Abstract
OBJECTIVE: To compare the effectiveness of outpatient vs residential treatment for tobacco dependence in a large referral practice.
PATIENTS AND METHODS: We analyzed data from 2 cohorts of cigarette smokers who received either comprehensive outpatient or intensive 8-day residential treatment for tobacco dependence between January 1, 2004, and December 31, 2007. Self-reported 7-day point prevalence abstinence from smoking at 6 months was obtained via telephone interview. Logistic regression was used to assess the likelihood of increased abstinence with residential treatment.
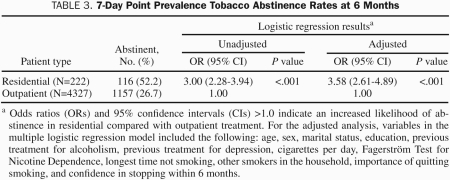
RESULTS: Overall, 4327 cigarette smokers received comprehensive outpatient treatment for tobacco dependence, and 226 smokers received treatment in an intensive 8-day residential program. Compared with outpatients, residential patients smoked more cigarettes per day (mean ± SD, 31.1±14.4 vs 21.2±11.2), had more severe nicotine dependence (Fagerström Test for Nicotine Dependence score, 6.9±2.0 vs 5.1±2.3), and were more likely to have been treated for alcoholism (58/222 [26%] vs 649/4327 [15%]) or depression (124/222 [56%] vs 1817/4327 [42%]; P<.001 for all comparisons). The 6-month smoking abstinence rate was significantly higher for residential patients compared with outpatients (115/222 [52%] vs 1168/4327 [27%]; unadjusted odds ratio, 3.0; 95% confidence interval, 2.3-3.9), with similar findings after adjusting for baseline characteristics (adjusted odds ratio, 3.58; 95% confidence interval, 2.6-4.9).
CONCLUSION: Compared with smokers who received outpatient treatment, those who received residential treatment had more severe tobacco dependence. Residential treatment for tobacco dependence was associated with a significantly greater odds of 6-month smoking abstinence compared with outpatient treatment among smokers in a referral clinic setting.
Compared with smokers who received outpatient treatment, those who received residential treatment had more severe tobacco dependence; residential treatment for tobacco use was associated with significantly greater odds of 6-month smoking abstinence compared with outpatient treatment.
CI = confidence interval; FTND = Fagerström Test for Nicotine Dependence; NDC = Nicotine Dependence Center; OR = odds ratio; TTS = tobacco treatment specialist
Cigarette smoking is the most preventable cause of morbidity, mortality, and excess health care costs in the United States, causing 442,398 deaths annually and $157 billion in health-related economic losses.1,2 The prevalence of cigarette smoking among adults in the United States declined from 42% in 1965 to 20.8% in 2006; however, it has not declined further and has remained virtually unchanged through 2009.3
Tobacco dependence is a chronic condition that often requires repeated courses of effective treatment to achieve long-term smoking abstinence. Effective treatment of tobacco dependence involves both targeted pharmacotherapy and counseling.4 Treatment of tobacco dependence is limited by the overall low efficacy related to both an initial failure to achieve abstinence and a high relapse rate among smokers who stop smoking.5 Most smokers (70%-80%) enrolled in clinical trials are not able to achieve smoking abstinence for more than 6 months with standard pharmacotherapy and behavioral support. For many chronic medical conditions (eg, diabetes, coronary heart disease, hypertension), more intensive therapy is provided when therapeutic targets and desired patient outcomes are not achieved with less intensive therapy. A similar approach is required in the treatment of tobacco use and dependence. To improve smoking abstinence rates, multiple therapeutic options are needed, including more intensive pharmacological treatment (eg, combinations of medications, extended duration of treatment, and higher doses of available first-line medications) and more intensive behavioral support. More intensive treatment may especially be needed during the first weeks of an attempt to quit smoking when relapse rates are highest.
We previously described a unique residential treatment program for tobacco dependence that provided intensive behavioral and pharmacological treatment.6,7 In that study, we matched residential patients and outpatients on several characteristics and compared residential treatment to standard outpatient intervention. Residential treatment was significantly more effective for promoting 6-month smoking abstinence.6 The aim of the current study was to extend these findings among a current cohort of smokers. We compared a cohort of patients that completed our 8-day residential treatment program with a contemporary cohort of outpatients who received comprehensive outpatient intervention for tobacco dependence.
PATIENTS AND METHODS
Study Setting
Since 1988, the Nicotine Dependence Center (NDC) at Mayo Clinic in Rochester, MN, has provided tobacco dependence treatment services to more than 50,000 patients. The NDC provides consultations, behavioral counseling, and pharmacotherapy for patients in multiple clinical settings, including medical outpatients, bedside counseling for hospitalized patients, and those in an intensive 8-day residential treatment program. Most patients treated in the NDC are seen as outpatients and are referred by a physician or self-referred for consultation; medical and surgical treatment teams request NDC consultation for their hospitalized patients. The treatment model used by the NDC includes a comprehensive intervention that combines behavioral, chemical dependence, relapse prevention, and pharmacological approaches and scheduled follow-up either in person or by telephone.
The NDC staff consists of physicians and tobacco treatment specialist (TTS) counselors who have at least a master's degree in counseling or a related field, are experienced in behavioral counseling, and have completed a TTS certification training program. The NDC provides evidence-based treatment in accordance with the US Public Health Service Clinical Practice Guidelines4 and current research.
Procedures
All outpatients and hospitalized patients are initially seen for an individual consultation by a TTS counselor, which involves 45 to 60 minutes of face-to-face assessment and counseling. The consultation includes assessment of current tobacco use, readiness and motivation to stop smoking, medical and mental health comorbidity history, and intratreatment support. The Fagerström Test for Nicotine Dependence (FTND)8 is completed by all patients. By incorporating information gathered during the initial session, the TTS counselor and the patient together develop an individually tailored treatment plan that consists of cognitive and behavioral strategies combined with pharmacological treatment. Counselors use motivational interviewing to enhance motivation, and they provide practical counseling on topics such as management of cravings, recognition of triggers to smoke, relaxation, stress management, and practical behavioral skills. Physicians in the NDC provide oversight of the program and prescribe a tailored pharmacotherapy plan to aid in smoking cessation. Follow-up by telephone or in person for 3 to 4 sessions of 10 to 15 minutes each is attempted for all patients who complete the initial consultation. Completion of follow-up counseling is not mandatory but is strongly recommended at the time of the initial consultation. The schedule for follow-up contact is determined by patient need and availability, not by a predetermined protocol.
In 1992, an intensive residential model of treatment of tobacco dependence was developed on the basis of the results of a pilot study.7 The residential treatment program is an 8-day program that uses a residential unit (private room, dining facility, meeting rooms, and exercise room located within designated clinical space but not in a hospital) to provide patients with intensive individual and group counseling as well as a comprehensive educational program in a protected, smoke-free milieu. Didactic sessions are conducted on a number of topics, including stress management, communication skills, medical complications of smoking, spirituality, relapse prevention, and healthy lifestyles. Services are provided by TTS counselors who are supervised by a physician. Tailored pharmacological therapy is provided to all patients. During daily rounds, patient progress is assessed, and pharmacological therapy may be adjusted. During the program, each patient is guided through the development of a detailed and personalized treatment and relapse prevention plan. The average total counseling time for each residential patient (both group and individual) is 12 hours during the 8-day program. For approximately 1 to 3 months after discharge from the program, patients are scheduled to receive 3 to 4 follow-up telephone or office counseling sessions from the TTS counselor, with each brief follow-up session lasting from 10 to 15 minutes. Similar to follow-up of outpatients, completion of follow-up counseling is not mandatory but is strongly recommended, with the follow-up contact schedule determined by patient need and availability. Patients who enter residential treatment are generally self-selected; however, a small number are referred by a physician or NDC counselor.
Average cost for the residential 8-day treatment program is $5000 and includes all follow-up counseling. This contrasts with the outpatient costs of approximately $488 for an initial counseling visit and completion of 4 brief follow-up sessions. Payments for services (both outpatient and residential) are received through third-party payer (private/commercial or government) or are made by the patient. Approximately 20% of patients in residential treatment receive financial support for all or a portion of the program charges based on an assessment of financial need.
Current smoking status is ascertained by systematic telephone contact with each patient treated at the NDC (both outpatients and residential patients) at 6 months (±30 days) after the initial TTS counseling session. Self-report 7-day point prevalence smoking abstinence is determined by asking, “Have you had even a puff in the past 7 days?” Patients who cannot be contacted after 3 attempts or decline to answer the self-report question are adjudicated as smoking. Because of database entry procedures, we cannot distinguish between patients who self-reported tobacco use from those who were adjudicated as smoking because of failure to contact or refusal to answer at telephone follow-up. Additionally, we cannot provide biochemical confirmation of smoking abstinence at 6 months because of the wide geographic distribution of patients treated in the NDC. For residential program patients only, we obtain expired air carbon monoxide verification of smoking abstinence (not smoking is confirmed by a value <8 parts per million) each day of the 8-day program.
Study Participants
The 2 cohorts consisted of patients who underwent tobacco dependence consultation at the NDC during a 4-year period (January 1, 2004, to December 31, 2007) in either the outpatient or residential setting. Participants were eligible for inclusion if they received either outpatient or residential treatment in the specified time window, provided general clinical research authorization, used no tobacco products other than cigarettes, and were at least 18 years of age at the time of the initial consultation. Patients included in this study received NDC consultation either through self-referral or physician referral. Patients admitted to residential treatment were self-selected, referred by a medical professional, or recommended by the TTS counselor after outpatient consultation. Besides referral to the residential program, no other specific criteria are required for admission.
Statistical Analyses
We compared baseline characteristics and smoking abstinence outcomes between outpatient and residential smokers. Baseline characteristics were summarized across the 2 cohorts. Patient characteristics were summarized using mean ± SD for continuous variables and frequency percentages for categorical variables, and they were compared between treatment groups using the t test or χ2 test, respectively.
Smoking abstinence rates at 6 months after the initial counseling date were summarized and compared across programs using the χ2 test. Participants were considered abstinent from smoking at the 6-month follow-up if they self-reported no cigarette use (“not even a puff”) for the 7 days before the follow-up date.
Smoking abstinence was also analyzed using a multiple logistic regression analysis. All program-by-covariate interaction effects were tested to determine whether any associations between baseline characteristics and abstinence differed between programs. Findings from the multivariable analysis were summarized using odds ratios (ORs) and corresponding 95% confidence intervals (CIs). In all cases, 2-sided P≤.05 was used to denote statistical significance.
RESULTS
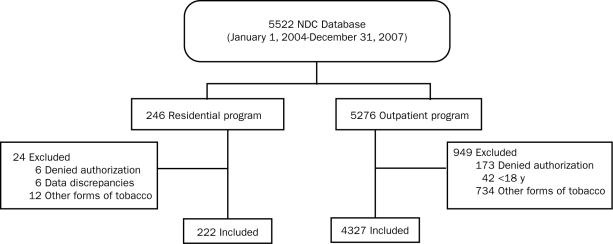
During the specified 4-year period, 5522 patients were treated at the NDC (5276 adult tobacco users in the outpatient setting and 246 patients in the 8-day residential setting [Figure]). Of these 5522 patients, 973 were excluded (949 outpatients and 24 residential patients) for the following reasons: 746 were using a tobacco product other than cigarettes (734 outpatients and 12 residential), 179 had not given research authorization (173 outpatients and 6 residential), 42 were younger than 18 years (all outpatients), and 6 (all residential patients) had irresolvable data discrepancies (attendance in the residential program within the study time frame could not be confirmed from a primary data source).
FIGURE.
Flow diagram of participant inclusion and exclusion criteria. NDC = Nicotine Dependence Center.
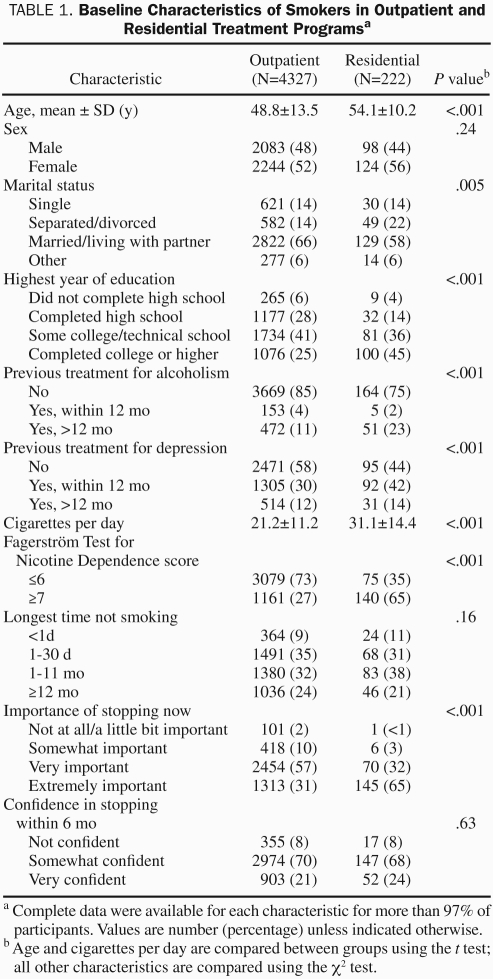
Compared with outpatients, residential patients were older, more often separated or divorced, more educated, and more often previously treated for alcoholism or depression (Table 1). In addition, residential patients smoked significantly more cigarettes per day and had more severe nicotine dependence as measured by the FTND score (6.9±2.0 for residential patients vs 5.1±2.3 for outpatients; P<.001). Moreover, they were significantly more likely to be in the preparation or action stage of readiness and believed that it was extremely important to stop smoking. Furthermore, residential patients were less likely to have other smokers in their household compared with outpatients.
TABLE 1.
Baseline Characteristics of Smokers in Outpatient and Residential Treatment Programsa
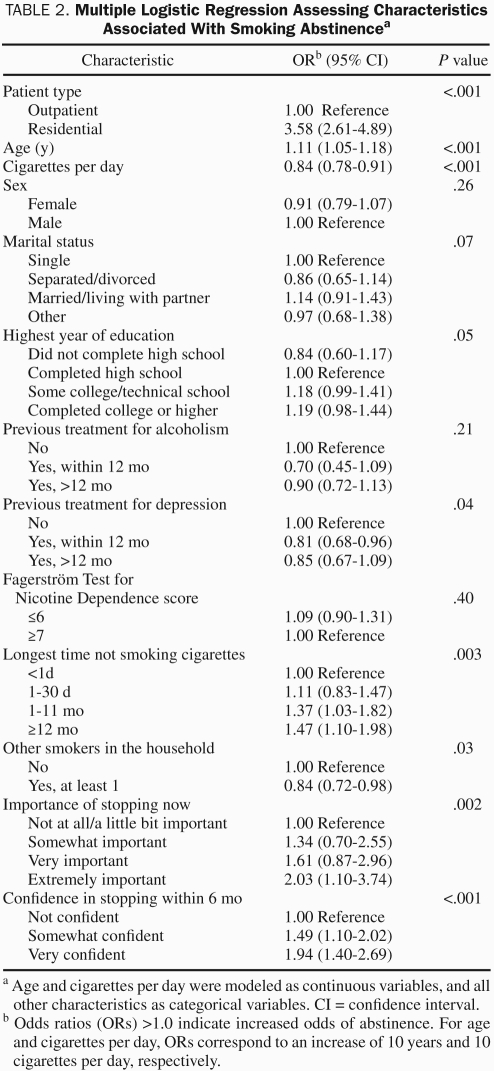
A number of patient characteristics were assessed for their association with 6-month smoking abstinence (Table 2). Not unexpectedly, there were a number of positive (age, longest time not smoking, and importance of quitting smoking now) and negative (cigarettes smoked per day, previous treatment for depression, and presence of other smokers in the household) correlations with 6-month smoking abstinence. The patient's treatment setting (outpatient or residential) showed the single strongest association with 6-month smoking abstinence. The smoking abstinence rate at 6 months after treatment was significantly higher for residential patients compared with outpatients (Table 3). Similar findings were observed using a multiple logistic regression analysis, which adjusted for all baseline covariates listed in Tables 1 and 2.
TABLE 2.
Multiple Logistic Regression Assessing Characteristics Associated With Smoking Abstinencea
TABLE 3.
7-Day Point Prevalence Tobacco Abstinence Rates at 6 Months
DISCUSSION
Residential treatment for tobacco dependence results in smoking abstinence rates that are significantly greater than those achieved with treatment in a standard outpatient setting. We have demonstrated long-term abstinence rates greater than 50% among a cohort of heavy smokers with high levels of nicotine dependence. This compares with our outpatient treatment abstinence rates of about 27% at 6 months. This striking difference is particularly notable because the outpatient cohort smoked less, had lower levels of nicotine dependence, were less likely to have a history of alcohol problems, and often reported less comorbid depression compared with residential patients. This extends our earlier findings in a study of outpatients and residential patients almost 10 years ago who were matched on multiple variables, including extent of smoking.6 When comparing residential treatment vs outpatient treatment in the current study, the OR for prolonged smoking abstinence was 3.58 (95% CI, 2.61-4.89) compared with an OR of 2.74 (95% CI, 1.60-4.71) in our earlier study. In addition, the 6-month smoking abstinence rate improved in the current study compared with our earlier one (52.2% and 45.2%, respectively). The improved result in the current study may reflect differences in the patients being treated or changes in the program content and treatment approaches (eg, newer pharmacotherapeutic approaches) over time.
Few reports of residential treatment for tobacco dependence have been published. Follow-up of patients who completed a 5-day residential program reported long-term smoking abstinence of 65%.9 The average length of self-reported smoking abstinence at the time of the survey was 120 weeks; however, follow-up was performed 5 or more years after program completion. These results cannot be directly compared with ours in that the self-report of smoking status was provided by mailed questionnaire with a 47% response rate, and nonresponders were not counted as smoking. If a conservative intent-to-treat approach of counting nonresponders as smoking is used, the estimated long-term abstinence rate approaches 30%. In a more recent pilot study of a 4-day residential program for tobacco dependence, Green et al10 reported a 6-month abstinence rate of about 26%. A large percentage of the study participants were male veterans (61%), and duration of the residential program was shorter than ours, making direct comparison of results difficult. In a study of smokers with chronic obstructive lung disease, participants were randomly assigned to 14 days of hospital treatment compared with usual outpatient care.11 One year after treatment, 52% of hospital-treated participants were abstinent from smoking compared with 7% in the usual outpatient care group. The treatment provided to the hospitalized patients appears to closely approximate the treatment intensity in our residential treatment program, and smoking abstinence rates are comparable. The main distinction between our study and the study by Sundblad et al11 is that their study focused on smokers with mild to severe chronic obstructive lung disease, whereas our study consisted of a general population of smokers. The similar results reported with in-hospital treatment11 and our residential treatment program suggest that intensive interventions comparable to our residential treatment should result in improved smoking abstinence rates compared with less intensive outpatient care.
As we noted in our previous report of residential treatment of tobacco dependence, a substantial proportion of patients have comorbid medical and psychiatric conditions. About one-quarter of our patients had been previously treated for alcohol abuse, and more than one-half received treatment for depression (current or past). These results are comparable to our previous report6 and consistent with observations made by others.10 These findings are not unexpected because alcohol abuse and/or mental illness is common among smokers, and both are more common among heavier smokers.12 Patients in our residential program were highly nicotine dependent as suggested by their high FTND scores and were heavy smokers, smoking an average of more than 30 cigarettes per day.
The reasons for the apparent efficacy of residential treatment are many. A clear dose-response relationship exists between the amount of direct contact in behavioral therapy and long-term smoking abstinence.4 Patients in our residential program undergo an average of 12 hours of group and individual face-to-face counseling as well as time in educational discussions and skills training with counseling and medical staff. Previous research in a consecutive sample of our residential patients has also shown improvement in a number of psychosocial variables that may affect the ability to maintain long-term smoking abstinence.13 Unlike many medical treatments, residential treatment for tobacco dependence is usually not completely covered by insurance. The financial and time investment required for completion of residential treatment compared with outpatient therapy may positively affect motivation and commitment for remaining abstinent from smoking. Finally, research has demonstrated that tailoring pharmacotherapy by using higher doses of nicotine replacement and combinations of first-line medications result in improved smoking abstinence rates compared with single-agent treatment or standard doses of nicotine replacement therapy.14-17 By virtue of daily contact for 8 days, all patients in the residential program receive individually tailored pharmacotherapy with adjustments to treatment based on daily observation of treatment response. A similar approach to initial pharmacotherapy is used among our outpatients, but day-to-day adjustments are not possible because of the limited contact with outpatients.
The observational nature of the current study limits our ability to make causal inferences about the higher smoking abstinence rates observed with residential treatment. The 2 cohorts may have had different characteristics that were not measured and could not be accounted for in statistical adjustments. For example, the economic status of the groups may have differed because residential patients often paid out of pocket for an expensive treatment. Residential treatment patients may have had far more past experience with tobacco treatment, which may have positively influenced response to treatment. Despite these important limitations, the magnitude of the difference between the treatment modalities, the reproducible results obtained in the current study compared with our previous study, and the known dose-response relationship between behavioral therapy contact time and improved smoking abstinence outcomes all suggest that residential treatment increases smoking abstinence rates compared with outpatient treatment. Admittedly, whether residential treatment is truly superior to other less intensive methods of treatment can only be determined with a high degree of certainty from a randomized, controlled trial of smokers desiring treatment for tobacco dependence.
Our study has other limitations. Because our patients were not randomly selected and were seen at a tertiary care medical center, referral bias may affect our results, and our results may not be generalizable to other populations of smokers. In addition, because of the wide geographic distribution of our referral patient population and the added expense to patients, we are not able to provide biochemical confirmation of 6-month smoking abstinence for either residential patients or outpatients. We recognize that self-report without biochemical confirmation may overestimate abstinence rates, but this seems to be less of a problem when a patient knows that follow-up is part of the treatment plan.18,19 Self-report alone may overestimate the 6-month smoking abstinence rates, but the magnitude of the inflation of smoking abstinence is usually small in general population studies.20 The inflation of self-reported smoking abstinence may be slightly greater among residential patients because of the intensity of therapeutic contact during the program compared with outpatients, although significant overreporting of smoking abstinence was not observed in an intensive hospital treatment program for tobacco dependence that closely resembles our residential treatment program.11 Because follow-up frequency and intensity and the follow-up procedures for obtaining the smoking self-report were identical between groups, differential bias should be minimal.20
We have observed that residential treatment for tobacco dependence is associated with significantly greater odds of 6-month smoking abstinence compared with outpatient treatment. These results extend our earlier findings and suggest that over time there has been an incremental improvement in the outcomes for residential treatment. In relatively ideal circumstances among highly selected smokers in clinical trials of various pharmacotherapies, smoking abstinence rates at 6 months are often 20% to 25%,4 whereas real-world outcomes are usually lower. Residential treatment provides an opportunity to increase smoking abstinence rates substantially, even among smokers in whom other treatments have failed or who have medical and psychiatric comorbid conditions. Although the initial cost of residential treatment is high (approximately $5000 at our institution), it is likely to be cost-effective because long-term smoking abstinence rates may be double or triple those achieved with typical outpatient treatment. Previous studies have shown that among primary and secondary preventive therapies, tobacco dependence treatment is one of the most cost-effective.4,21,22 In a recent Dutch study, the cost-effectiveness of even the most intensive interventions for tobacco dependence was significantly better than that of commonly recommended medical and surgical interventions.23 Although our type of residential treatment cannot be used in every medical center and hospital, similarly intensive treatment tailored to local and regional needs should be made available at referral centers. Our consistent results suggest that a residential treatment alternative, if expanded, may provide effective tobacco dependence treatment for even the most recalcitrant smoker.
CONCLUSION
Residential treatment for tobacco use and dependence is associated with a significantly greater 6-month smoking abstinence rate compared with standard outpatient treatment and provides an approach that is effective for heavy smokers who are severely tobacco dependent. Additional studies of residential treatment in randomized, controlled clinical trials are needed to prove its efficacy.
Supplementary Material
Acknowledgments
We are grateful for the skillful and compassionate care of patients provided by the staff of the Mayo Clinic NDC.
REFERENCES
- 1. Centers for Disease Control (CDC) Annual smoking-attributable mortality, years of potential life lost, and economic costs–United States, 1995-1999. MMWR Morb Mortal Wkly Rep. 2002;51(14)300-303 [PubMed] [Google Scholar]
- 2. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000 [published corrections appear in JAMA. 2005;293(3):293-294 and 2005;293(3):298]. JAMA. 2004;291(10)1238-1245 [DOI] [PubMed] [Google Scholar]
- 3. Center for Disease Control (CDC) Prevalence of current smoking among adults aged 18 years and over: United States, 1997-June 2009. National Health Interview Survey. www.cdc.gov/nchs/data/nhis/earlyrelease/200912_08.pdf Accessed January 19, 2011
- 4. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Dept of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 5. Lancaster T, Hajek P, Stead LF, West R, Jarvis MJ. Prevention of relapse after quitting smoking: a systematic review of trials. Arch Intern Med. 2006;166(8)828-835 [DOI] [PubMed] [Google Scholar]
- 6. Hays JT, Wolter TD, Eberman KM, Croghan IT, Offord KP, Hurt RD. Residential (inpatient) treatment compared with outpatient treatment for nicotine dependence. Mayo Clin Proc. 2001;76(2)124-133 [DOI] [PubMed] [Google Scholar]
- 7. Hurt RD, Dale LC, Offord KP, Bruce BK, McClain FL, Eberman KM. Inpatient treatment of severe nicotine dependence. Mayo Clin Proc. 1992;67(9)823-828 [DOI] [PubMed] [Google Scholar]
- 8. Fagerström KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerström Tolerance Questionnaire. J Behav Med. 1989;12(2):159-182 [DOI] [PubMed] [Google Scholar]
- 9. Hoffman EH, Blackburn C, Cullari S. Brief residential treatment for nicotine addiction: a five-year follow-up study. Psychol Rep. 2001;89(1)99-105 [DOI] [PubMed] [Google Scholar]
- 10. Green A, Yancy WS, Braxton L, Westman EC. Residential smoking therapy. J Gen Intern Med. 2003;18(4)275-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sundblad BM, Larsson K, Nathell L. High rate of smoking abstinence in COPD patients: smoking cessation by hospitalization. Nicotine Tob Res. 2008;10(5):883-890 [DOI] [PubMed] [Google Scholar]
- 12. Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11)1107-1115 [DOI] [PubMed] [Google Scholar]
- 13. Ames SC, Croghan IT, Clark MM, et al. Change in perceived stress, partner support, decisional balance, and self-efficacy following residential nicotine dependence treatment. J Addict Dis. 2008;27(1)73-82 [DOI] [PubMed] [Google Scholar]
- 14. Ebbert JO, Burke MV, Hays JT, Hurt RD. Combination treatment with varenicline and nicotine replacement therapy. Nicotine Tob Res. 2009;11(5):572-576 [DOI] [PubMed] [Google Scholar]
- 15. Ebbert JO, Croghan IT, Sood A, Schroeder DR, Hays JT, Hurt RD. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine Tob Res. 2009;11(3)234-239 [DOI] [PubMed] [Google Scholar]
- 16. Hurt RD, Dale LC, Fredrickson PA, et al. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow-up: one-year outcome and percentage of nicotine replacement. JAMA. 1994;271(8):595-600 [PubMed] [Google Scholar]
- 17. Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;(1):CD000146 [DOI] [PubMed] [Google Scholar]
- 18. Rohren CL, Croghan IT, Hurt RD, Offord KP, Marusic Z, McClain FL. Predicting smoking cessation outcome in a medical center from stage of readiness: contemplation versus action. Prev Med. 1994;23(3)335-344 [DOI] [PubMed] [Google Scholar]
- 19. Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull. 1992;111(1)23-41 [DOI] [PubMed] [Google Scholar]
- 20. SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2)149-159 [DOI] [PubMed] [Google Scholar]
- 21. Croghan IT, Offord KP, Evans RW, et al. Cost-effectiveness of treating nicotine dependence: the Mayo Clinic experience. Mayo Clin Proc. 1997;72(10):917-924 [DOI] [PubMed] [Google Scholar]
- 22. Cromwell J, Bartosch WJ, Fiore MC, Hasselblad V, Baker T, Agency for Health Care Policy and Research Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. JAMA. 1997;278(21)1759-1766 [PubMed] [Google Scholar]
- 23. Feenstra TL, Hamberg-van Reenen HH, Hoogenveen RT, Rutten-van Molken MP. Cost-effectiveness of face-to-face smoking cessation interventions: a dynamic modeling study. Value Health. 2005;8(3)178-190 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.