Abstract
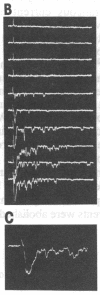
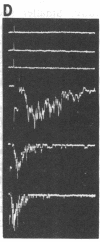
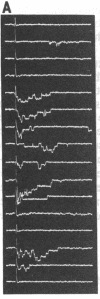
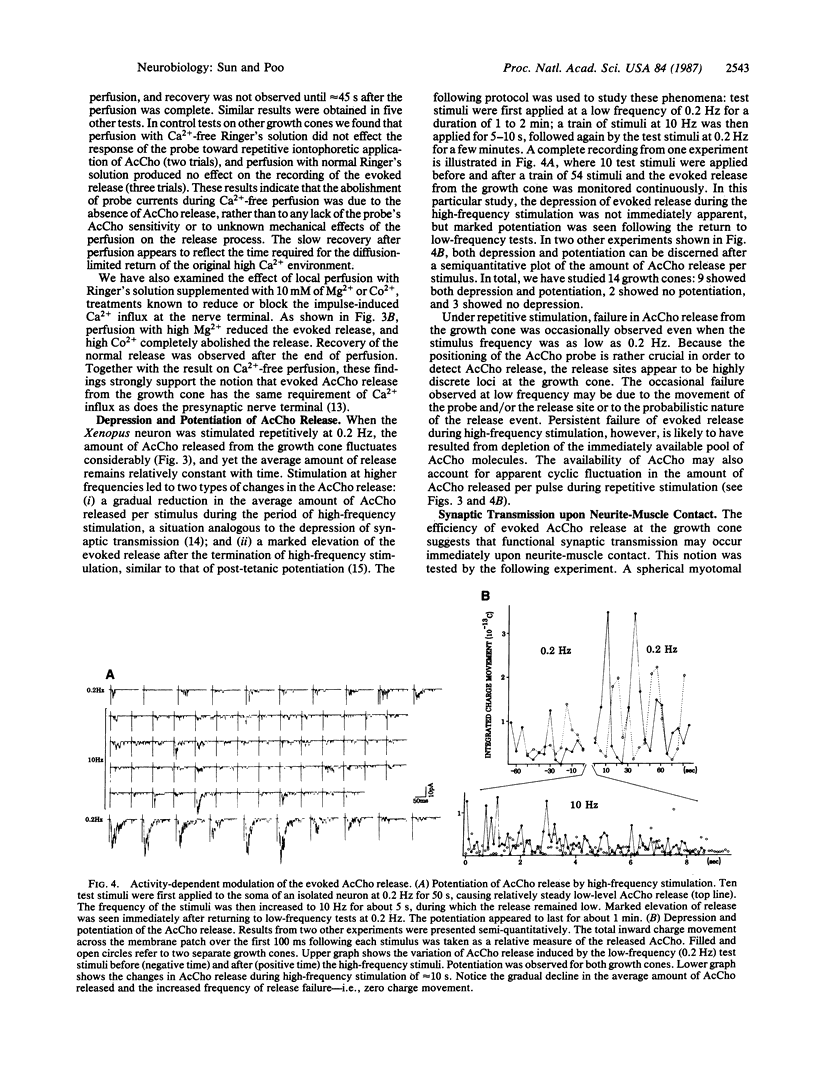
An excised patch of embryonic muscle membrane was used as a probe for measuring the release of acetylcholine (AcCho) from growing spinal neurons in Xenopus cell culture. The neuron was stimulated extracellularly at the soma, and the evoked AcCho release was monitored at the growth cone, along the neurite, and near the soma. For a majority of the neurons studied, a brief suprathreshold stimulation of the soma triggered a pulse of AcCho release from the growth cone. This release showed many of the characteristics reminiscent of the transmitter release at the nerve terminal of a mature neuromuscular synapse: it occurs within a few ms following the stimulation, depends on extracellular Ca2+ concentration, and exhibits depression and potentiation during and after high-frequency stimulation, respectively. Similar evoked release was also observed only at selected points along the neurite, and prolonged suprathreshold stimulus was required to induce release from the soma. These results indicate that some of the growing spinal neurons have acquired a substantial number of AcCho molecules as well as an efficient mechanism for excitation-secretion coupling at the growth cone, ready for establishing functional contact with the target muscle cell. This notion was further supported by the finding that the evoked AcCho release is capable of inducing suprathreshold excitation of the muscle cell within the first minute following neurite-muscle contact.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Cohen M. W., Zorychta E. Effects of innervation on the distribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977 Jul;268(3):731–756. doi: 10.1113/jphysiol.1977.sp011879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby J. L., Spitzer N. C. The appearance and development of neurotransmitter sensitivity in Xenopus embryonic spinal neurones in vitro. J Physiol. 1984 Aug;353:143–155. doi: 10.1113/jphysiol.1984.sp015328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow I., Poo M. M. Release of acetylcholine from embryonic neurons upon contact with muscle cell. J Neurosci. 1985 Apr;5(4):1076–1082. doi: 10.1523/JNEUROSCI.05-04-01076.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Patlak J. Single channel currents from excised patches of muscle membrane. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6930–6934. doi: 10.1073/pnas.77.11.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume R. I., Role L. W., Fischbach G. D. Acetylcholine release from growth cones detected with patches of acetylcholine receptor-rich membranes. Nature. 1983 Oct 13;305(5935):632–634. doi: 10.1038/305632a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The timing of calcium action during neuromuscular transmission. J Physiol. 1967 Apr;189(3):535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Yeh E. Initial synaptic transmission at the growth cone in Xenopus nerve-muscle cultures. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6727–6731. doi: 10.1073/pnas.79.21.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. W. Plasminogen activator release at the neuronal growth cone. Science. 1981 Sep 25;213(4515):1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- Kullberg R. W., Lentz T. L., Cohen M. W. Development of the myotomal neuromuscular junction in Xenopus laevis: an electrophysiological and fine-structural study. Dev Biol. 1977 Oct 1;60(1):101–129. doi: 10.1016/0012-1606(77)90113-0. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. Long term changes in augmentation, potentiation, and depression of transmitter release as a function of repeated synaptic activity at the frog neuromuscular junction. J Physiol. 1976 May;257(2):471–494. doi: 10.1113/jphysiol.1976.sp011379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N. C., Lamborghini J. E. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky E. J., Patterson P. H. Potassium-stimulated purine release by cultured sympathetic neurons. J Neurosci. 1985 Jul;5(7):1680–1687. doi: 10.1523/JNEUROSCI.05-07-01680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z. P., Poo M. M. Initial events in the formation of neuromuscular synapse: rapid induction of acetylcholine release from embryonic neuron. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7069–7073. doi: 10.1073/pnas.83.18.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. H., Poo M. M. Spontaneous release of transmitter from growth cones of embryonic neurones. Nature. 1983 Oct 13;305(5935):634–637. doi: 10.1038/305634a0. [DOI] [PubMed] [Google Scholar]
- Young S. H., Poo M. M. Topographical rearrangement of acetylcholine receptors alters channel kinetics. Nature. 1983 Jul 14;304(5922):161–163. doi: 10.1038/304161a0. [DOI] [PubMed] [Google Scholar]