Abstract
A20 or tumor necrosis factor–induced protein 3 is a negative regulator of nuclear factor κB signaling. A20 has been shown previously to attenuate cardiac hypertrophy in vitro and postmyocardial infarction remodeling in vivo. In the present study, we tested the hypothesis that overexpression of A20 in the murine heart would protect against cardiac hypertrophy in vivo. The effects of constitutive human A20 expression on cardiac hypertrophy were investigated using in vitro and in vivo models. Cardiac hypertrophy was produced by aortic banding in A20 transgenic mice and control animals. The extent of cardiac hypertrophy was quantitated by echocardiography, as well as by pathological and molecular analyses of heart samples. Constitutive overexpression of human A20 in the murine heart attenuated the hypertrophicresponse and markedly reduced inflammation, apoptosis, and fibrosis. Cardiac function was also preserved in hearts with increased A20 levels in response to hypertrophic stimuli. Western blot experiments further showed A20 expression markedly blocked transforming growth factor-β–activated kinase 1–dependent c-Jun N-terminal kinase/p38 signaling cascade but with no difference in either extracellular signal-regulated kinase 1/2 or AKT activation in vivo and in vitro. In cultured neonatal rat cardiac myocytes, [3H]proline incorporation and Western blot assays revealed that A20 expression suppressed transforming growth factor-β–induced collagen synthesis and transforming growth factor-β–activated kinase 1–dependent Smad 2/3/4 activation. In conclusion, A20 improves cardiac functions and inhibits cardiac hypertrophy, inflammation, apoptosis, and fibrosis by blocking transforming growth factor-β–activated kinase 1–dependent signaling.
Keywords: A20, cardiac remodeling, inflammation, apoptosis
Introduction
Heart failure is increasing in prevalence and is a debilitating disease with high rates of mortality and morbidity (1, 2). Cardiac hypertrophy is a common precursor to many forms of heart failure, whose molecular and cellular determinants remain largely unknown. After a period of compensatory adaptation, hypertrophy is associated with functional and histological deterioration of the myocardium, fibrosis, inflammation, and altered cardiac gene expression (3, 4). Accumulating evidence suggests that the nuclear factor-kappa B (NF-κB) signaling system is a critical regulator of this process (5–7). Modulation of NF-κB signaling in the heart may provide a novel approach to attenuate the development of heart failure after cardiac hypertrophy.
A20 is a zinc finger protein originally identified as a tumor necrosis factor (TNF) responsive gene in endothelial cells (8). It is an inducible and broadly expressed cytoplasmic protein that inhibits TNF-induced NF-κB activity. Recent studies showed that A20 expression protects various cell types from TNF-mediated apoptosis (9, 10). We also found that A20 expression protects against oxidized low density lipoprotein (OxLDL)-induced macrophage apoptosis and inhibits the proliferation of vascular smooth muscle cells (11, 12). A20-deficient mice demonstrate spontaneous inflammation, cachexia, and premature death, and A20-deficient fibroblasts cannot properly terminate TNF-induced NF-κB activity (13). A20 is also an inducible ubiquitin-editing enzyme that restricts both toll-like receptor (TLR) and TNF-induced responses by regulating the ubiquitination of key signaling proteins (14). Our data demonstrated that forced expression of A20 in the heart resulted in markedly improved functional recovery, decreased inflammation, reduced apoptosis, and diminished interstitial fibrosis after acute myocardial infarction (MI) (15). Cook and colleagues (16) reported that A20 is dynamically regulated during acute biomechanical stress in the heart and functions to attenuate cardiac hypertrophy in vitro. Despite the potentially significant roles of A20 in attenuating NF-κB-dependent apoptotic, inflammatory and hypertrophic signaling, it has remained unclear whether A20 could regulate cardiac hypertrophy in vivo, and whether targeted myocardial overexpression of A20 is cardio-protective. Thus in the present study, our aim is to investigate the role of A20 in cardiac hypertrophy mediated by pressure overload, and to clarify the related molecular mechanisms.
Methods and Materials
Materials
The antibodies against ERK1/2, P38, JNK, Caspase-3/8/9, phospho-Smad2, TAK1, phospho-p65, IKKα, IKKβ, phospho-IκBα, and IκBα were purchased from Cell Signaling Technology. [3H]-leucine and [3H]-proline were purchased from Amersham. The BCA protein assay kit was purchased from Pierce and the IKK activity kit was obtained from B&D Bioscience. All other antibodies were purchased from Santa Cruz Biotechnology. TGF-β1 was purchased from R&D Systems. Fetal calf serum (FCS) was obtained from Hyclone. Wild type rat A20 cDNA (AdA20) and siA20 adenoviral (AdsiA20) were made as described previously (11). Cell culture reagents and all other reagents were obtained from Sigma.
Animals, Aortic banding surgery, Blood pressure and Echocardiography
All protocols were approved by institutional guidelines. All surgeries and subsequent analyses were performed in a fashion blinded for genotype. Transgenic mice were produced as described previously. We used 8–10 week-old male mice with cardiac-specific expression of human A20 and their control littermates. Genotyping was performed by polymerase chain reaction (PCR) as described previously (15). Aortic banding (AB) was performed as described previously (17). Age- and sex-matched WT and TG mice were anesthetized with isoflurane. A 7.0 nylon suture ligature was tied against a 27-gauge needle at the transverse aorta to produce a 65–70% constriction following removal of the needle. Doppler analysis was performed to ensure that physiologic constriction of the aorta was induced. Hearts and lungs of sacrificed mice were dissected and weighed to compare heart weight/body weight (HW/BW, mg/g) and lung weight/body weight (LW/BW, mg/g) in TG and control mice. A microtip catheter transducer (SPR-839, Millar Instruments, and Houston, Tex) was inserted into the right carotid artery and advanced into the left ventricle under pressure control. After stabilization for 15 minutes, the pressure signals and heart rate were recorded continuously with an ARIA pressure-volume conductance system coupled with a Powerlab/4SP A/D converter, stored, and displayed on a personal computer as described previously (17). Echocardiography was performed by SONOS 5500 ultrasound (Philips Electronics, Amsterdam) with a 15-MHz linear array ultrasound transducer. The LV was assessed in both parasternal long-axis and short-axis views at a frame rate of 120 Hz. End-systole or end-diastole was defined as the phase in which the smallest or largest area of LV, respectively, was obtained. LVEDD and LVESD were measured from the M-mode tracing with a sweep speed of 50 mm/s at the mid-papillary muscle level.
Histological analysis and determination of apoptosis
Hearts were excised, washed with saline solution, and placed in 10% formalin. Hearts were cut transversely close to the apex to visualize the left and right ventricles. Several sections of heart (4–5 µm thick) were prepared and stained with hematoxylyn and eosin (H&E) for histopathology or Picrosirius Red (PSR) for collagen deposition, then visualized by light microscopy. For myocyte cross-sectional area, a single myocyte was measured with an image quantitative digital analysis system (NIH Image 1.6). The outline of 100 to 200 myocytes was traced in each group. Cell death by apoptosis was evaluated by a TUNEL assay that was performed in sections with use of the CardiaoTACS in situ Apoptosis Detection Kit (R&D Systems, Minneapolis, USA) according to the manufacture's recommendations. Caspase-3/8/9 activities were also used to examine the effects of A20 on apoptosis.
Western Blot Analysis and Northern blot
All procedures were performed as previously described. Protein extracts from different groups of myocardium (50 µg) were fractionated on a 10% polyacrylamide gel under reducing conditions, transferred to nitrocellulose membranes, and probed with various antibodies. After incubation with a secondary peroxidase-conjugated antibody, signals were visualized by Chemiluminescence kit (Amersham, Sunnyvale, CA). We Northern blot to detect mRNA levels of ANP, BNP, β-MHC, α-MHC, α-skeletal actin, and sarcoplasmic reticulum Ca2+ ATPase (SERCA2a), as well as fibrosis markers including TGFβ1, TGFβ2, CTGF, Collagen I and Collagen III. Total RNA was extracted from frozen, pulverized mouse tissues using TRIzol (Invitrogen). The detailed information for Northern blot was described in previous work (15). We normalized results against glyceraldehydes-3-phosphate dehydrogenase (GAPDH) gene expression.
Electrophoretic Mobility Shift Assay, IKK Assay and TAK1 kinase Assay
Electrophoretic mobility shift assays (EMSA) were performed according to the manufacturer's instructions (Gel Shift Assay System E3300, Promega, Madison, WI). Nuclear proteins were isolated using our previously method (6, 7). Protein concentrations were measured by BCA Protein Assay Reagents (PIERCE, Rockford, IL) using bovine serum albumin (BSA) as a standard. To determine the effect of A20 on IKK activation, the IKK assay was performed as described previously (6, 7). TAK1 immunoprecipitates were assayed using His-MKK6 as substrate as described previously (18).
Cultured neonatal rat cardiac myocytes and fibroblasts
Primary cultures of cardiac myocytes were prepared as described previously (5, 6). Cells from the hearts of 1- to 2-day-old Sprague-Dawley rats (Charles River Laboratories) were seeded at a density of 1×106/well onto 6-well culture plates coated with fibronectin (Becton Dickinson) in plating medium consisting of F10 medium supplemented with 10% FCS and penicillin/streptomycin. After 48 hours, the culture medium was replaced with F10 medium containing 0.1% FCS and BrdU (0.1 mM), then infected with different adenoviruses followed by Ang II (1 µM) treatment. Viability was determined by cell number, frequency of contractions, cellular morphology, and trypan blue exclusion. Cultures of neonatal rat ventricular nonmyocytes, which have been shown to be predominantly fibroblasts, were prepared as described previously (19). The purity of these cultures was greater than 95% cardiac fibroblasts as determined by positive staining for vimentin and negative staining for smooth muscle actin and von Willebrand factor. For the cell infections, 1×106/well cardiac myocytes or cardiac fibroblasts were cultured in 6-well plates and exposed to 2×108 pfu of each virus in 1 ml of serum-free medium for 24 hours. The cells were then washed and incubated in serum-containing media for 24 hours.
[3H]-Leucine incorporation and surface area
[3H]-Leucine incorporation was measured as described previously (6, 7). Briefly, cardiac myocytes were infected with different adenoviruses for 24 hours and subsequently stimulated with Ang II (1 µM) and coincubated with [3H]-leucine (2 µCi/mL) for the indicated time. At the end of the experiment, cells were washed with Hanks' solution, scraped off the well, and then treated with 10% trichloroacetic acid at 4°C for 60 minutes. The precipitates were then dissolved in NaOH (1 N) and subsequently counted with a scintillation counter. For surface areas, the cells were fixed with 3.7% formaldehyde in PBS, permeabilized in 0.1% Triton X–100 in PBS, and stained with α-actinin (Sigma) at a dilution of 1:100 by standard immunocytochemical techniques.
Collagen synthesis assay
Collagen synthesis was evaluated by measuring [3H]-proline incorporation as described previously (17). In brief, cardiac fibroblasts were infected with different adenoviruses, made quiescent by culturing in 0.1% FCS DMEM for 24 h, and subsequently incubated with TGF-β1 and 5 µCi/ml [3H]-proline for the indicated time. Cells were washed with PBS twice, treated with ice-cold 5% trichloroacetic acid (TCA) for one hour and washed with distilled water twice. Cells were then lysed with 1 N NaOH solutions and counted in a liquid scintillation counter. The count representing the amount of newly synthesized collagen was normalized to the cell number.
Reporter assays
Cardiac myocytes or cardiac fibroblasts were seeded in triplicate in 6-well plates. Cells were infected with different adenoviruses for 24 hours and then transfected with 0.5 µg of ANF luciferase reporter construct, and internal control plasmid DNA using 10 µl of LipofectAMINE reagent (Invitrogen), according to the manufacturer's instructions. Cardiomyocytes were then treated with Ang II and fibroblasts with TGF-β1. Cells were harvested using passive lysis buffer (Promega) according to the manufacturer's protocol. The luciferase activity was normalized by control plasmid. All experiments were done in triplicate and repeated at least three times.
Statistical Analysis
All values are expressed as mean±SEM. Differences between two groups were determined by a Student’s t test. Comparison between groups on Western blotting data was assessed by One-Way ANOVA followed by a Bonferroni correction. A value of P<0.05 was considered statistically significant.
Results
Forced A20 Expression Attenuates Pathological Cardiac Hypertrophy
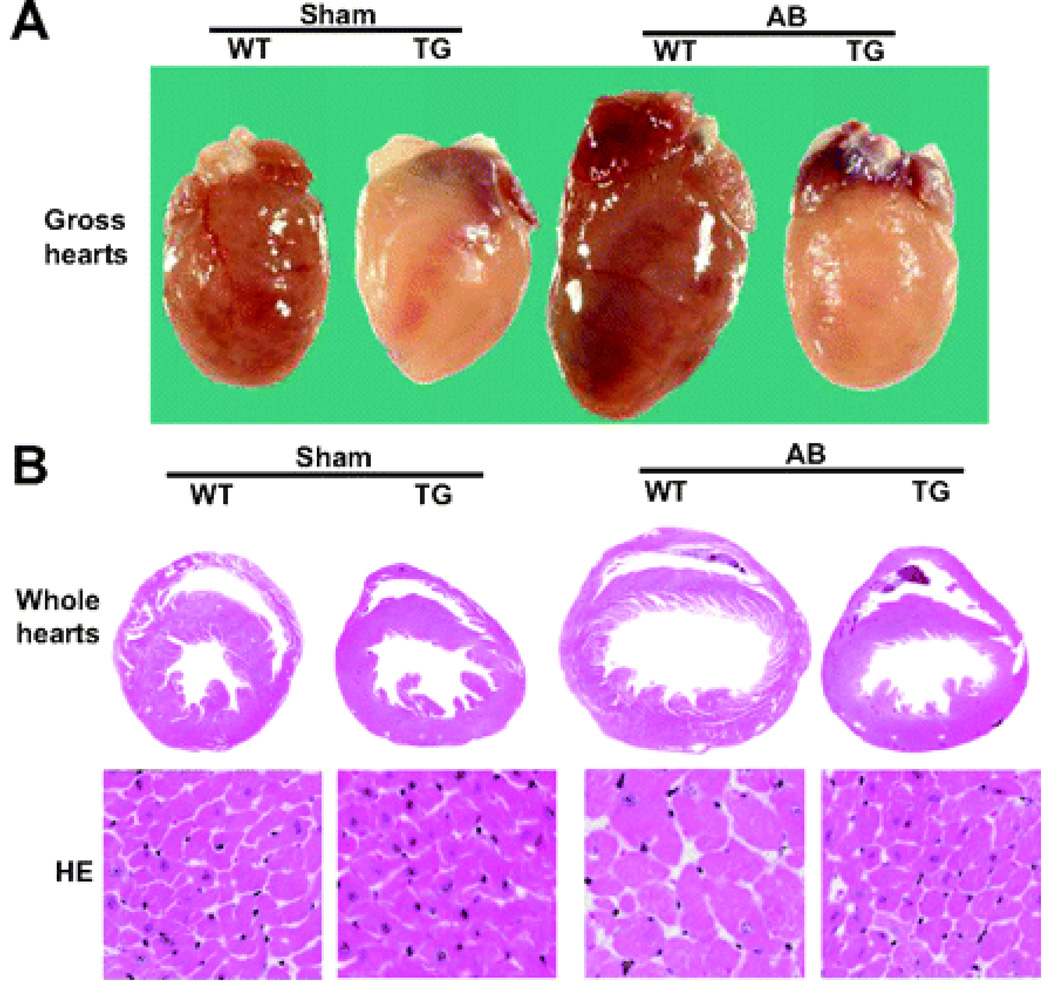
To investigate the role of A20 in biomechanical stress in the heart, we performed AB surgery on 8− to 10-week–old TG and wild-type (WT) mice. As shown in Table S1, heart weight:body weight and lung weight:body weight ratios were significantly decreased in TG mice compared with WT mice. Cardiac function was examined by echocardiography after 8 weeks of surgery. The increases in left ventricle chamber dimensions and wall thickness induced by pressure overload were also markedly reduced during both systole and diastole in TG mice compared with WT littermates (Table S1). Gross heart and hematoxylin-eosin staining further confirmed the inhibitory effect of A20 on cardiac remodeling in response to AB (Figure 1). We examined the expression of several cardiac hypertrophy markers in TG and WT mice after AB surgery by Northern blot analysis. Expression levels of atrial natriuretic peptide, brain natriuretic peptide, and β-myosin heavy chain were induced to a higher level in WT mice after AB, and such increases were markedly attenuated in TG mice (Figure S1). These results indicate that A20 overexpression in cardiomyocytes decreases the expression of cardiac hypertrophy markers atrial natriuretic peptide, brain natriuretic peptide, and β-myosin heavy chain and results in attenuated cardiac hypertrophy induced by pressure overload.
Figure 1.
Forced A20 expression attenuates pathological cardiac hypertrophy. Gross hearts, whole hearts, and hematoxylin-eosin (HE) staining of sham and AB mice at 8 weeks postsurgery (n=5).
Forced A20 Expression Attenuates Mechanical Stress-Mediated p38/JNK1/2 Signaling
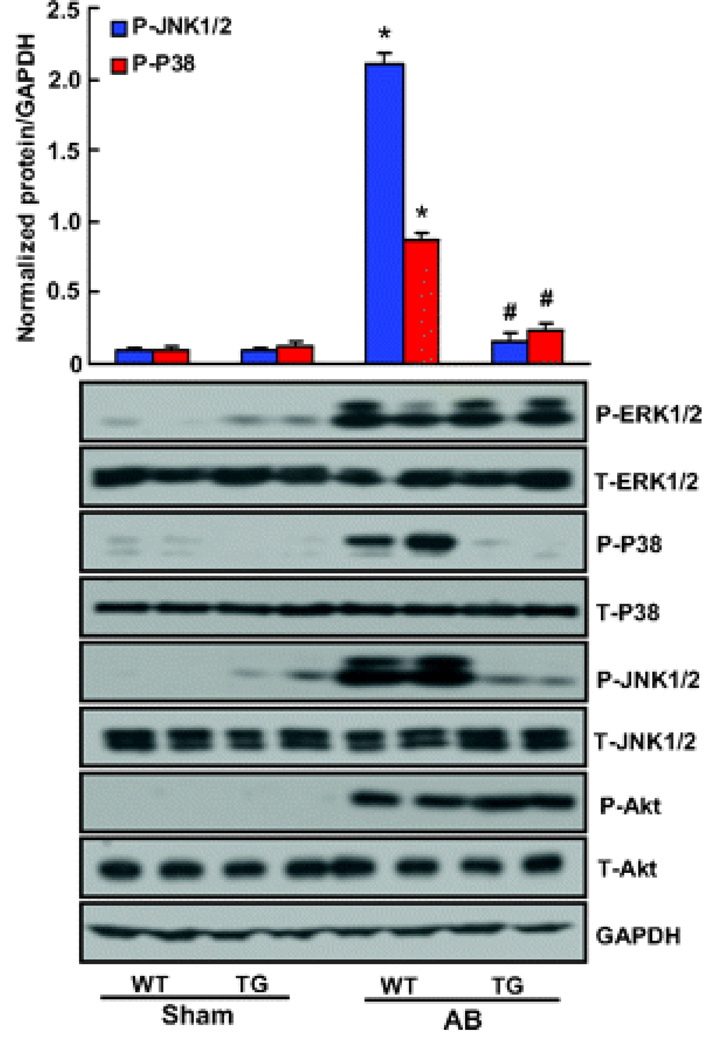
To examine the molecular mechanisms of A20 on cardiac hypertrophy, we investigated activation of the mitogen-activated protein kinase (MAPK) pathway in our hypertrophic models. We found that the phosphorylated levels of p38, JNK1/2, and ERK1/2 were significantly increased by AB in WT hearts. However, the phosphorylation of p38 and JNK1/2 was almost completely blocked in TG hearts, whereas ERK1/2 activation was similar in the 2 groups after AB (Figure 2). Although AKT signaling plays a crucial role in the regulation of cardiac remodeling and apoptosis, we did not observe any differences in AKT activation between WT and TG mice, as determined by immunoblotting for phosphorylation of AKT (Figure 2). Collectively, these data suggest that A20 overexpression suppresses the activation of p38 and JNK, although it has no effects on ERK1/2 or AKT activation in hearts subjected to AB. In vitro studies further demonstrated that p38 and JNK phosphorylation levels were enhanced after the reduction of A20 expression by RNA interference in response to hypertrophic stimuli. In contrast, p38 and JNK activations were almost completely blocked by increased A20 expression in cultured cardiac myocytes (Figure S2). These findings suggest that p38/JNK signaling was critical to the influence of A20 on cardiac hypertrophy.
Figure 2.
Forced A20 expression attenuates mechanical stress-mediated activation of stress kinase activation. The level of total and phosphorylated ERK1/2, P38, JNK1/2, and AKT in hearts tissues of mice in indicated groups (n=4). Top, Quantitative results. Bottom, Representative blots. Values are mean±SEM. *P<0.01 vs WT/sham. #P<0.01 vs WT/AB after AB.
Forced A20 Expression Impairs TAK1 Signaling Involved in Hypertrophy
Activation of TAK1, an upstream regulator of p38 and JNK, has been shown to participate in cardiac dysfunction after the development of hypertrophy. We, therefore, determined cardiac TAK1 activation by in vitro kinase activity assay. TAK1 activity was markedly increased in response to AB in WT mice (Figure 3). In the AB model, TAK1 activity was increased at 24 hours, peaked after 4 weeks, and then decreased, although the level remained higher than in the sham group. In contrast, the activity of TAK1 in response to AB was significantly abolished in TG hearts (Figure 3), suggesting that A20 overexpression may suppress TAK1 activation. The total protein level of TAK1 was not different among all of the tested groups. Consistent with our in vivo results, in vitro results showed that overexpression of A20 by infection of AdA20 blocked angiotensin (Ang) II–induced TAK1 activation, whereas downregulation of A20 expression by infection with AdsiA20 promoted angiotensin II–induced TAK1 activation (Figure S3). To further investigate the molecular mechanisms of the function of A20, we examined the effects of TAK1 activation on p38/JNK and cardiac hypertrophy. Blocking TAK1 activation by dominant-negative TAK1 (AddnTAK1) abrogated angiotensin II–mediated p38/JNK phosphorylation and cardiac hypertrophy, whereas activation of TAK1 by constitutively active TAK1 (AdcaTAK1) augmented these effects, as demonstrated by Western blot, ANF promoter activity, and [3H]-leucine incorporation (Figure S4 and S5). These results indicate that A20 attenuates cardiac hypertrophy by blocking TAK1-dependent JNK/p38 signaling pathways.
Figure 3.
Forced A20 expression impairs TAK1 signaling involved in hypertrophy. The TAK1 activity and TAK1 protein expression in hearts tissues of mice from indicated groups in WT and TG mice (n=5). Values are mean±SEM. *P<0.01 for difference from WT/AB after AB.
Forced A20 Expression Attenuates Fibrosis in vivo
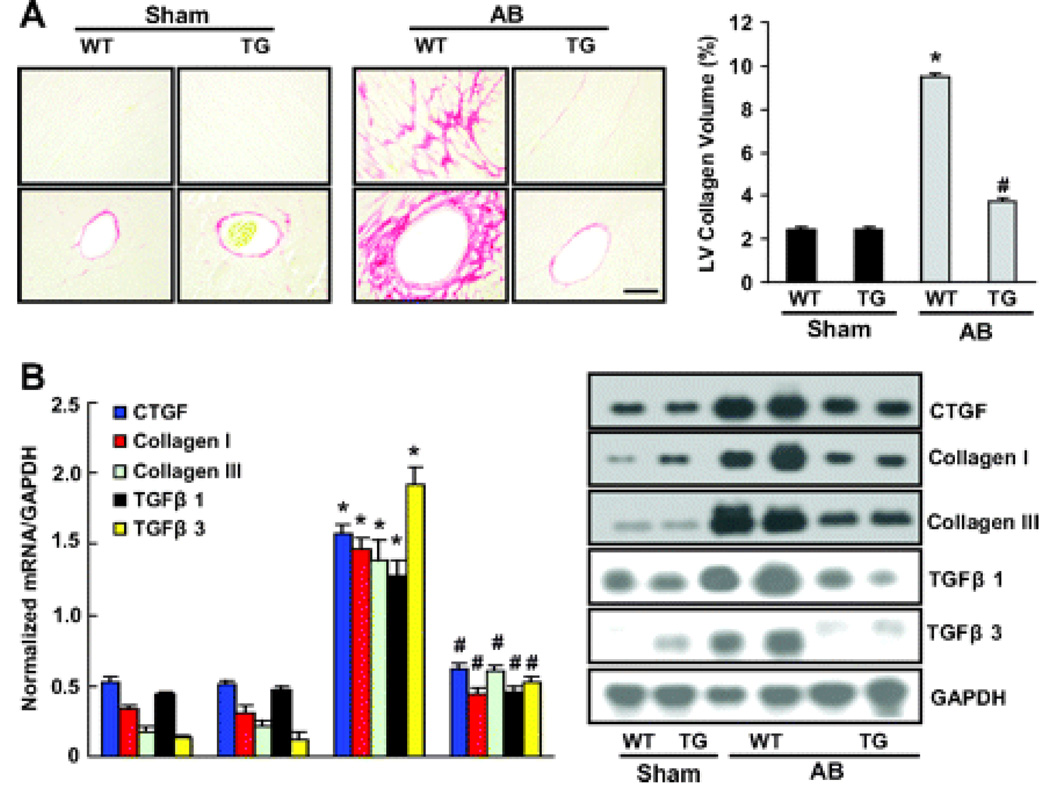
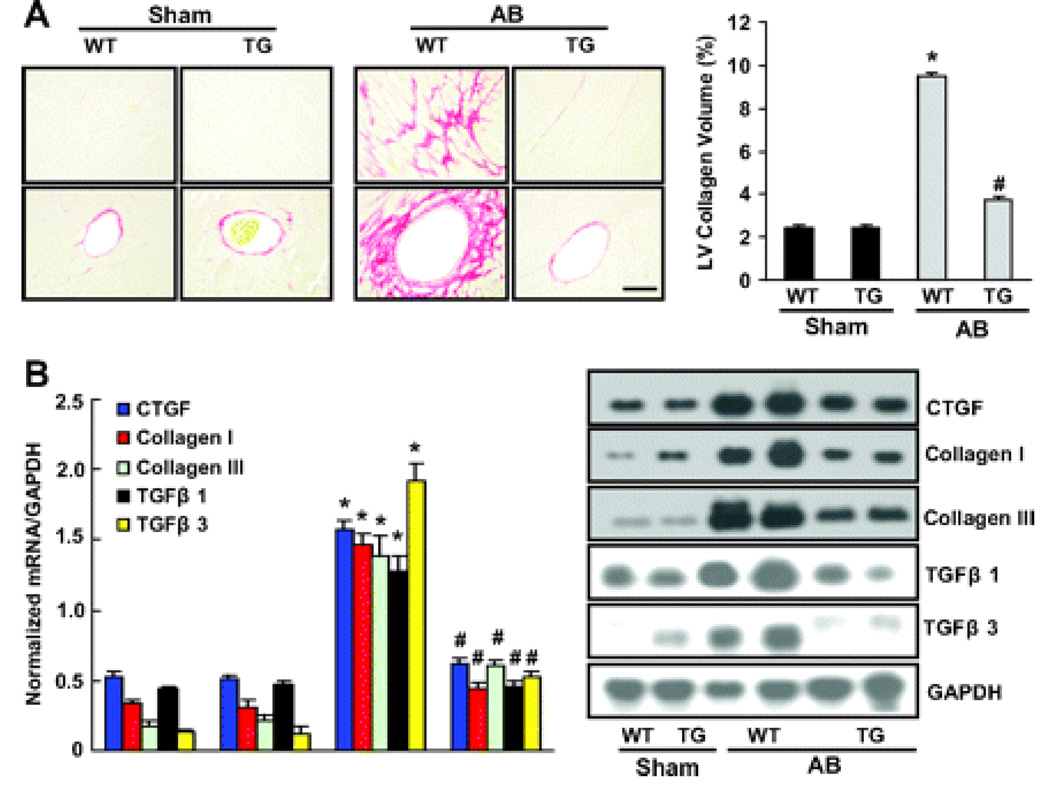
Heart sections were stained with PSR to detect fibrosis. In both groups, collagen continued to accumulate in the heart after 8 weeks of AB. As shown in Figure 4A, increased collagen deposition was observed in WT mice, but this was markedly reduced in TG mice. Quantitative analysis also showed reduced collagen volume in the myocardium of TG mice compared to WT mice. Reduced fibrosis in TG mice may represent increased collagen degradation or decreased collagen synthesis in response to tissue damage. We therefore examined the synthesis of collagen by examining the expression of mRNA and protein encoding CTGF, collagen I, collagen III, TGF-β1 and TGF-β3, known to be involved in the proliferation of cardiac fibroblasts and the biosynthesis of ECM proteins. The results showed that CTGF, collagen I, collagen III, TGF-β1 and TGF-β3 mRNA and protein expressions were significantly lower in TG than WT mice in response to hypertrophic stimuli (Figure 4, B and C). We then assessed the regulatory role of A20 in Smad cascade activation. TG animals showed suppressed Smad-2 phosphorylation, almost complete inhibition of Smad 2/3/4 nuclear translocation, but negligible effects on Smad 2/4/7 protein expression (Figure 4D).
Figure 4.
The effects of A20 on fibrosis in vivo. A, Picrosirius red staining on histological sections of the left ventricle (LV) was performed on indicated groups after 8 weeks AB. The magnification of images were X400. Fibrotic areas from histological sections were quantified using an image-analyzing system (n=5). *P<0.01 vs WT/sham. #P<0.01 vs WT/AB after AB. B, Northern blot analyses of connective tissue growth factor (CTGF), collagen I, collagen III, TGF-β1, and TGF-β3 were performed to determine mRNA expression levels in indicated groups. GAPDH was used as the normalization control. Data represent typical results of 3 different experiments as mean±SEM (n=4 to 5 mice per group). *P<0.01 vs WT/sham. #P<0.01 vs WT/AB after AB.
Forced A20 expression inhibits collagen synthesis induced by TGF-β1 in vitro
To confirm our in vivo fibrosis data, we examined the potential antifibrotic effect of A20 by [3H]-proline incorporation assay in cardiac fibroblasts. Cells were infected with AdA20 or AdsiA20 for 24 hours, then serum-starved for 24 hours in 0.5% FCS, and subsequently treated with different concentrations of TGF-β1 for 48 hours or with 15 ng/ml TGF-β1 for the indicated time. TGF-β1 stimulated [3H]-proline incorporation in a time- and dose-dependent manner (data not shown). More importantly, overexpression of A20 by infection of AdA20 inhibited TGF-β1-induced [3H]-proline incorporation, CTGF and Collagen I/III protein expression. Conversely, downregulation of A20 by infection AdsiA20 expression promoted these effects (Figure 5, A and B). The inhibition of collagen synthesis and protein expression of fibrotic markers by A20 expression was sustained for all tested times periods. To further investigate the molecular mechanisms of A20 on fibrosis, we examined the effects of A20 on Smad signaling in vitro. Western blot analysis revealed significant phosphorylation of Smad 2 and translocation of Smad 2/3/4 without any significant alterations in Smad 2/4/7 protein expression after TGF-β1 treatment in AdGFP and Adsi-control groups (Figure 5C). AdA20 infection, however, almost completely suppressed Smad 2 phosphorylation as well as Smad 2/3/4 nuclear translocation, whereas Adsi-A20 enhanced these effects (Figure 5C).
Figure 5.
The effects of A20 on fibrosis in vitro. The effects of A20 on TGF-β1–induced [3H]-proline incorporation and protein expression of collagen I and collagen III. Cardiac fibroblasts were infected with AdA20, Adsi-control, AdGFP, or AdsiA20 for 24 hours and then incubated with 10 ng/mL of TGF-β1 for an indicated time to observe [3H]-proline incorporation and protein expression. *P<0.01 vs AdGFP+10 ng/mL of TGF-β1 group at the 0 time point. Data represent typical results of 3 different experiments as mean±SEM.
We then examined the effects of A20 on TGF-β1-induced TAK1 activity. Our further experiments demonstrated that forced expression of A20 significantly blocked TAK1 activity mediated by TGF-β1, whereas decreased A20 expression promoted TAK1 activity in cultured cardiac fibroblasts (Figure 5D). Confluent cardiac fibroblasts were infected with AdGFP, AdcaTAK1, or AddnTAK1, and incubated with TGF-β1 for indicated time. Activation of TAK1 induced a significant increase in collagen synthesis by TGF-β1, whereas blocking TAK1 activity by infection with AddnTAK1 almost completely abrogated the TGFβ1-induced responses (Figure 5E). Furthermore, immunoblot analysis demonstrated that TGF-β1 incubation of cardiac fibroblasts infected with AdcaTAK1 resulted in markedly increased phosphorylation of Smad 2 and nuclear translocation of Smad 2/3/4 in response to TGF-β1. Conversely, infection with AddnTAK1 almost completely blocked these effects (Figure 5F).
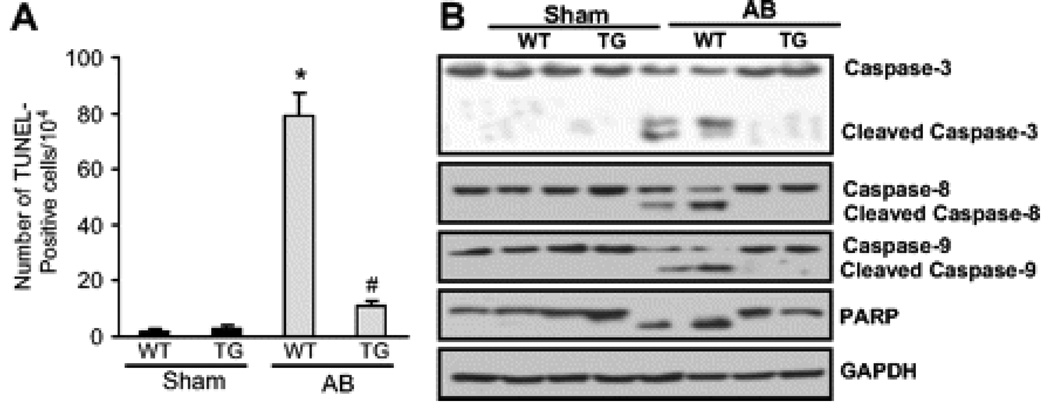
A20 Expression Inhibits Apoptosis and Inflammatory Response Induced by AB
We next examined the effects of A20 on apoptosis by TUNEL assays after 8 weeks of AB. Apoptotic cells were detected in TG and control mice, and the fraction of apoptotic versus total cells was significantly lower in TG mice than in WT mice (Figure 6A). To determine whether TG mice are resistant to apoptotic signals, we examined the cleavage of caspase 3, caspase 8, and caspase 9, as well as that of poly (ADP-ribose) polymerase (PARP). As expected, TG mice displayed a significant delay of cleavage of caspase 3, caspase 8, and caspase 9, as well as PARP degradation in response to AB (Figure 6B). To determine whether expression of A20 prevents the inflammatory responses in the hearts, cytokine induction was characterized by Western blot analyses. TG mice have significantly lower TNF-α, interleukin 6, and monocyte chemoattractant protein 1 protein levels in cardiac tissue after 8 weeks of surgery compared with WT mice (Figure S12). To determine the molecular mechanisms by which A20 attenuated cytokine induction in vivo, we analyzed NF-κB signaling pathways. We detected NF-κB activation, IKKβ, and IκBα phosphorylation, as well as IκBαdegradation, clearly after 8 weeks of AB in WT mice. Interestingly, NF-κB activation, IKKβ and IκBα phosphorylation, and IκBα degradation were evidently blocked in TG mice (Figure S13).
Figure 6.
The effect of A20 on apoptosis. A, TUNEL-positive cells from histological sections were quantified (n=5). *P<0.01 vs WT/sham, #P<0.01 vs WT/AB after AB. B, Western blot analysis of the cleavage caspase 3, caspase 8, caspase 9, and PARP in response to AB (n=5).
Discussion
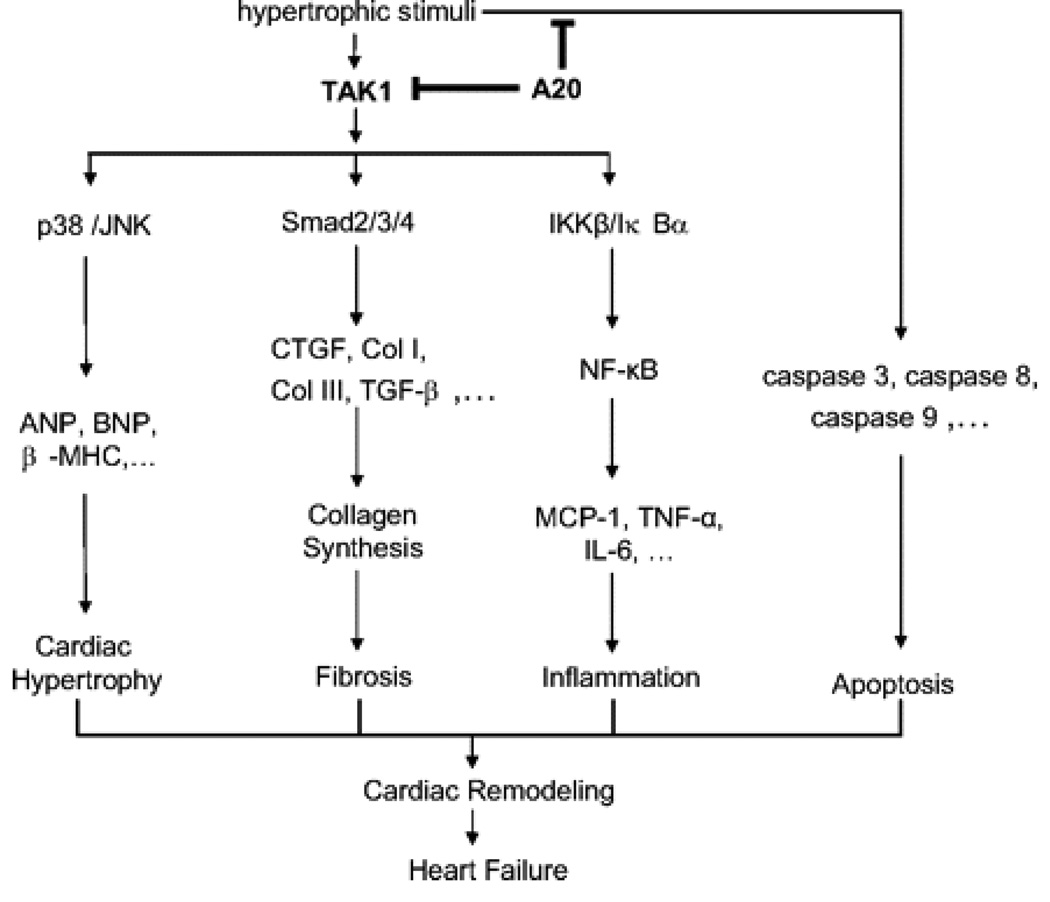
In the present study, we demonstrate that the expression of A20 in the heart protects against cardiac hypertrophy. The cardioprotection of A20 is mediated by interruption of TAK1 activity–dependent signaling pathways (Figure 7). This results in the protection of the host from the combined deleterious effects of cardiac hypertrophy, apoptosis, inflammation, and fibrosis (Figure 7). The ability of A20 to prevent cardiac dysfunction and hypertrophy mediated by sustained pressure overload suggests that it may be an effective therapeutic candidate.
Figure 7.
Proposed model of the process of A20. In the present study we demonstrated that the inhibitory effects of A20 are achieved by blocking 4 proposed signaling pathways. First, activated TAK1 results in activation of p38 and JNK signaling pathways, enhances hypertrophic markers expression, and subsequently leads to cardiac hypertrophy. Second, TAK1 is shown to promote the Smad signaling, increase expression of fibrotic markers, and result in collagen synthesis and fibrosis. Third, activation of TAK1 leads to signaling through the IKKβ/IκBα/NF-κB pathway, promoting proinflammatory cytokine expression and leading to inflammation. Fourth, hypertrophic stimuli also activate apoptotic signaling, ultimately leading to apoptosis. A20 blocks these proposed TAK1-dependent signaling pathways and apoptotic signaling and then protects against cardiac hypertrophy, fibrosis, inflammation, and apoptosis, finally preventing the progression of cardiac remodeling and heart failure.
The MAPK signaling cascade is initiated in cardiac myocytes by activation of G protein–coupled receptors, receptor tyrosine kinases, and stress stimuli.19,20 Once activated, downstream p38, JNKs, and ERKs each phosphorylate a wide array of intracellular targets, including numerous transcription factors, resulting in the reprogramming of cardiac gene expression. A significant finding of the present study is that the increase in JNK and p38 phosphorylation levels in response to hypertrophic stimuli was almost completely blocked in TG mice. The phosphorylations of ERK1/2 and AKT in myocytes were not affected by A20 expression. Further in vitro studies showed that inhibition of A20 expressionsignificantly enhanced the activation of JNK and p38 but not that of ERK1/2 and AKT. Therefore, JNK/p38 signaling was the mediator of influences of A20 on cardiac myocyte growth. Suppression of the JNK/p38 signaling pathway by A20 in the heart attenuates cardiac remodeling. However, there is still some controversy on whether activation of the JNK and p38 MAPK pathway is protective or detrimental. Blocking JNK or p38 signaling by either genetic or pharmacological approaches has previously demonstrated cardioprotective effects.21,22 In contrast, other studies suggest that JNK/p38 signaling may protect against apoptosis.23,24 Another study found that dual JNK/p38 inhibition also leads to increased apoptosis in the heart25; however, this report also shows that the proapoptotic effects of the dual JNK/p38 inhibitor are possibly attributable to suppression of JNK, as opposed to p38 MAPK. These previous studies suggest that, although inhibition/activation of either p38 or JNK pathways produces the same cardiac phenotype, the temporal manifestation of the disease possibly depends on the overall extent of cellular signaling inhibition/activation andespecially the upstream molecules of JNK/p38MAPK. This view is further supported by 2 recent studies on mixed-lineage kinase 7 and heat shock protein 20.26,27 Mixed-lineage kinase 7 was reported to activate both JNK and p38 MAPK, and overexpression of mixed-lineage kinase 7 resulted in cardiac hypertrophy and promoted cell death in the heart, indicating that dual activation of JNK/p38 is more catastrophic than either alone. Another study showed that heat shock protein 20 overexpression blocks cardiac hypertrophy and fibrosis through inhibition of the ASK1-p38/JNK cascade.27 These findings suggest that inhibition of the upstream regulator of p38 and JNK may be beneficial in halting cardiac remodeling and the progression of heart failure.
To further investigate the molecular mechanisms by which A20 inhibits cardiac hypertrophy, we examined another protein upstream of p38/JNK, TAK1. TAK1 is an MAPK kinase kinase family member originally identified as a mediator in the TGF-β signaling pathway and can be activated in response to stress stimuli.28 Genetic and biochemical evidence has established TAK1 as a key kinase that mediates the activation of IKK, p38, and JNK by diverse cellular stimuli.29 Recent studies showed that TAK1 is critically important in the cardiac hypertrophic response.30 We found that A20 not only suppressed TAK1 activity in vivo in response to hypertrophic stimuli but also blocked TAK1 activity induced by angiotensin II in vitro. Our in vitro study also showed that a decreased A20 expression level effectively enhanced TAK1 activity resulting from angiotensin II. In addition, our data confirmed that inhibition of TAK1 activity abrogated theactivation of JNK/p38, whereas activation of TAK1 activity augmented the phosphorylation of JNK/p38 in response to hypertrophic stimuli in vitro. These findings indicate that A20 attenuates cardiac hypertrophy by blocking TAK1-JNK/p38 signaling.
Cardiac fibrosis is another classic feature of pathological hypertrophy and is characterized by the expansion of the extracellular matrix attributed to the accumulation of collagen.31 Thus, it is important to understand the mechanisms that stimulate collagen deposition in the heart and define approaches to limit these processes. We found that A20 blocks cardiac fibrosis in vivo and inhibits collagen synthesis in vitro. Our study demonstrated, for the first time, that A20 blocks AB-induced fibrosis in vivo and TGF-β1–induced collagen synthesis in cardiac fibroblasts. In addition, our data suggest, for the first time, that A20 abrogates Smad 2 phosphorylation and Smad 2/3/4 translocation in both cardiac fibroblasts and hypertrophied hearts, thus inhibiting collagen synthesis and fibrosis. There is considerable evidence for synergy between the TAK-dependent and Smad-dependent TGF-β signaling pathways. TAK1 has been reported to interact with Smad 7 to inhibit TGF-β signaling by a negative feedback mechanism. More recently, TAK1 has been shown to interact with Smads and to inhibit BMP signaling.32 The relative contributions, however, of TAK-dependent and Smad-dependent pathways to cardiac fibrosis remain undetermined. We demonstrated that blocking TAK1 activation led to complete inhibition, whereas activation of TAK1 led to upregulation of collagen synthesis and Smad 2/3/4activation in vitro. We also showed that TGF-β1–induced collagen synthesis depends on TAK1 signaling, indicating that the inhibitory effects of A20 on fibrosis and collagen synthesis are mediated by blocking TAK1-dependent signaling.
Cardiac myocyte apoptosis plays an important role in the transition of cardiac hypertrophy to heart failure.33 The experimental findings here show a correlation between an increase in the frequency of apoptosis and the extent of cardiac remodeling. Consistent with previous reports, A20 expression in the heart markedly decreased the number of apoptotic cells in response to long-term pressure overload. Furthermore, it has been demonstrated that A20 blocks apoptosis of various cell types, associated with inhibition of caspase-3, -8, and -9 activities.9,10 Indeed, we found that overexpression of A20 attenuates myocardial apoptosis and is associated with abrogated cleavages of caspase-3 and -9, as well as that of PARP. The effects of A20 on these signaling molecules may explain the protection from apoptosis observed in TG hearts subjected to AB. In addition to apoptosis, there is evidence that proinflammatory cytokines play a role in pathological cardiac hypertrophy and heart failure.34,35 We found a marked induction of cytokine expression in the heart in response to hypertrophic stimuli that was observably attenuated by cardiac forced expression of A20. One possible mechanism for such a protective effect is that A20 expression directly blocks NF-κB activation, attenuating the inflammatory response and subsequent myocardial hypertrophy. Our present data suggest that A20 abrogates NF-κB activation by disrupting DNA binding and phosphorylation of IκB. By blocking NF-κB signaling, A20 may inhibit the early steps of inflammation and modulate the amplification of multiple cytokine signaling cascades. In summary, the present work demonstrates that A20 protects against cardiac remodeling and heart failure in response to hypertrophic stimuli. The mechanism underlying the protective effects of A20 appears to involve the inhibition of the TAK1-JNK/p38 signaling pathway. The potential for A20 as a therapeutic target should be considered in future studies.
Perspectives.
The current study provides a new insight into the role of A20 in the development of cardiac hypertrophy and fibrosis induced by pressure overload. Our findings suggest that A20 behaves as an endogenous and negative regulator of hypertrophic response, which may provide a novel therapeutic target for cardiac hypertrophy and fibrosis.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by the National Natural Science Foundation of China (30900524, 30972954, and 30770733) and by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (2006-331).
Footnotes
Disclosures
None.
References
- 1.Mehra MR, Uber PA, Francis GS. Heart failure therapy at a crossroad: are there limits to the neurohormonal model. J Am Coll Cardiol. 2003;41:1606–1610. doi: 10.1016/s0735-1097(03)00245-6. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Robbins J. Heart failure and protein quality control. Circ Res. 2006;99:1315–1328. doi: 10.1161/01.RES.0000252342.61447.a2. [DOI] [PubMed] [Google Scholar]
- 3.Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 5.Li HL, Wang AB, Huang Y, Liu DP, Wei C, Williams GM, Zhang CN, Liu G, Liu YQ, Hao DL, Hui RT, Lin M, Liang CC. Isorhapontigenin, a new resveratrol analog, attenuates cardiac hypertrophy via blocking signaling transduction pathways. Free Radic Biol Med. 2005;38:243–257. doi: 10.1016/j.freeradbiomed.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Li HL, Huang Y, Zhang CN, Liu G, Wei YS, Wang AB, Liu YQ, Hui RT, Wei C, Williams GM, Liu DP, Liang CC. Epigallocathechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and -independent signal pathways. Free Radic Biol Med. 2006;40:1756–1775. doi: 10.1016/j.freeradbiomed.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Li HL, She ZG, Li TB, Wang AB, Yang Q, Wei YS, Wang YG, Liu DP. Overexpression of myofibrillogenesis regulator-1 aggravates cardiac hypertrophy induced by angiotensin II in mice. Hypertension. 2007;49:1399–1408. doi: 10.1161/HYPERTENSIONAHA.106.085399. [DOI] [PubMed] [Google Scholar]
- 8.Vereecke L, Beyaert R, van Loo G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 2009;30:383–391. doi: 10.1016/j.it.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Malynn BA, Ma A. A20 takes on tumors: tumor suppression by an ubiquitin-editing enzyme. J Exp Med. 2009;206:977–980. doi: 10.1084/jem.20090765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coornaert B, Carpentier I, Beyaert R. A20: central gatekeeper in inflammation and immunity. J Biol Chem. 2009;284:8217–8221. doi: 10.1074/jbc.R800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li HL, Wang AB, Zhang R, Wei YS, Chen HZ, She ZG, Huang Y, Liu DP, Liang CC. A20 inhibits oxidized low-density lipoprotein-induced apoptosis through negative Fas/Fas ligand-dependent activation of caspase-8 and mitochondrial pathways in murine RAW264.7 macrophages. J Cell Physiol. 2006;208:307–318. doi: 10.1002/jcp.20665. [DOI] [PubMed] [Google Scholar]
- 12.Wang AB, Li HL, Zhang R, She ZG, Chen HZ, Huang Y, Liu DP, Liang CC. A20 attenuates vascular smooth muscle cell proliferation and migration through blocking PI3k/AKT singling in vitro and in vivo. J Biomed Sci. 2007;14:357–371. doi: 10.1007/s11373-007-9150-x. [DOI] [PubMed] [Google Scholar]
- 13.Heyninck K, Beyaert R. A20 inhibits NF-kappaB activation by dual ubiquitin-editing functions. Trends Biochem Sci. 2005;30:1–4. doi: 10.1016/j.tibs.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Hitotsumatsu O, Ahmad RC, Tavares R, Wang M, Philpott D, Turer EE, Lee BL, Shiffin N, Advincula R, Malynn BA, Werts C, Ma A. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity. 2008;28:381–390. doi: 10.1016/j.immuni.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li HL, Zhuo ML, Wang D, Wang AB, Cai H, Sun LH, Yang Q, Huang Y, Wei YS, Liu PP, Liu DP, Liang CC. Targeted cardiac overexpression of A20 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circulation. 2007;115:1885–1894. doi: 10.1161/CIRCULATIONAHA.106.656835. [DOI] [PubMed] [Google Scholar]
- 16.Cook SA, Novikov MS, Ahn Y, Matsui T, Rosenzweig A. A20 is dynamically regulated in the heart and inhibits the hypertrophic response. Circulation. 2003;108:664–667. doi: 10.1161/01.CIR.0000086978.95976.41. [DOI] [PubMed] [Google Scholar]
- 17.Tang Q, Cai J, Shen D, Bian Z, Yan L, Wang YX, Lan J, Zhuang GQ, Ma WZ, Wang W. Lysosomal cysteine peptidase cathepsin L protects against cardiac hypertrophy through blocking AKT/GSK3beta signaling. J Mol Med. 2009;87:249–260. doi: 10.1007/s00109-008-0423-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D, Gaussin V, Taffet GE, Belaguli NS, Yamada M, Schwartz RJ, Michael LH, Overbeek PA, Schneider MD. TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med. 2000;6:556–563. doi: 10.1038/75037. [DOI] [PubMed] [Google Scholar]
- 19.Muslin AJ. MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin Sci (Lond) 2008;115:203–218. doi: 10.1042/CS20070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Liang Q, Molkentin JD. Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animal models. J Mol Cell Cardiol. 2003;35:1385–1394. doi: 10.1016/j.yjmcc.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation. 2007;116:1413–1423. doi: 10.1161/CIRCULATIONAHA.106.679589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyoi S, Otani H, Matsuhisa S, Akita Y, Tatsumi K, Enoki C, Fujiwara H, Imamura H, Kamihata H, Iwasaka T. Opposing effect of p38 MAP kinase and JNK inhibitors on the development of heart failure in the cardiomyopathic hamster. Cardiovasc Res. 2006;69:888–898. doi: 10.1016/j.cardiores.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J Mol Cell Cardiol. 2005;38:47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Petrich BG, Wang Y. Stress-activated MAP kinases in cardiac remodeling and heart failure; new insights from transgenic studies. Trends Cardiovasc Med. 2004;14:50–55. doi: 10.1016/j.tcm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Fan GC, Yuan Q, Song G, Wang Y, Chen G, Qian J, Zhou X, Lee YJ, Ashraf M, Kranias EG. Small heat-shock protein Hsp20 attenuates beta-agonist-mediated cardiac remodeling through apoptosis signal-regulating kinase 1. Circ Res. 2006;99:1233–1242. doi: 10.1161/01.RES.0000251074.19348.af. [DOI] [PubMed] [Google Scholar]
- 27.Christe M, Jin N, Wang X, Gould KE, Iversen PW, Yu X, Lorenz JN, Kadambi V, Zuckerman SH, Bloem LJ. Transgenic mice with cardiac-specific over-expression of MLK7 have increased mortality when exposed to chronic beta-adrenergic stimulation. J Mol Cell Cardiol. 2004;37:705–715. doi: 10.1016/j.yjmcc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Delaney JR, Mlodzik M. TGF-beta activated kinase-1: new insights into the diverse roles of TAK1 in development and immunity. Cell Cycle. 2006;5:2852–2855. doi: 10.4161/cc.5.24.3558. [DOI] [PubMed] [Google Scholar]
- 29.Xiao H, Zhang YY. Understanding the role of transforming growth factor-beta signalling in the heart: overview of studies using genetic mouse models. Clin Exp Pharmacol Physiol. 2008;35:335–341. doi: 10.1111/j.1440-1681.2007.04876.x. [DOI] [PubMed] [Google Scholar]
- 30.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 31.Cai J, Yi FF, Yang L, Shen DF, Yang Q, Li A, Ghosh AK, Bian ZY, Yan L, Tang QZ, Li H, Yang XC. Targeted expression of receptor-associated late transducer inhibits maladaptive hypertrophy via blocking epidermal growth factor receptor signaling. Hypertension. 2009;53:539–548. doi: 10.1161/HYPERTENSIONAHA.108.120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shim JH, Greenblatt MB, Xie M, Schneider MD, Zou W, Zhai B, Gygi S, Glimcher LH. TAK1 is an essential regulator of BMP signalling in cartilage. EMBO J. 2009;28:2028–2041. doi: 10.1038/emboj.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anilkumar N, Sirker A, Shah AM. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci. 2009;14:3168–3187. doi: 10.2741/3443. [DOI] [PubMed] [Google Scholar]
- 34.Jadhav A, Torlakovic E, Ndisang JF. Interaction among heme oxygenase, nuclear factor-kappaB, and transcription activating factors in cardiac hypertrophy in hypertension. Hypertension. 2008;52:910–917. doi: 10.1161/HYPERTENSIONAHA.108.114801. [DOI] [PubMed] [Google Scholar]
- 35.Di Zhang A, Cat AN, Soukaseum C, Escoubet B, Cherfa A, Messaoudi S, Delcayre C, Samuel JL, Jaisser F. Cross-talk between mineralocorticoid and angiotensin II signaling for cardiac remodeling. Hypertension. 2008;52:1060–1067. doi: 10.1161/HYPERTENSIONAHA.108.117531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.