus plaques of WT and IL-17A−/− mice (n = 5 in each group). Means and SD are shown (*p < 0.05).
Mouse Aortic Endothelial Cells Express IL-17 Receptor A and Respond to IL-17A by IL-6 and CCL2 Release and Enhance Subsequent Foam Cell Formation by Macrophages
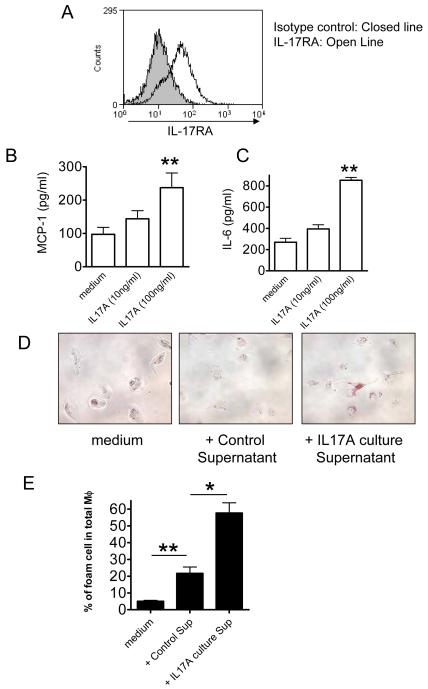
IL-17A was shown to act on non-hematopoietic cells previously (21). Additionally, Smith et al showed that IL-17A treatment of whole aorta isolated from ApoE−/− mice promotes aortic CXCL1 expression and monocyte adhesion in an ex vivo adhesion assay (23). Roussel et al recently demonstrated that ECs produce chemokine and upregulate VCAM and E-selectin in response to IL-17(38). To evaluate whether murine endothelial cells (ECs) do express IL-17RA and respond to IL-17A, we isolated primary mouse aortic endothelial cells (MAECs) and stained the cells with an IL-17RA antibody and analyzed by Flow cytometry. We observed that IL-17RA is expressed on the surface of MAECs (Fig. 4A). We therefore reasoned that IL-17A could stimulate EC to release various cytokines, which in turn would lead to enhanced leukocyte–EC adhesion, one of the pivotal early events in development of atherosclerotic plaques, and may facilitate foam cell formation in macrophages. To address this possibility, we stimulated WT MAECs with recombinant IL-17A or PBS only, and measured IL-6 and CCL2 release. As anticipated, MAECs stimulated with IL-17A lead to significantly increased IL-6 and CCL2 release (Fig 4B and 4C). These results suggest the IL-17A is able to activate mouse aortic EC and induce the production of various cytokines, which would increased leukocyte-EC adhesion and be proatherogenic. We next explored whether the cytokine milieu induced by IL-17A-stimulated endothelial cells might accelerate the oxidized LDL (ox-LDL) induced foam cell formation in macrophages in vitro. We isolated peritoneal-derived macrophages from WT mice and cultured with ox-LDL in the presence of supernatant of IL-17A-stimulated EC and measured foam cell formation as we described previously (33). As anticipated, treatment of macrophages with ox-LDL (100 μg/ml) and IL-17A-stimulated EC supernatant induced a significant increase in foam cell formation (Fig 4D and 4E) compared to controls. Importantly, the addition of recombinant IL-17A alone to the macrophages did not alter foam cell formation (data not shown). These findings demonstrate that IL-17A can induce EC to release pro-inflammatory cytokines that facilitate foam cell formation in macrophages, thus providing another mechanism by which IL-17A may be proatherogenic in this model.
Figure 4. IL-17A increases macrophage foam cell formation via murine aortic endothelial cells.
A, Flow cytometry analysis on MAECs derived from aortas of WT mice to determine the presence of IL-17RA. Shaded histogram: Isotype control. Open histogram: Anti-IL-17RA. B, MAECs derived from aortas of WT mice were stimulated with recombinant IL-17A (100 ng/ml) for 24 h. The supernatants were collected and IL-6 and levels were measured by ELISA. C, MAECs derived from aortas of WT mice were stimulated with recombinant IL-17A (100 ng/ml) for 24 hr. CCL2 levels were measured by ELISA. Data shown are derived from one representative experiment out of three independent experiments. Stimulations were performed in quadruplicates. Means ± SD are shown. D, Micrographs of Oil Red O- stained wild type peritoneal macrophages treated with culture supernatants of MAEC with and without IL-17A stimulation. E, The percentages of foam cells in total macrophages were quantified. Means and SD are shown (*p < 0.05, **<p<0.01). Control Sup: culture supernatants of MAEC without IL-17A stimulation.
DISCUSSION
IL-17 and Th17 cells have quickly become an important paradigm in immunology and the IL-23-IL-17 axis has emerged as a critical regulatory system that bridges the innate and adaptive arms of the immune system and have been linked to the pathogenesis of several chronic inflammatory diseases (39-42). The role of Th17 cells and IL-17 in various stages of atherogenesis remains controversial and is only beginning to be elucidated. While IL-17 is predominantly a proinflammatoryy cytokine, it has pleotrophic and regulatory functions as well and has been implicated both as an instigator in the pathogenesis of inflammatory disorders as well as protective in certain inflammatory disease models (17). Here we show that genetic deficiency of IL-17A reduces the size of atherosclerotic plaques in the aortic sinus and the aorta en face preparations in C57BL/6 mice under high fat diet. Furthermore, IL-17A deficiency was associated with significant reductions in lipid composition of the plaques, the number of infiltrating macrophages as well as expression of the proinflammatory enzyme, COX-2 and T cell infiltration in the aortic sinus lesions. The atheroprotective effect of IL-17A deficiency was not due to altered serum cholesterol or lipoproteins. We observed significantly reduced circulating proinflammatory cytokines IL-12p40, CCL2 and IFNγ in IL-17A−/− mice, suggesting that the atheroprotective effects of IL-17A deficiency may result in part from reduced systemic inflammation. Collectively, our data obtained from IL-17-deficient mice indicate that IL-17A is proatherogenic in the hypercholesterolemic mouse model, and that Th17 cells and IL-17A contribute to atherogenesis by both systemic and local effects.
Consistent with previous studies (25, 28, 34, 43), we also found that C. pneumoniae infection of hypercholesterolemic C57BL/6 mice (WT) resulted in accelerated atherosclerosis associated with significantly increased lesion size, lipid content, numbers of macrophage and COX-2 in lesions, and serum levels of proinflammatory cytokines (IL-12p40, CCL2) compared with mock-infected controls. In contrast, while C. pneumoniae infection was still able to accelerate atherosclerosis in IL-17A−/−, the infection-mediated acceleration of lesion size and lipid composition of the aorta as well as serum levels of IL-12p40 and CCL2 increases were significantly inhibited in IL-17A-deficient mice compared to infected WT mice, suggesting that C. pneumoniae infection-induced acceleration in lesion development in hypercholesterolemic mice, is at least partially driven by an IL-17A-dependent manner. However, the data also suggest that pathogen-induced acceleration can still occur in an IL-17A-independent manner. Importantly, since we did not observe any influence of IL-17A gene deficiency on bacterial replication and clearance in the lungs during Cpn infection (data not shown), we believe that the residual infection-induced acceleration of atherosclerosis in IL-17A-deficient mice is not due to a significantly altered course of infection.
In this study we used a well-described and widely-used high-fat diet that contains cholate (Paigen diet) (44) that has been shown to induce atherosclerotic lesions in C57BL/6 mice by several investigators (45, 46), (28, 47), While there have been some concerns that cholate containing high-fat diets may be associated with induction of fibrosis related genes in the liver in addition to inflammation genes induced by non-cholate containing high-fat diets (30), we did not observed any liver fibrosis in our groups and lipoprotein levels were similar in both WT and IL-17-deficient mice that received the same diet. The hypercholesterolemia induced by this atherogenic diet in C57BL/C mice is milder than in genetically altered mice such as ApoE-deficient mice (48),(34). Therefore, this model seems to be a useful model for studying the accelerating role of C. pneumoniae in atherosclerosis at an earlier stage.
Our data showing that IL-17A is proatherogenic, using IL-17A-deficient mice, is in agreement with several previous studies (21, 22). Kuiper van Es et al observed that irradiated LDLR-deficient recipient mice transplanted with IL-17RA-deficient bone marrow resulted in a 46% reduction in lesion size in the aortic root plaque under a Western-type diet (21). However, one limitation to this model is that stromal cells are known to be important responders to IL-17A (49, 50), thus this model should be considered only as a partial phenotype. Erbel et al. administered blocking antbody agianst IL-17A that resulted in reduced atherosclerotic lesion development and decreased plaque vulnerability, cellular infiltration, and tissue activation in ApoE-deficient mice (22). One limitation of this study is that blocking IL-17A will not prevent the formation of Th17 cells, as well as other IL-17 producing cell types, which may also have effects aside from IL-17A production. Additionally, as with all blocking strategies, the prospect of leaky signaling, and or cross reactivity of the antibody cannot be ignored. Another recent study by Smith et al found that IL-17A treatment of whole aorta isolated from ApoE−/− mice promots aortic CXCL1 expression and monocyte adhesion in an ex- vivo adhesion assay(23), also supporting a proatherogenic role for IL-17A. However, in contrast to our results and these earlier studies, Taleb et al published that loss of suppressor of cytokine signaling-3 (SOCS-3) in mouse T cells increases IL-17A production, inducing an anti-inflammatory macrophage phenotype, which results in a reduction in lesion development and vascular inflammation, suggesting that IL-17 may have a protective role in atherogenesis (24). Additionally, these investigators have also shown that in vivo administration of rIL-17A to LDLR−/− mice resulted in reduced endothelial VCAM-1 expression, as well as reduced vascular T cell infiltration and atherosclerotic lesion development (24). These investigators concluded that endogenous expression of SOCS3 in T cells interrupts a major regulatory pathway in atherosclerosis through inhibition of IL-17A production and that IL-17A functions as an atheroprotective cytokine. However, one caveat to this study is the fact that in addition to upregulated IL-17A, the authors also found an increase in IL-10 production, a potent regulatory cytokine, which is atheroprotective (51-55). Additionally, SOCS-3 plays a role in several different signaling cascades, thus it is difficult to assign cause and effect for the role of IL-17A in the SOCS-3 knockout model (56). In summary while the precise role of IL-17 in various stages of atherogenesis remains controversial, our study together with recently published studies (21-23) now provide a more direct evidence that IL-17A maybe predominantly proatherogenic. Nevertheless, IL-17 is a pleiotropic cytokine with environment-specific inflammatory or protective and regulatory functions(17). Therefore, the role of IL-17 in various stages of lesion development are probably complex and needs to be further evaluated.
Many studies have documented the importance of Th1 responses and atherogenesis (57-62). We have gone over in some detail the current literature on this subject and reviewed the current controversy on the role of Th17 cells and atherosclerosis(63). It has become increasingly clear that Th17 and Th1 cells regulate each other, apparently in both directions (16, 64-66). Th1 cells have been found to be necessary for the downstream accumulation of Th17 cells (67). In a recent study published in Immunity, Lin Y et al found that IL-17A is required for induction of IL-12, a critical cytokine in Th1 skewing, and host resistance to infection (16). If IL-17 was required for proper Th1 responses in atherogenesis, then one would predict that IL-17A−/− mice would have reduced atherosclerotic lesions, as we have seen in our study. We also found that IL-17A could induce proinflammatory cytokine production (CCL2, IL-6) in primary aortic endothelial cells, and that these supernatants could then drive foam cell formation and contribute to atherogenesis. Taken together with the previous study that showed IL-17A could induce monocyte adhesion and CXCL1 expression on aortic endothelial cells, one could argue that IL-17A most likely also has a direct effect on lesion development (23).
Adding to the complexity in assessing our study and the other investigations for the role of IL-17 is the fact that IL-17 is a family of cytokines, including various isoforms. Our study utilized the IL-17A knockout mouse, but presumably IL-17F would be unaffected in this mouse model. Additionally, IL-17E (IL-25), an anti-inflammatory cytokine, was found to be expressed in normal and atherosclerotic vessels, and might play a role in regulating inflammatory processes in the vessel wall (68). Therefore further work investigations should be done to define the specific role of the different IL-17 isoforms (such as IL-17F or IL-17E) in atherosclerosis.
REFERENCES
- 1.Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 2.Coffman RL. Origins of the T(H)1-T(H)2 model: a personal perspective. Nat Immunol. 2006;7:539–541. doi: 10.1038/ni0606-539. [DOI] [PubMed] [Google Scholar]
- 3.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 4.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 5.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine. 2008;41:84–91. doi: 10.1016/j.cyto.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther. 2005;7:29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 14.Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, Puccetti P, Iwamoto I, Nakajima H. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178:1023–1032. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 15.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, Onishi R, Nyugen N, Walter MJ, Pociask D, Randall TD, Gaffen SL, Iwakura Y, Kolls JK, Khader SA. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connor W, Jr., Zenewicz LA, Flavell RA. The dual nature of T(H)17 cells: shifting the focus to function. Nat Immunol. 11:471–476. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 18.Song L, Schindler C. IL-6 and the acute phase response in murine atherosclerosis. Atherosclerosis. 2004;177:43–51. doi: 10.1016/j.atherosclerosis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, Yao R, Chen Y, Liao YH. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97. doi: 10.1016/j.clim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 21.van Es T, van Puijvelde GH, Ramos OH, Segers FM, Joosten LA, van den Berg WB, Michon IM, de Vos P, van Berkel TJ, Kuiper J. Attenuated atherosclerosis upon IL-17R signaling disruption in LDLr deficient mice. Biochem Biophys Res Commun. 2009;388:261–265. doi: 10.1016/j.bbrc.2009.07.152. [DOI] [PubMed] [Google Scholar]
- 22.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Bockler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 23.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, Yoshimura A, Tedgui A, Mallat Z. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206:2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naiki Y, Sorrentino R, Wong MH, Michelsen KS, Shimada K, Chen S, Yilmaz A, Slepenkin A, Schroder NW, Crother TR, Bulut Y, Doherty TM, Bradley M, Shaposhnik Z, Peterson EM, Tontonoz P, Shah PK, Arditi M. TLR/MyD88 and liver X receptor alpha signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol. 2008;181:7176–7185. doi: 10.4049/jimmunol.181.10.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 27.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blessing E, Campbell LA, Rosenfeld ME, Chough N, Kuo CC. Chlamydia pneumoniae infection accelerates hyperlipidemia induced atherosclerotic lesion development in C57BL/6J mice. Atherosclerosis. 2001;158:13–17. doi: 10.1016/s0021-9150(00)00758-9. [DOI] [PubMed] [Google Scholar]
- 29.de Haan JB, Witting PK, Stefanovic N, Pete J, Daskalakis M, Kola I, Stocker R, Smolich JJ. Lack of the antioxidant glutathione peroxidase-1 does not increase atherosclerosis in C57BL/J6 mice fed a high-fat diet. J Lipid Res. 2006;47:1157–1167. doi: 10.1194/jlr.M500377-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Getz GS, Reardon CA. Diet and murine atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:242–249. doi: 10.1161/01.ATV.0000201071.49029.17. [DOI] [PubMed] [Google Scholar]
- 31.Nishina PM, Lowe S, Verstuyft J, Naggert JK, Kuypers FA, Paigen B. Effects of dietary fats from animal and plant sources on diet-induced fatty streak lesions in C57BL/6J mice. J Lipid Res. 1993;34:1413–1422. [PubMed] [Google Scholar]
- 32.Qiao JH, Tripathi J, Mishra NK, Cai Y, Tripathi S, Wang XP, Imes S, Fishbein MC, Clinton SK, Libby P, Lusis AJ, Rajavashisth TB. Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteopetrotic mice. Am J Pathol. 1997;150:1687–1699. [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S, Sorrentino R, Shimada K, Bulut Y, Doherty TM, Crother TR, Arditi M. Chlamydia pneumoniae-induced foam cell formation requires MyD88-dependent and -independent signaling and is reciprocally modulated by liver X receptor activation. J Immunol. 2008;181:7186–7193. doi: 10.4049/jimmunol.181.10.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moazed TC, Campbell LA, Rosenfeld ME, Grayston JT, Kuo CC. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. J Infect Dis. 1999;180:238–241. doi: 10.1086/314855. [DOI] [PubMed] [Google Scholar]
- 35.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, Jiao L, Yao Z, Yang X. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol. 2009;183:5886–5895. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, Chen Q, Moore J, Kolls JK, Halperin S, Wang J. Critical role of the interleukin-17/interleukin-17 receptor axis in regulating host susceptibility to respiratory infection with Chlamydia species. Infect Immun. 2009;77:5059–5070. doi: 10.1128/IAI.00403-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Gao L, Lei L, Zhong Y, Dube P, Berton MT, Arulanandam B, Zhang J, Zhong G. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J Immunol. 2009;183:1291–1300. doi: 10.4049/jimmunol.0803075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roussel L, Houle F, Chan C, Yao Y, Berube J, Olivenstein R, Martin JG, Huot J, Hamid Q, Ferri L, Rousseau S. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol. 184:4531–4537. doi: 10.4049/jimmunol.0903162. [DOI] [PubMed] [Google Scholar]
- 39.Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med. doi: 10.1016/j.rmed.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Lafdil F, Miller AM, Ki SH, Gao B. Th17 cells and their associated cytokines in liver diseases. Cell Mol Immunol. doi: 10.1038/cmi.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarra M, Pallone F, Macdonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. doi: 10.1002/ibd.21248. [DOI] [PubMed] [Google Scholar]
- 42.Algood HM, Allen SS, Washington MK, Peek RM, Jr., Miller GG, Cover TL. Regulation of gastric B cell recruitment is dependent on IL-17 receptor A signaling in a model of chronic bacterial infection. J Immunol. 2009;183:5837–5846. doi: 10.4049/jimmunol.0901206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothstein NM, Quinn TC, Madico G, Gaydos CA, Lowenstein CJ. Effect of azithromycin on murine arteriosclerosis exacerbated by Chlamydia pneumoniae. J Infect Dis. 2001;183:232–238. doi: 10.1086/317941. [DOI] [PubMed] [Google Scholar]
- 44.Ishida BY, Albee D, Paigen B. Interconversion of prebeta-migrating lipoproteins containing apolipoprotein A-I and HDL. J Lipid Res. 1990;31:227–236. [PubMed] [Google Scholar]
- 45.Paigen B. Genetics of responsiveness to high-fat and high-cholesterol diets in the mouse. Am J Clin Nutr. 1995;62:458S–462S. doi: 10.1093/ajcn/62.2.458S. [DOI] [PubMed] [Google Scholar]
- 46.Ishida BY, Blanche PJ, Nichols AV, Yashar M, Paigen B. Effects of atherogenic diet consumption on lipoproteins in mouse strains C57BL/6 and C3H. J Lipid Res. 1991;32:559–568. [PubMed] [Google Scholar]
- 47.Chesebro BB, Blessing E, Kuo CC, Rosenfeld ME, Puolakkainen M, Campbell LA. Nitric oxide synthase plays a role in Chlamydia pneumoniae-induced atherosclerosis. Cardiovasc Res. 2003;60:170–174. doi: 10.1016/s0008-6363(03)00389-4. [DOI] [PubMed] [Google Scholar]
- 48.Hu H, Pierce GN, Zhong G. The atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. J Clin Invest. 1999;103:747–753. doi: 10.1172/JCI4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saitoh T, Kusunoki T, Yao T, Kawano K, Kojima Y, Miyahara K, Onoda J, Yokoi H, Ikeda K. Role of interleukin-17A in the eosinophil accumulation and mucosal remodeling in chronic rhinosinusitis with nasal polyps associated with asthma. Int Arch Allergy Immunol. 151:8–16. doi: 10.1159/000232566. [DOI] [PubMed] [Google Scholar]
- 50.Rafei M, Birman E, Forner K, Galipeau J. Allogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitis. Mol Ther. 2009;17:1799–1803. doi: 10.1038/mt.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 52.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 53.Pinderski Oslund LJ, Hedrick CC, Olvera T, Hagenbaugh A, Territo M, Berliner JA, Fyfe AI. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–2853. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- 54.Von Der Thusen JH, Kuiper J, Fekkes ML, De Vos P, Van Berkel TJ, Biessen EA. Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in LDLr−/− mice. FASEB J. 2001;15:2730–2732. doi: 10.1096/fj.01-0483fje. [DOI] [PubMed] [Google Scholar]
- 55.Pinderski LJ, Fischbein MP, Subbanagounder G, Fishbein MC, Kubo N, Cheroutre H, Curtiss LK, Berliner JA, Boisvert WA. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient Mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 56.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dansky HM, Charlton SA, Harper MM, Smith JD. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1997;94:4642–4646. doi: 10.1073/pnas.94.9.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daugherty A, Pure E, Delfel-Butteiger D, Chen S, Leferovich J, Roselaar SE, Rader DJ. The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein E−/− mice. J Clin Invest. 1997;100:1575–1580. doi: 10.1172/JCI119681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 60.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Interferon Cytokine Res. 2002;22:661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 62.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol. 2000;157:1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen S, Crother TR, Arditi M. Emerging role of IL-17 in atherosclerosis. J Innate Immun. 2:325–333. doi: 10.1159/000314626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Connor RA, Prendergast CT, Sabatos CA, Lau CW, Leech MD, Wraith DC, Anderton SM. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181:3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, Idu MM, van Maldegem F, Aten J, van der Wal AC. Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J Pathol. 2009;220:499–508. doi: 10.1002/path.2667. [DOI] [PubMed] [Google Scholar]