Abstract
Background
A DNA prime, poxvirus (COPAK) boost vaccination regime with four antigens, i.e. a combination of two Plasmodium knowlesi sporozoite (csp/ssp2) and two blood stage (ama1/msp142) genes, leads to self-limited parasitaemia in 60% of rhesus monkeys and survival from an otherwise lethal infection with P. knowlesi. In the present study, the role of the blood stage antigens in protection was studied in depth, focusing on antibody formation against the blood stage antigens and the functionality thereof.
Methods
Rhesus macaques were immunized with the four-component vaccine and subsequently challenged i.v. with 100 P. knowlesi sporozoites. During immunization and challenge, antibody titres against the two blood stage antigens were determined, as well as the in vitro growth inhibition capacity of those antibodies. Antigen reversal experiments were performed to determine the relative contribution of antibodies against each of the two blood stage antigens to the inhibition.
Results
After vaccination, PkAMA1 and PkMSP119 antibody titres in vaccinated animals were low, which was reflected in low levels of inhibition by these antibodies as determined by in vitro inhibition assays. Interestingly, after sporozoite challenge antibody titres against blood stage antigens were boosted over 30-fold in both protected and not protected animals. The in vitro inhibition levels increased to high levels (median inhibitions of 59% and 56% at 6 mg/mL total IgG, respectively). As growth inhibition levels were not significantly different between protected and not protected animals, the ability to control infection appeared cannot be explained by GIA levels. Judged by in vitro antigen reversal growth inhibition assays, over 85% of the inhibitory activity of these antibodies was directed against PkAMA1.
Conclusions
This is the first report that demonstrates that a DNA prime/poxvirus boost vaccination regimen induces low levels of malaria parasite growth inhibitory antibodies, which are boosted to high levels upon challenge. No association could, however, be established between the levels of inhibitory capacity in vitro and protection, either after vaccination or after challenge.
Background
Malaria is a leading cause of morbidity and mortality affecting billions of people worldwide. It is estimated that malaria is responsible for the annual death of 800,000 people, mostly children under the age of five [1]. In the face of increasing resistance of Plasmodium parasites to anti-malarial (prophylactic) drugs, development of an effective malaria vaccine is generally considered a public health priority [2]. Feasibility of a successful malaria vaccine has been demonstrated by immunization with irradiated sporozoites and subsequent malaria infection in rodent, non-human primate and human models [3-5]. Furthermore, natural long-term exposure to the parasite is associated with an age-related decrease in the incidence, prevalence and density of infection [6].
The traditional approach for malaria vaccine development is based on recombinant proteins administered in combination with novel adjuvants, directed either to erythrocytic or pre-erythrocytic stages of the parasite. Early clinical trials conducted with the pre-erythrocytic particulate protein vaccine RTS,S showed moderate levels of efficacy [7]. Protein subunit vaccines do have a number of disadvantages. One is that they require the use of adjuvants that may induce to adverse effects and may be difficult to get access to, due to intellectual property rights. Moreover, antigen conformation and stability (with or without adjuvant) at ambient temperatures are also major issues that may complicate the use of subunit vaccines.
To circumvent these caveats, alternative vaccine delivery platforms have been developed. These include, among others, viral vector approaches, DNA vaccination and virosomal delivery systems, combinations of DNA and viral vector in prime-boost strategies, and protein/adjuvant booster strategies [8-13].
Previous studies with the malaria murine challenge model have shown that DNA vaccines encoding Plasmodium antigens are able to induce CD4+ and antibody responses, as well CD8+, CTL and IFNγ responses required to attack parasites as they develop inside hepatocytes [14-16]. Phase I/IIa clinical trials have established the safety, tolerability and immunogenicity of DNA vaccines encoding malaria parasite antigens in healthy individuals [2,17].
A DNA prime (3x), poxvirus (COPAK) boost (1x) vaccination regimen comprising two sporozoite (csp/ssp2) and two blood stage (ama1/msp142) antigens (Pk4x3/COPAK) was developed at the Naval Medical Research Centre. This reproducibly yields high levels (>60%) of protection in the rhesus macaque/Plasmodium knowlesi sporozoite challenge model [12,18,19]. The immunological analysis of these studies [19] focused on the cellular immune response. The parameter measured (IFN-γ ELIspot) did not correlate with protection. It was noted that immunization with a similar vaccine, containing two sporozoite antigens (csp/ssp2), using the same immunization schedule, resulted in a one-day delay in the onset of parasitaemia, but not in protection. This delay was not accompanied by lower parasite growth rates in the blood stage, when compared to naive animals [19]. This suggested that protection is critically depended on the blood stage antigens included in the Pk4x3/COPAK vaccine. Therefore, in this study the titres and functionality of the antibodies from blood samples of the above studies (before and after challenge) were analysed using ELISA and in vitro growth inhibition assays. Subsequently, GIA inhibition levels (after vaccination and after challenge) were compared between protected and not protected animals, in order to establish potential correlates of protection.
Methods
Plasmid DNA vaccines and poxvirus
The DNA plasmid and COPAK poxvirus immunization vector (Virogenetics, Troy, N.Y) encoding two (csp/ssp2) or four P. knowlesi genes (csp/ssp2/ama1/msp142) are previously described. COPAK is derived from the Copenhagen strain of vaccinia virus [12].
Antigen preparation
PkMSP119 was produced and purified as described previously [20]. PkAMA1 was expressed in Pichia pastoris and produced as described previously [21]. Briefly, a synthetic gene, comprising domain I-II-III of P. knowlesi H strain AMA1 (Accession code XM_002259303) and a hexa-histidine tag, codon optimized for expression in Pichia pastoris (DNA20, Menlo Park, CA), was cloned into the pPicZαA vector (Invitrogen, Leek, The Netherlands) and transformed into P. pastoris Km71H.
Rhesus monkeys, immunization regimen and challenge
The immunization and challenge phase of these studies have been published, as well as the immunological analysis focusing on the sporozoite antigens [19]. Briefly, in these studies three vaccination groups were used: 1) a four antigen (Pk4) (csp/ssp2/ama1/msp142) vaccine regimen, 2) a two antigen (csp/ssp2) vaccine regimen, and 3) a control vaccine (mock vaccine; an empty DNA plasmid and empty COPAK virus). The immunization regimen included a prime with three injections of DNA (dose of 0.5 mg of each plasmid in a volume of 1 mL) given at day 0, 28, 56. Four months later (day 168) the monkeys received a booster immunization with 2 ×108 pfu of COPAK virus, for each individual antigen [19].
Three weeks after the COPAK booster (day 189), pre-challenge blood samples were collected. One week later (day 196), animals were challenged by intravenous injection of 100 P. knowlesi (H strain) sporozoites [19]. Animals were termed 'protected' when able to control the parasitaemia below 1.5% and eventually to undetectable levels, after challenge. Animals unable to control the parasitaemia below 1.5% were treated with chloroquine [19] and were termed 'not protected'. A summary of the outcomes is presented in Table 1. Four weeks after challenge (day 224), a final blood sample was taken. At day 224, animals not yet treated were given chloroquine.
Table 1.
Observed parasitaemia in Pk4 vaccinated, CSP/SSP2 vaccinated and control (mock vaccine) groups.
| Experiment # | Vaccine group | Rhesus ID | day1st parasitaemia | Mean 1st day parasitaemia | day >1.5% parasitaemia | Mean day >1.5% parasitaemia |
|---|---|---|---|---|---|---|
| 1 | Mock* | 20H | 7 | 7.0 | 12 | 11.5 |
| 1 | Mock | 205 | 7 | 12 | ||
| 1 | Mock | AB07† | 7 | 11 | ||
| 1 | Mock | AB58 | 7 | 12 | ||
| 1 | Mock | Q121 | 7 | 11 | ||
| 2 | csp/ssp2 | IIG | 9 | 8.2 | 11 | 11.2 |
| 2 | csp/ssp2 | AB67 | 8 | 11 | ||
| 2 | csp/ssp2 | AC70 | 8 | 12 | ||
| 2 | csp/ssp2 | Q134 | 8 | 10 | ||
| 2 | csp/ssp2 | AK52 | 8 | 12 | ||
| 2 | csp/ssp2 | IIG | 9 | 11 | ||
| 2 | Pk4** | 281 | 11 | 9.5 | 14 | 13 |
| 2 | Pk4 | 284 | 9 | 13 | ||
| 2 | Pk4 | 3000 | 9 | 13 | ||
| 2 | Pk4 | 3129 | 9 | 13 | ||
| 2 | Pk4 | 19159 | 9 | 13 | ||
| 2 | Pk4 | 262 | 9 | 9.5 | Never | Never |
| 2 | Pk4 | 299 | 10 | Never | ||
| 2 | Pk4 | 3086 | 11 | Never | ||
| 2 | Pk4 | 3098 | 9 | Never | ||
| 2 | Pk4 | AB34 | 9 | Never | ||
| 1 | Pk4 | Q120 | 9 | Never | ||
| 1 | Pk4 | T152 | 10 | Never | ||
| 3 | Pk4 | 228 | 10 | Never | ||
† Animal died before the end of the study. No post challenge sample was available.
* Mock, vaccine comprised of empty DNA vector and empty COPAK vaccinia virus
** Pk4, vaccine comprised of four Plasmodium knowlesi antigens, csp/ssp2/ama1/msp142
Day to 1st parasitaemia is the day of patency of parasites on thin smear. Day >1.5% parasitaemia is the day of drug treatment. All vaccinated animals were primed with 3 DNA vaccine injections and boosted with recombinant poxviruses encoding the Pk4 antigens. Control (mock) animals received mock DNA vaccine and empty poxvirus. Subjects were taken from three experiments (#1-3), under identical vaccination regimens.
ELISA
ELISA's were performed in 96-well flat bottom micro titre plates (Greiner, Alphen a/d Rijn, The Netherlands), coated with either 0.5 μg mL-1 of PkMSP119 or PkAMA1 according to published methods [22]. Titres are expressed as arbitrary units (AU), where 1 AU yields an optical density of 1.0 over background. Thus, the AU-value of a sample is the reciprocal dilution at which the absorbance at 405 nm equals 1.0. All assays were performed in duplicate.
IgG purification
Total IgG was isolated on protein A columns (Sigma, St. Louis, MO). Elution buffer was exchanged for RPMI 1640 by repeated concentration/dilution using Amicon Ultra-15 concentrators (30-kDa cutoff; Millipore BV, Amsterdam, The Netherlands). IgG fractions were filter sterilized and stored at -20°C until use. IgG concentrations were determined using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE).
Parasite growth inhibition assay
The ability of protein A purified rhesus IgG to inhibit in vitro parasite growth was assessed in triplicate using 96-well flat-bottomed tissue culture plates (Greiner, Alphen a/d Rijn, The Netherlands) with in vitro matured and synchronized P. knowlesi (H strain) schizonts at a starting parasitaemia of 0.8-1.0%, a haematocrit of 2.0%, in RPMI 1640 fortified with 10% normal human serum and 20 μg mL-1 gentamicin, in a final volume of 100 μL.
For antigen reversal GIA experiments, PkAMA1 and PkMSP119 antigens were dialyzed against RPMI 1640, concentrated and filter-sterilized. Then they were serially diluted and incubated with isolated total rhesus IgG, at a concentration that was determined to result in an inhibition of 80% (in the absence of added antigens). Incubation was in incomplete culture medium (total volume 50 μL per well) for 45 min at room temperature, followed by 15 min of incubation at 37°C in a 96-well tissue culture plate. A parasite suspension containing schizonts in culture medium with 40% normal human serum was prepared and added to the plate to adjust the cultures to the same parasitaemia and haematocrit levels as used in the standard GIA described above.
After incubation of 24 to 26 hours, 30 μL of the resuspended culture was added to 200 μL ice-cold PBS, pH 7.4. After brief centrifugation, the supernatant was removed and pellets were frozen. Parasite lactate dehydrogenase levels were determined in the thawed pellets, as previously described [23]. From the pLDH levels, parasite growth inhibition reported as percentage was calculated as follows: 100-[(A650 of infected RBCs with test IgG - A650 of uninfected RBCs)/(A650 of infected RBCs with test IgG, at T = 0 - A650 uninfected RBCs, at T = 0) × 100]. All GIA and reversal GIA results reported are the averages of two independent GIA assays.
Statistics
All statistical analyses were performed using R software version 2.8.1 (R foundation for Statistical Computing, Vienna, Austria). IgG titres were log-transformed to obtain normality and significance was assessed by t-tests; a correction for unequal variances was applied where necessary. IgG antibody levels are presented as geometric means with 95% confidence intervals. The statistical significance of changes in IgG titres between time points were assessed using a paired t-test and presented as a ratio with 95% confidence intervals. Between group comparisons of GIA titres were performed using Mann-Whitney U test and titres are presented as medians with quartile ranges. Changes in GIA titres between time points were assessed using a paired t-test and data are presented as a difference (in percent points) with the corresponding 95% confidence intervals. The relation between GIA titre and PkAMA1 or PkMSP119 antibody levels was assessed by Spearman's rank correlation, the correlation is presented as Spearman's Rho. Two-sided P values less than 0.05 were considered significant.
Results
This study is a further analysis of samples obtained during a vaccination and challenge study, published by Weiss and co-workers [19]. In that study, approximately 60% (8 out of 13) of monkeys were protected from challenge with P. knowlesi, after receiving three injections with DNA encoding 4 Plasmodium knowlesi antigens (csp/ssp2/ama1/msp142) and a booster with a mixture of 4 COPAK viruses, encoding the same antigens. Monkeys receiving a similar vaccination regimen, but with DNA and poxvirus comprising sporozoite antigens (csp/ssp2) only, or mock vaccine, were not able to control the parasitaemia (not protected). A summary of the outcomes of this study is presented in Table 1.
Antibody responses induced by DNA prime/viral boost vaccination
ELISA titres for the two blood stage antigens (PkMSP119/PkAMA1) were determined in serum samples obtained from csp/ssp2 and csp/ssp2/ama1/msp142 animals, both before and after challenge, and run in a single experiment (Figure 1, Table 2). Pre-immune sera were all negative. Following Pk4x3 prime and poxvirus (COPAK) booster vaccination, all monkeys seroconverted for both PkMSP119 and PkAMA1 antigens. Geometric mean titre for PkMSP119 was 332 AU/mL (95% CI: 97-1,136) while the titre for PkAMA1 appeared higher at 2,305 AU/mL (95% CI: 855-6,214).
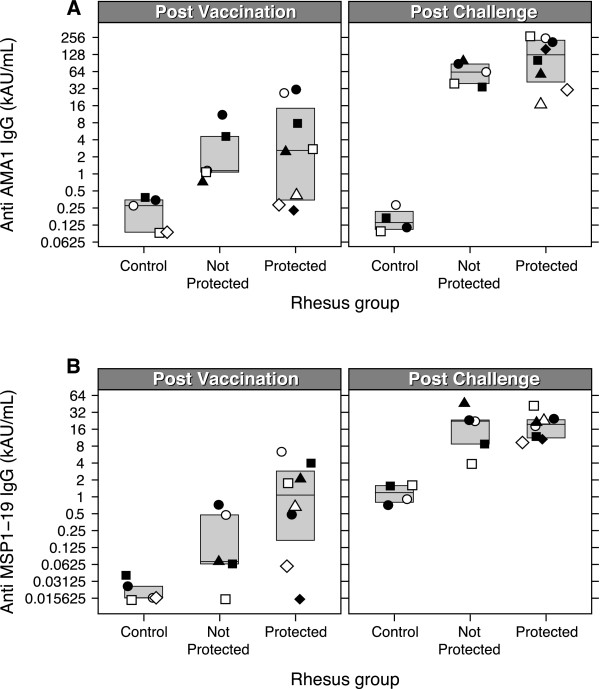
Figure 1.
ELISA titters against PkMSP119 and PkAMA1 before and after challenge. IgG antibody titres were measured by ELISA. The superimposed box around the data points indicate the upper and lower quartiles, the line in the middle indicates the median value. A) Antibody titres to PkMSP119 in the control (mock vaccine receiving) animals and Pk4-vaccinated animals (protected or not protected) before challenge (left panel) and after challenge (right panel). B) Antibody titres to PkAMA1 in the control (mock vaccine receiving) animals and vaccinated animals (protected or not protected) before challenge (left panel) and after challenge (right panel). Geometric shapes represent individual animals in each group, throughout all figures. For one animal in the control group no post challenge data are available, as it died for study-unrelated reasons.
Table 2.
Geometric means of antibody titres as determined by ELISA.
| All PkAMA1 | Protected PkAMA1 | Not protected PkAMA1 | All PkMSP119 | Protected PkMSP119 | Not protected PkMSP119 | |
|---|---|---|---|---|---|---|
| Post vaccination | 2,305 [855-6,214] | 2,417 [469-12,451] | 2,137 [508-8,990] | 332 [97-1,136] | 633 [109-3,671] | 118 [16-853] |
| Post challenge | 79,030 [46,803-133,444] | 94,870 [40,055-224,699] | 59,000 [32,980-105,548] | 16,784 [11,104-25,369] | 17,875 [11,730-27,240] | 15,175 [4,562-50,477] |
In brackets the 95% confidence intervals. No significant differences were observed between protected or not-protected groups of animals. For both antigens and all groups, antibody levels are significantly higher after challenge.
After vaccination, there were no significant differences between the PkMSP119 and PkAMA1 antibody titres from protected and non-protected monkeys, respectively (T-test, P = 0.133 and P = 0.889, for PkMSP119 and PkAMA1, respectively) (Figure 1, Table 2).
Four weeks after challenge, antibody levels to PkAMA1 and PkMSP119 were boosted significantly. For PkAMA1 the antibody level was 34 fold [95% CI: 15-80, P = 9.9 e-7] higher post-challenge compared to pre-challenge. For PkMSP119 the increase in the antibody titre after challenge was 50 fold [95% CI: 17-149, P = 4.2 e-6]. Again no significant differences were observed between protected and not protected animals (PkMSP119; P = 0.94/PkAMA1; P = 0.35, t-test) (Figure 1, Table 2).
After challenge, control animals (mock-vaccinated) showed elevated antibody levels against PkMSP119, while no increase in anti-PkAMA1 levels was observed. Before challenge, antibodies against PkAMA1 and PkMSP119 were not detected in these animals (Figure 1).
Functionality of the antibodies
Growth inhibition assays were performed to assess the functionality of the anti-blood stage antigen antibodies. Plasma samples from 13 animals were selected from previous Pk4 vaccination/challenge experiments with identical vaccination regimens [19]. These were divided into two groups, comprised of protected (N = 8) or not protected animals (N = 5). Samples from animals that received a mock vaccine (N = 5) and from animals that received csp/ssp2 vaccine (N = 5) were also included in the analysis (Table 1).
Figure 2 shows the results of parasite growth inhibition assays after vaccination (Panel A) and after challenge (Panel B) of Pk4-vaccinated animals, csp/ssp2-vaccinated animals and controls.
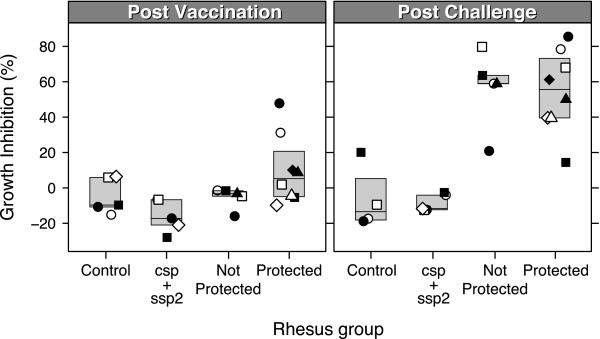
Figure 2.
Parasite growth inhibition activity of protected and non-protected monkeys. A) Post vaccination B) post challenge. Mean GIA inhibition levels of total IgG isolated from monkey serum from animals in control (mock) group, CSP/SSP2 group, and Pk4x3/COPAK vaccinated animals (protected or not protected animals are shown). Final IgG concentration added to P. knowlesi parasite culture was 6 mg/mL. Geometric shapes represent individual animals in each group, throughout all figures. For one animal in the control group no post challenge data are available, as it died for study-unrelated reasons.
After vaccination, total IgG isolated from serum of protected animals inhibited growth of P. knowlesi between -10 and 48% (median inhibition 5.3 at 6 mg/mL IgG concentration). The animals with the highest growth inhibition levels (31% and 48% at 6 mg/mL, circles in Figure 2) were able to control the infection. Virtually no growth inhibitory antibodies were present in total IgG fractions isolated from plasma samples from animals that were not protected (Figure 2). No inhibition was observed using purified total IgG from plasma samples from animals receiving the sporozoite antigen vaccine or mock vaccine.
Four weeks after sporozoite challenge, a significant increase of 51% ([95% CI: 41-61%], P = 1.1 e-7; paired t-test) in the level of inhibition was observed in all Pk4 vaccinated animals (Figure 2). Purified total IgG isolated from plasma samples from protected animals inhibited parasite growth ranging from 14% to 80% (median inhibition of 56% at 6 mg/mL IgG concentration), while not protected animals had inhibition levels ranging from 21 to 80% (median inhibition of 59%).
GIA levels between protected and not protected animals were not significantly different (t-test), either before (P = 0.07234) or after challenge (P = 0.8884). This suggests that there is also no association between GIA levels and protection.
Other studies have shown a positive correlation between antibody levels and GIA inhibition (f.e. [24]). In this study this correlation was confirmed, by Spearman's rank correlation test (for PkMSP119: rho = 0.683, P = 3.5 e-6; for PkAMA1: rho = 0.754, P = 8.7 e-7).
Purified total IgG from CSP/SSP2 vaccinated monkeys and mock-vaccinated monkeys did not show any inhibition to P. knowlesi parasites in vitro, either before or after challenge (Figure 2).
Specificity of the parasite growth inhibitory antibodies
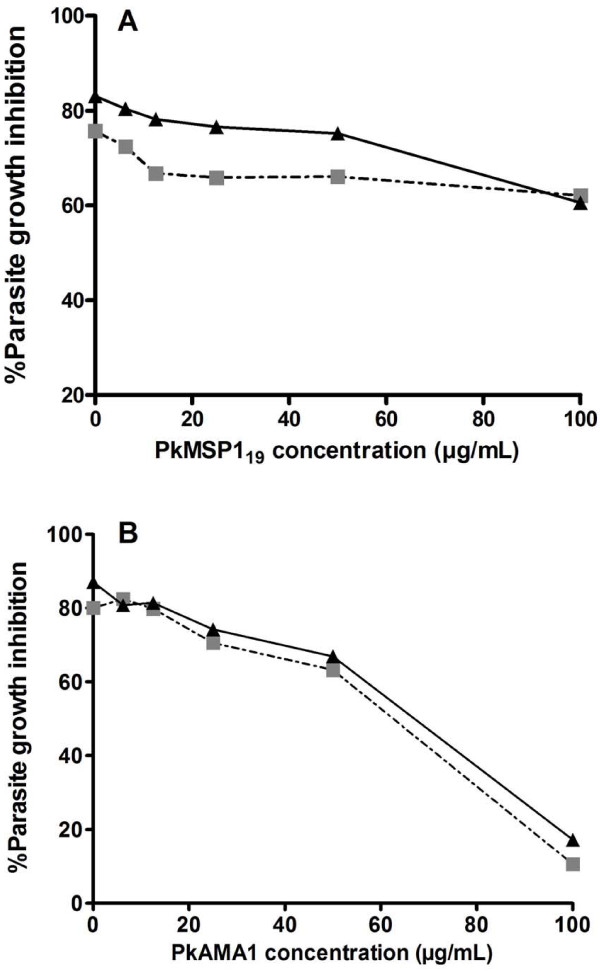
The ability of PkAMA1 and PkMSP119 proteins to reverse growth inhibition was evaluated in the in vitro assay, in order to analyse the specificity of antibodies induced by DNA prime, poxvirus boost vaccination and P. knowlesi challenge. This was done using a pool of total IgG isolated from plasma of protected animals obtained after challenge and total IgG of a single protected animal (3086), also only after challenge. Antibodies were pre-incubated with increasing concentrations of PkAMA1 or PkMSP119, prior to addition to a growth inhibition assay. Growth inhibition could be reversed to over 85% by addition of the PkAMA1 antigen at 100 μg/mL (Figure 3A). PkMSP119 protein, at the same concentration, could only reverse inhibition by 10% (Figure 3B). The same degree of inhibition was obtained with post-challenge IgG isolated from a plasma sample of a single protected monkey (Figure 3). These results show that in this assay the larger part of the inhibitory activity is mediated by anti-PkAMA1 antibodies.
Figure 3.
Reversal of growth inhibitory activity by PkAMA1 and PkMSP119. Purified IgG from monkey 3086 (protected), post-challenge (black triangle). Mixture of pool purified IgG taken from Pk4-vaccinated monkeys (262, 299, 3086, 3098, AB34, Q120, T152, 228), post-challenge (black square). IgG was pre-incubated with either PkMSP119 (Panel A) or PkAMA1 (Panel B) in a five-fold serial dilution, prior to mixing with P. knowlesi parasites. Results shown are the mean of two independent assays.
Discussion and conclusions
A DNA prime, poxvirus boost vaccination regimen that has been shown to protect a high percentage of macaques from potentially lethal parasitaemia after P. knowlesi challenge [12,18,19] was further investigated. An important question left open from these studies was the nature of the immune response responsible for protection. Of the four malaria antigens included in the Pk4 vaccine, CSP and SSP2 are expressed on sporozoites and may be present in the early stage infected liver cells [25,26]. Although AMA1 and MSP1 are generally considered to be blood stage antigens [27,28], expression for both antigens in late liver schizonts has been demonstrated [29,30], while AMA1 is also expressed on the sporozoite surface membrane [29]. Thus, both sets of antigens can be considered to have added value as multi-stage vaccine candidates.
Rhesus monkeys receiving csp and ssp2 only demonstrated a delay in appearance of parasites in the blood (>1 day), but were not able to control the infection below 1.5% parasitaemia [19]. By contrast, monkeys that received the Pk4x3/COPAK vaccine including ama1 and msp142, showed a significant delay in the appearance of parasites in the blood (>2 days) and 60% of these monkeys could control the infection below 1.5% parasitaemia. This is a strong indication that the immune response to the blood stage antigens was necessary for controlling parasite growth.
The cellular immune responses measured in the original study [19] did not correlate with protection. As antibodies are generally believed to be the key mediators for protection against blood stage malaria, the antibody responses to PkAMA1 and PkMSP119 after vaccination were determined and found to be present at low levels, with corresponding low growth inhibition activity (median inhibition 5.3% in protected animals versus -3.3% in not protected animals). Challenge of the animals initially resulted in high parasite growth rates, for all animals. Obviously, in vivo parasite growth was not or only marginally inhibited. This is supported by the observation that functional antibody levels after vaccination, as determined in GIA, are low.
Although antibody levels against PkMSP119 and PkAMA1 appear to be higher in protected animals compared to those in not protected animals (Figure 1), the differences are not statistically significant, likely to be the result of the low values and corresponding high variance of the data. Similarly, after vaccination there was no correlation between the (low) GIA levels and protection. Low GIA values (<15% inhibition) are difficult to interpret. For low inhibitions the two terms in the upper part of the equation used to calculate the inhibition (See methods), are relatively large and nearly equal to each other. A small deviation in either the control or the sample value will have a strong impact on the magnitude of the inhibition. The variance in the outcomes of the growth experiments is also one of the reasons why negative values are frequently observed in the GIA.
Sporozoite challenge boosted the levels of antibodies against PkMSP119 and PkAMA1 in animals receiving the Pk4 vaccines over 30 fold, but not in monkeys receiving mock vaccines (Figure 1), supportive for the hypothesis that DNA prime followed by a poxvirus boost induces T-cell responses to the vaccine antigens. The induced CD4 T-cells provide help to B-cells upon (re-)exposure to the vaccine antigens, which may explain the increase in PkAMA1 and PkMSP119 specific antibodies during challenge.
Although antibody levels were significantly elevated post challenge, no significant difference was observed between the antibody levels of protected and not protected animals, either for PkAMA1 or for PkMSP119. Similarly, GIA inhibitions were not significantly different between protected and not protected animals after challenge (Figure 2).
The measured antibody titres and inhibition levels (GIA), four weeks after challenge are lower than the levels reported for PkAMA1/adjuvant immunization studies [21]. In these studies, even at high inhibition levels (~70% inhibition at 6 mg/mL total IgG) some animals were not able to control the infection. This is an indication that immune responses other than antibodies are likely to be involved in protection.
Interestingly, PkMSP119 antibody levels were detected after challenge of naïve monkeys, while PkAMA1 antibodies were not (Figure 1). As in naturally exposed humans in endemic areas the anti-PfAMA1 antibodies titres are normally higher than those against PfMSP119, [31,32], this may be explained by assuming that this is the result of a single exposure to the parasite, reflecting the difference in abundance of MSP1 and AMA1 on the parasite's surface, MSP1 being the most abundant protein on the merozoite surface, while AMA1 is poorly abundant.
In Figure 2, the values for the growth inhibition are negative for most groups, indicative for a small stimulation of growth in the presence of the antibodies that are added. This observation is not uncommon, and, as explained above, is related to the calculation of growth inhibition.
Almost complete reversal of the growth inhibition can be achieved by addition of 100 μg/mL PkAMA1 protein in the GIA assay. Titration of PkMSP119 at the same concentration of protein leads to approximately 10% reversal of inhibition (Figure 3), implying that the larger proportion of the (GIA) inhibitory antibodies in the sera are directed against PkAMA1 rather than PkMSP119. This observation does not necessarily imply that anti-PkMSP119 antibodies do not contribute to the ability to control the infection. It is known that several mechanisms of antibody-mediated inhibition exist, some with the aid of immune cells (such as antibody dependent cellular inhibition (ADCI) [33]) that will not give a response in GIA. For anti-PfMSP119 antibodies, it has been shown that Fc-tail mediated antibody responses may be important for protection in a humanized mouse model [34]. Moreover, part of the anti-MSP119 antibodies may be growth-inhibitory rather than invasion inhibitory [35]. These mechanisms may result in an underestimating of the inhibitory capacity of the anti PkMSP119 antibodies.
It has to be noted that all analyses of the response against the PkMSP142 part of the vaccine was done using PkMSP119 protein. It cannot be excluded that this may have lead to an underestimation of the antibody titres and of the ability to reverse the inhibition in the GIA reversal experiments. However, a protein/adjuvant immunization study with PfMSP142 in humans has shown that the antibody response against PfMSP142 is strongly directed to the PfMSP119 part [36], warranting the conclusion that the main part of the GIA activity is directed against PkAMA1.
The present study could not establish a correlation between the levels of inhibitory antibodies, either before or after challenge, with the ability to control the infection. Previous analysis of these experiments has shown that also no correlation could be established between T-cell mediated immunity (ELIspot antigen-induced IFN-γ production), and protection [19].
The boost in functional antibody levels observed after sporozoite challenge is very interesting, especially in relation with the observed course of parasitaemia in the Pk4x3/COPAK vaccinated animals [19]. Boosting of the antibody levels will, per definition, take place after the parasites emerge from the liver and enter the circulation, as only then the antigens will be "visible" for the immune system. As the immune response against the blood stage antigens is the determining factor for protection in this model, it can be imagined that the growth rate of the parasites versus the increase over time in inhibiting capacity of the immune system, irrespective of the exact nature of this inhibiting capacity, determines whether an animal will be protected or not. Obviously, in protected animals the immune response is increasing in magnitude over time, while the parasitaemia increases to levels very near to 1.0%. The day the animals become patent with parasites (day 11/12) parasites are multiplying with a multiplication factor higher than one, resulting in higher parasitaemia the next day. Obviously the inhibitory capacity of the immune system is not high enough to arrest parasite multiplication. After two to three days, in the protected animals parasite levels become more or less constant, at 1% parasitaemia, and it can be argued that the animals' immune system has been built up to an extent that the inhibition equals the multiplication rate. In most protected animals this situation is maintained for a number of days, after which the parasites are cleared from the circulation. In non-protected animals the immune system is obviously not able to catch up with the growth of parasites. As P. knowlesi has a multiplication rate of, on average, 10 per 24 hours (one cycle) [37] (i.e. each ruptured schizont gives rise to 10 freshly infected RBCs), a value confirmed by the parasitaemia profile of the controls (Figure 1A, in [19]), the inhibition level at this point has to be close to 90%. For some animals, protected or not, the levels of functional antibodies, determined four weeks after challenge, are not too far off of this value. This shows that antibody levels may be of key importance for protection for some animals, but given the low inhibition values of other protected monkeys, other immunological responses may be of key importance for protection in these animals. A final note, something that has not been appreciated so far, is that the kinetics of the immune response may be of great importance for the outcome of an infection. The time it takes to reach the required inhibition levels, in relation to the course of the infection, may be as important as the final magnitude. Frequent, daily sampling (starting at the day of challenge) may reveal whether (functional) antibody production rates versus the course of the parasitaemia is correlated with protection. Another interesting possibility would be a second challenge, four weeks after the first, to investigate whether the high levels of antibodies that are present at that time may lead to protection of animals with high functional antibody titres.
Importantly, this study shows that in a vaccination regimen that is not focused on the production of antibodies, these are produced and their levels are significantly boosted after sporozoite challenge. These antibodies may play a direct role in protection alongside the cellular and antibody-mediated cellular immunity induced after vaccination and challenge.
The above studies show that the P. knowlesi-rhesus macaque challenge model could be instrumental for the eventual elucidation of factors that contribute to protection upon challenge with P. knowlesi.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MMaH, BF, ER and WW made substantial contributions to the conception and the design of the study. MMaH did the work in lab, analysis and interpretation of data was done by MMaH, IH, AH, WW, ER and BF. MMaH drafted the manuscript, DN, AT, CK, ER, WW and BF revising it critically. MMaH, ER and BF gave final approval of the version to be published; all authors read and approved the final manuscript.
Contributor Information
Muzamil Mahdi Abdel Hamid, Email: mahdi@iend.org.
Edmond J Remarque, Email: remarque@bprc.nl.
Ibrahim M El Hassan, Email: ibrahimelhassan@iend.org.
Ayman A Hussain, Email: aymanhussain@gmail.com.
David L Narum, Email: dnarum@niaid.nih.gov.
Alan W Thomas, Email: thomas@bprc.nl.
Clemens HM Kocken, Email: kocken@bprc.nl.
Walter R Weiss, Email: walter.weiss@verizon.net.
Bart W Faber, Email: faber@bprc.nl.
Acknowledgements
This work received financial support from the European Malaria Vaccine Initiative (EMVI) and the Biomedical Primate Research Centre. This research was supported in part by the Intramural Research Program of NIAID, NIH. We thank Vanessa Riasat for excellent technical assistance.
References
- Greenwood BM, Fidock DA, Kyle DE, Kappe SH, Alonso PL, Collins FH, Duffy PE. Malaria: progress, perils, and prospects for eradication. J Clin Invest. 2008;118:1266–1276. doi: 10.1172/JCI33996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard MP, Reed ZH, Friede M, Kieny MP. A review of human vaccine research and development: malaria. Vaccine. 2007;25:1567–1580. doi: 10.1016/j.vaccine.2006.09.074. [DOI] [PubMed] [Google Scholar]
- Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, Gordon DM, Stoute JA, Church LW, Sedegah M, Heppner DG, Ballou WR, Richie TL. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- Thomas AW, Slierendregt B, Mons B, Druilhe P. Chimpanzees and supporting models in the study of malaria pre-erythrocytic stages. Mem Inst Oswaldo Cruz. 1994;89(Suppl 2):111–114. doi: 10.1590/s0074-02761994000600023. [DOI] [PubMed] [Google Scholar]
- Baird JK. Age-dependent characteristics of protection v. susceptibility to Plasmodium falciparum. Ann Trop Med Parasitol. 1998;92:367–390. doi: 10.1080/00034989859366. [DOI] [PubMed] [Google Scholar]
- Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, Mshamu S, Lang T, Gould J, Dubois MC, Demoitie MA, Stallaert JF, Vansadia P, Carter T, Njuguna P, Awuondo KO, Malabeja A, Abdul O, Gesase S, Mturi N, Drakeley CJ, Savarese B, Villafana T, Ballou WR, Cohen J, Riley EM, Lemnge MM, Marsh K, von Seidlein L. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359:2521–2532. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennink JR, Yewdell JW, Smith GL, Moller C, Moss B. Recombinant vaccinia virus primes and stimulates influenza haemagglutinin-specific cytotoxic T cells. Nature. 1984;311:578–579. doi: 10.1038/311578a0. [DOI] [PubMed] [Google Scholar]
- Li S, Locke E, Bruder J, Clarke D, Doolan DL, Havenga MJ, Hill AV, Liljestrom P, Monath TP, Naim HY, Ockenhouse C, Tang DC, Van Kampen KR, Viret JF, Zavala F, Dubovsky F. Viral vectors for malaria vaccine development. Vaccine. 2007;25:2567–2574. doi: 10.1016/j.vaccine.2006.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Doolan DL, Le TP, Hedstrom RC, Coonan KM, Charoenvit Y, Jones TR, Hobart P, Margalith M, Ng J, Weiss WR, Sedegah M, de Taisne C, Norman JA, Hoffman SL. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- Gluck R. Immunopotentiating reconstituted influenza virosomes (IRIVs) and other adjuvants for improved presentation of small antigens. Vaccine. 1992;10:915–919. doi: 10.1016/0264-410X(92)90325-E. [DOI] [PubMed] [Google Scholar]
- Rogers WO, Baird JK, Kumar A, Tine JA, Weiss W, Aguiar JC, Gowda K, Gwadz R, Kumar S, Gold M, Hoffman SL. Multistage multiantigen heterologous prime boost vaccine for Plasmodium knowlesi malaria provides partial protection in rhesus macaques. Infect Immun. 2001;69:5565–5572. doi: 10.1128/IAI.69.9.5565-5572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin EM, Diemert DJ, McArthur JH, Perreault JR, Miles AP, Giersing BK, Mullen GE, Orcutt A, Muratova O, Awkal M, Zhou H, Wang J, Stowers A, Long CA, Mahanty S, Miller LH, Saul A, Durbin AP. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73:3677–3685. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RJ, Hannan CM, Gilbert SC, Laidlaw SM, Sheu EG, Korten S, Sinden R, Butcher GA, Skinner MA, Hill AV. Enhanced CD8+ T cell immune responses and protection elicited against Plasmodium berghei malaria by prime boost immunization regimens using a novel attenuated fowlpox virus. J Immunol. 2004;172:3094–3100. doi: 10.4049/jimmunol.172.5.3094. [DOI] [PubMed] [Google Scholar]
- Gilbert SC, Schneider J, Hannan CM, Hu JT, Plebanski M, Sinden R, Hill AV. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine. 2002;20:1039–1045. doi: 10.1016/S0264-410X(01)00450-9. [DOI] [PubMed] [Google Scholar]
- Hoffman SL, Sedegah M, Hedstrom RC. Protection against malaria by immunization with a Plasmodium yoelii circumsporozoite protein nucleic acid vaccine. Vaccine. 1994;12:1529–1533. doi: 10.1016/0264-410X(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Le TP, Coonan KM, Hedstrom RC, Charoenvit Y, Sedegah M, Epstein JE, Kumar S, Wang R, Doolan DL, Maguire JD, Parker SE, Hobart P, Norman J, Hoffman SL. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine. 2000;18:1893–1901. doi: 10.1016/S0264-410X(99)00407-7. [DOI] [PubMed] [Google Scholar]
- Rogers WO, Weiss WR, Kumar A, Aguiar JC, Tine JA, Gwadz R, Harre JG, Gowda K, Rathore D, Kumar S, Hoffman SL. Protection of rhesus macaques against lethal Plasmodium knowlesi malaria by a heterologous DNA priming and poxvirus boosting immunization regimen. Infect Immun. 2002;70:4329–4335. doi: 10.1128/IAI.70.8.4329-4335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss WR, Kumar A, Jiang G, Williams J, Bostick A, Conteh S, Fryauff D, Aguiar J, Singh M, O'Hagan DT, Ulmer JB, Richie TL. Protection of rhesus monkeys by a DNA prime/poxvirus boost malaria vaccine depends on optimal DNA priming and inclusion of blood stage antigens. PLoS ONE. 2007;2:e1063. doi: 10.1371/journal.pone.0001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman SC, Simcoke WN, Stowers AW, Garboczi DN. Structure of the C-terminal domains of merozoite surface protein-1 from Plasmodium knowlesi reveals a novel histidine binding site. J Biol Chem. 2003;278:7264–7269. doi: 10.1074/jbc.M210716200. [DOI] [PubMed] [Google Scholar]
- Mahdi M. PhD thesis. Khartoum: University of Khartoum; 2009. Development of the Plasmodium knowlesi Rhesus macaque model using the malaria vaccine candidate Apical Membrane Antigen 1 (AMA1) [Google Scholar]
- Remarque EJ, Faber BW, Kocken CH, Thomas AW. A diversity-covering approach to immunization with Plasmodium falciparum apical membrane antigen 1 induces broader allelic recognition and growth inhibition responses in rabbits. Infect Immun. 2008;76:2660–2670. doi: 10.1128/IAI.00170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MC, Wang J, Zhang Y, Miles AP, Chitsaz F, Saul A, Long CA, Miller LH, Stowers AW. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun. 2002;70:6948–6960. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusi KA, Faber BW, Riasat V, Thomas AW, Kocken CH, Remarque EJ. Generation of humoral immune responses to multi-allele PfAMA1 vaccines; effect of adjuvant and number of component alleles on the breadth of response. PLoS One. 2010;5:e15391. doi: 10.1371/journal.pone.0015391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair PL, Witney A, Haynes JD, Moch JK, Carucci DJ, Adams JH. Transcripts of developmentally regulated Plasmodium falciparum genes quantified by real-time RT-PCR. Nucleic Acids Res. 2002;30:2224–2231. doi: 10.1093/nar/30.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, Moch JK, Muster N, Sacci JB, Tabb DL, Witney AA, Wolters D, Wu Y, Gardner MJ, Holder AA, Sinden RE, Yates JR, Carucci DJ. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- Holder AA. The precursor to major merozoite surface antigens: structure and role in immunity. Prog Allergy. 1988;41:72–97. [PubMed] [Google Scholar]
- Kocken CH, Hundt E, Knapp B, Brazel D, Enders B, Narum DL, Wubben JA, Thomas AW. Immunization of Aotus monkeys with recombinant Plasmodium falciparum hybrid proteins does not reproducibly result in protection from malaria infection. Infect Immun. 1998;66:373–375. doi: 10.1128/iai.66.1.373-375.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvie O, Franetich JF, Charrin S, Mueller MS, Siau A, Bodescot M, Rubinstein E, Hannoun L, Charoenvit Y, Kocken CH, Thomas AW, Van Gemert GJ, Sauerwein RW, Blackman MJ, Anders RF, Pluschke G, Mazier D. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J Biol Chem. 2004;279:9490–9496. doi: 10.1074/jbc.M311331200. [DOI] [PubMed] [Google Scholar]
- Suhrbier A, Holder AA, Wiser MF, Nicholas J, Sinden RE. Expression of the precursor of the major merozoite surface antigens during the hepatic stage of malaria. Am J Trop Med Hyg. 1989;40:351–355. doi: 10.4269/ajtmh.1989.40.351. [DOI] [PubMed] [Google Scholar]
- Nebie I, Diarra A, Ouedraogo A, Soulama I, Bougouma EC, Tiono AB, Konate AT, Chilengi R, Theisen M, Dodoo D, Remarque E, Bosomprah S, Milligan P, Sirima SB. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect Immun. 2008;76:759–766. doi: 10.1128/IAI.01147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodoo D, Aikins A, Kusi KA, Lamptey H, Remarque E, Milligan P, Bosomprah S, Chilengi R, Osei YD, Akanmori BD, Theisen M. Cohort study of the association of antibody levels to AMA1, MSP119, MSP3 and GLURP with protection from clinical malaria in Ghanaian children. Malar J. 2008;7:142. doi: 10.1186/1475-2875-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh RS, Shi J, Jennings RM, Chappel JC, de Koning-Ward TF, Smith T, Green J, van Egmond M, Leusen JH, Lazarou M, van de Winkel J, Jones TS, Crabb BS, Holder AA, Pleass RJ. The importance of human FcgammaRI in mediating protection to malaria. PLoS Pathog. 2007;3:e72. doi: 10.1371/journal.ppat.0030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzewski AR, Ling IT, Hopkins JM, Grainger M, Margos G, Mitchell GH, Holder AA, Bannister LH. Formation of the food vacuole in Plasmodium falciparum: a potential role for the 19 kDa fragment of merozoite surface protein 1 (MSP1(19)) PLoS One. 2008;3:e3085. doi: 10.1371/journal.pone.0003085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin E, Long CA, Stowers AW, Zou L, Singh S, Macdonald NJ, Narum DL, Miles AP, Orcutt AC, Muratova O, Moretz SE, Zhou H, Diouf A, Fay M, Tierney E, Leese P, Mahanty S, Miller LH, Saul A, Martin LB. Phase 1 study of two merozoite surface protein 1 (MSP1(42)) vaccines for Plasmodium falciparum malaria. PLoS Clin Trials. 2007;2:e12. doi: 10.1371/journal.pctr.0020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coatney GRCW, Warren M, Contacos PG. The primate malarias. Washington: Washington: US Government Printing Office; 1971. [Google Scholar]