Abstract
OBJECTIVE: To assess in a US general adult population the effect of the functional single-nucleotide polymorphism rs198389 in the promoter region of the gene of brain-type natriuretic peptide (BNP) on 3 commonly used BNP assays, clinical phenotype, disease prevalence, overall survival, and diagnostic test characteristics of BNP as a biomarker.
PATIENTS AND METHODS: We genotyped for rs198389 in a random sample of the general population (aged ≥45 years; n=1970; enrolled between June 1, 1997, and September 30, 2000) from Olmsted County, Minnesota. Patients were characterized biochemically, clinically, echocardiographically, and regarding BNP molecular forms (2 assays for BNP and 1 assay for amino-terminal proBNP). Median follow-up was 9 years.
RESULTS: Genotype frequencies were in Hardy-Weinberg equilibrium (P=.98): TT genotype, n=645 (32.7%); TC genotype, n=983 (49.9%); and CC genotype, n=342 (17.4%). The C allele independently predicted higher BNP forms (P<.001 for all assays). Genotypes did not differ with regard to clinical and echocardiographic phenotype or overall survival. When previously reported genotype-unadjusted cut points for the detection of left ventricular ejection fraction less than or equal to 40% (n=37 [1.9%]) and less than or equal to 50% (n=116 [6.0%]) were used, sensitivity generally increased with the number of C alleles, whereas specificity decreased, both on average by more than 10% for the TT vs CC genotype.
CONCLUSION: The C allele of rs198389 is common in the general US population and is associated with higher concentrations of BNP molecular forms but not with cardiovascular phenotype or survival. The C allele confounds the test characteristics of commonly used assays.
The C allele of rs198389 is common in the general US population and is associated with higher concentrations of brain-type natriuretic peptide molecular forms but not with cardiovascular phenotype or survival; the C allele confounds the test characteristics of commonly used assays.
ANP = atrial natriuretic peptide; BNP = brain-type natriuretic peptide; BNP-IR = BNP immunoreactivity; cGMP = cyclic guanosine monophosphate; HF = heart failure; LV = left ventricular; NT-proBNP = amino-terminal proBNP; PCR = polymerase chain reaction; SNP = single-nucleotide polymorphism
Brain-type natriuretic peptide (BNP), a cardiac hormone with pleiotropic actions, plays an important role in cardiorenal regulation.1-3 Circulating BNP has emerged as a robust biomarker to aid in the diagnosis and prognosis of left ventricular (LV) dysfunction and heart failure (HF), as well as serving as a prognostic biomarker in the general population to identify those at higher risk of mortality and cardiovascular morbidity.4-12 The utility of BNP as a cardiac biomarker is secondary to its activation by myocardial stretch and ischemia, as is observed in HF and myocardial infarction. Age and sex are important factors that contribute to the regulation of plasma BNP.8,10,13
The BNP gene, NPPB, is located on chromosome 1, in tandem with NPPA, the gene for atrial natriuretic peptide (ANP).14 Several single-nucleotide polymorphisms (SNPs) have been reported for this region, some of which have recently been associated with higher BNP and ANP plasma levels and lower blood pressure and prevalence of hypertension.15 The SNP rs198389 (also referred to as BNP T-381C) is located in the promoter region of the BNP gene and has been associated with higher BNP levels in 3 studies.16-18 Meirhaeghe et al16 demonstrated that the C allele was associated with higher promoter activity in vitro. Currently, the prevalence of rs198389 in the general US population is unknown, as is its effect on cardiovascular phenotype, mortality, and assay test characteristics. In this study, we defined the prevalence of rs198389 in a large random sample of the general adult population in Olmsted County, Minnesota, and its effect on phenotype, specifically circulating forms of BNP as measured by different assays; cardiovascular, renal, and neurohumoral characteristics; and disease prevalence. We also compared survival among genotypes, which could conceivably be affected by elevated BNP levels. Further, we assessed the effect of rs198389 on the test characteristics of BNP for the detection of reduced LV function in the general community. We hypothesized that rs198389 would affect assays for BNP, including BNP, proBNP1-108, and amino-terminal proBNP (NT-proBNP), and that the C alleles would be associated with higher sensitivity but lower specificity if genotype-unadjusted cut points were used.
PATIENTS AND METHODS
This study consists of a well-characterized random sample of the general population aged 45 years or older in Olmsted County, Minnesota (n=1970). Data and blood samples were collected between June 1, 1997, and September 30, 2000. Echocardiography was performed and a blood sample was collected within days of each other.8-10,12,19,20 Mortality data on Olmsted County residents are routinely collected by reviewing community medical records, death certificates, and obituary notices as part of the Rochester Epidemiology Project. Patients were followed up until death or March 2008, at which time they were censored, providing a median follow-up of 9 person-years. The Mayo Clinic Institutional Review Board approved this study.
Hormone Assays
As previously described, plasma BNP was measured by radioimmunoassay (Shionogi & Co, Tokyo, Japan) and fluorescence immunoassay (Biosite, San Diego, CA), and NT-proBNP was measured by an electrochemiluminescence immunoassay (Roche Diagnostics, Indianapolis, IN).8,10 To measure proBNP1-108, we used a specific assay that is based on a previously reported antibody directed against the region of proBNP1-108, where the cleavage into NT-proBNP and BNP occurs (the assay was provided by Bio-Rad Laboratories, Hercules, CA).21 Atrial natriuretic peptide was measured by radioimmunoassay (Phoenix Pharmaceuticals, Burlingame, CA), as were cyclic guanosine monophosphate (cGMP; PerkinElmer, Boston, MA) and aldosterone (Siemens Medical Solutions Diagnostics, Los Angeles, CA).
Genotyping for rs198389 SNP
Genotyping was conducted by the restriction fragment length polymorphism method. The oligonucleotides used for the polymerase chain reaction (PCR) amplification were 5′CTGTGAGTCACCCCGTGCTC-3′ and 5′-GGCAGGAACGCGCTGGAGAC-3′. Polymerase chain reaction was conducted with standard reagents and 2-mM MgCl2 at an annealing temperature of 66°C, generating an amplicon of 186 bp. The PCR product was digested with 12 U of MspI (New England Biolabs, Hitchin, Hertfordshire, UK). The T allele was characterized by 2 fragments of 48 and 138 bp, whereas the C allele was characterized by 3 PCR fragments of 48, 67, and 71 bp, resolved on a 3.5% MetaPhor agarose gel (Cambrex Bio Science Rockland, Rockland, ME).
BNP Genotype and Diagnostic Test Characteristics
Given the increased BNP values with additional C alleles, we hypothesized that cutoffs for the screening of reduced LV systolic function optimized for sensitivity and specificity would be correspondingly higher for every additional C allele. To establish new cutoffs, a reasonable number of individuals with an ejection fraction less than or equal to 40% would have been required in every genotype. Because the number of individuals with an ejection fraction less than or equal to 40% was limited in our general community sample (n=37 [1.9%]), we deemed the numbers within genotypes too small to generate reliable cut points. However, given that Specificity = True Negatives/(True Negatives + False Positives), our sample was able to provide a good estimate of the effect of genotype on specificity. To illustrate the effect of genotype on diagnostic test characteristics for the detection of reduced ejection fraction, we calculated test characteristics for individual genotypes using cut points previously determined when all genotypes were combined.10 Positive and negative likelihood ratios and odds ratios were calculated. Assuming that a test result above the cut point would be verified with an imaging study, we also calculated the percentage of patients requiring echocardiography, the percentage showing no abnormalities on echocardiography, and the percentage with missed disease.
Statistical Analyses
Values are expressed as mean ± SD or percentage. Categorical variables were compared using the χ2 test and continuous variables using one-way analysis of variance. Given the additive nature of the effect of each C allele on BNP levels, additive models were used. Brain-type natriuretic peptide values were log-transformed before analysis. To assess the effect of rs198389 on BNP immunoreactivity (BNP-IR), we also performed a linear least squares regression with stepwise forward selection in which we included parameters known to potentially affect BNP-IR (age, sex, rs198389, body mass index, heart rate, left atrial volume index, LV mass index, LV dimension index, systolic and diastolic blood pressure, serum creatinine level, and estimated glomerular filtration rate [calculated by the Cockcroft-Gault equation]). The significance level for variables to be entered into the model was P=.15. Survival from entry into the study was estimated using the Kaplan-Meier method. A 2-sided P<.05 was considered to indicate statistical significance.
RESULTS
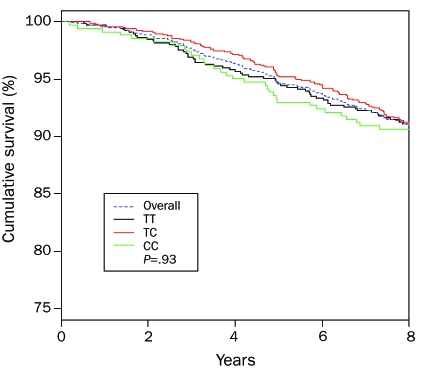
A total of 1970 patients were successfully genotyped and were included in this study. Clinical and echocardiographic characteristics are shown in Table 1. Genotype frequencies were TT, 32.7% (n=645); TC, 49.9% (n=983); and CC, 17.4% (n=342), corresponding to a minor allele frequency of 42.3%. This distribution was in Hardy-Weinberg equilibrium (P=.98). Genotypes did not differ with regard to clinical and echocardiographic parameters, such as age, body mass index, cardiovascular disease prevalence, or LV dimension, mass, or ejection fraction. Similarly, overall survival did not differ among genotypes (P=.93; Figure 1).
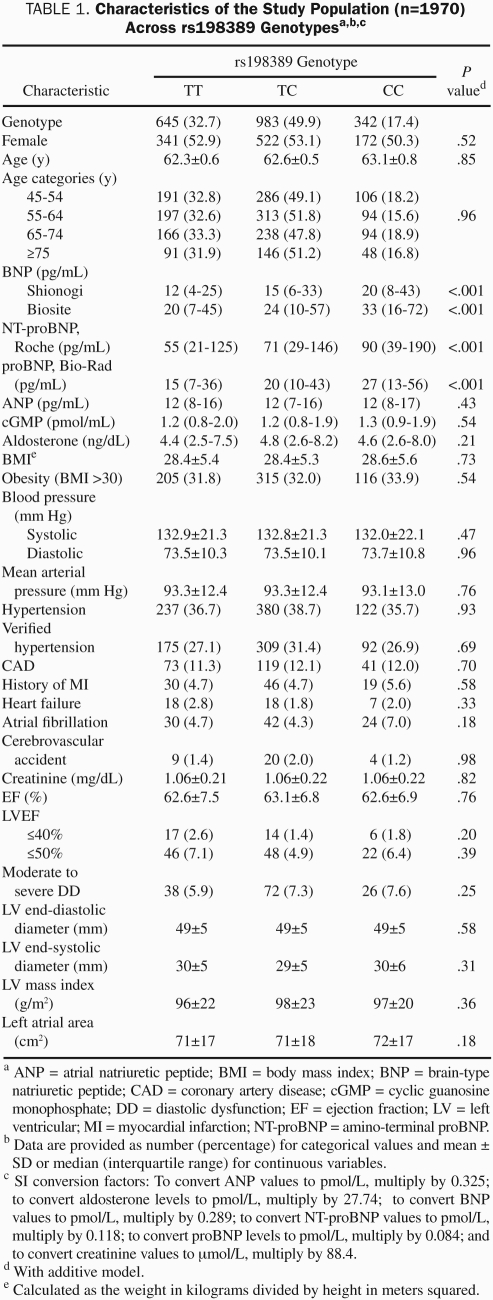
TABLE 1.
Characteristics of the Study Population (n=1970) Across rs198389 Genotypesa,b,c
FIGURE 1.
Overall survival in the population according to rs198389 genotype.
Effect of BNP Genotype on Immunoreactivity Measured by Commonly Used Assays
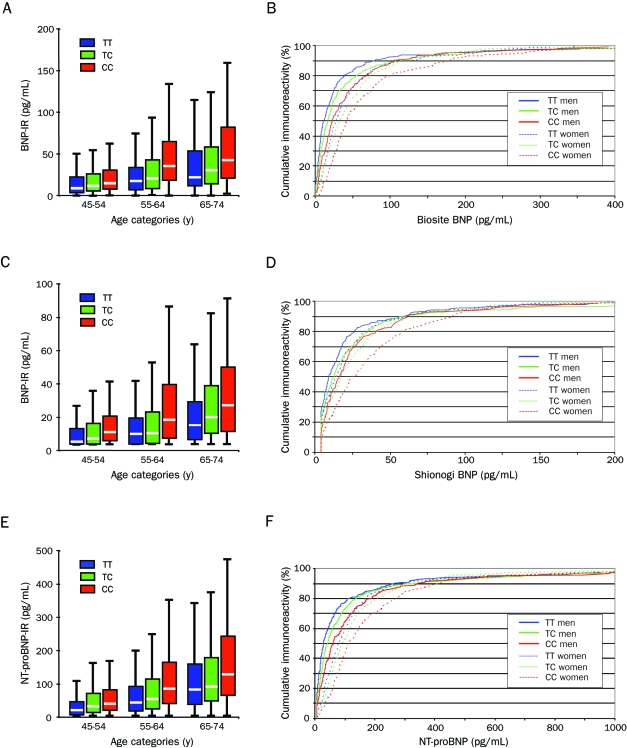
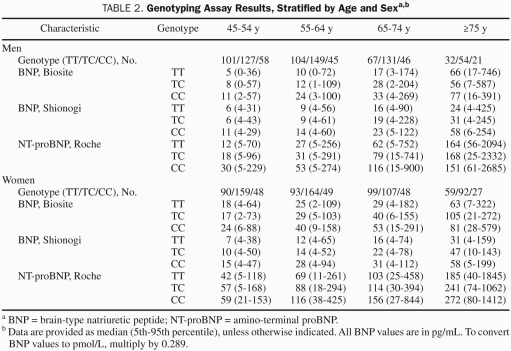
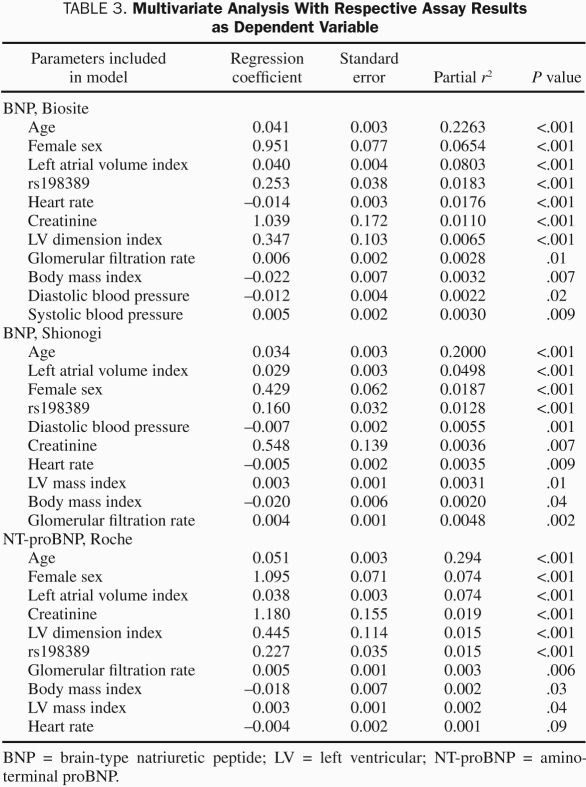
Figure 2 shows the distribution of BNP and NT-proBNP for genotypes in age categories as well as their cumulative frequencies separately for men and women. As can be seen, both genotype and sex affected BNP and NT-proBNP values, with female sex and every additional C allele increasing levels. Table 2 shows median and 5th and 95th percentiles of BNP for age and sex strata. In a multivariate analysis that included more parameters known to potentially affect BNP levels, rs198389 continued to be a significant determinant of BNP-IR (Table 3). In addition, plasma levels of proBNP1-108, the prohormone that is cleaved into BNP and NT-proBNP, increased with every additional C allele (Table 1). In contrast, plasma levels of ANP and cGMP, which is the second messenger of both ANP and BNP, did not vary by genotype, and the same was true for plasma aldosterone.
FIGURE 2.
Immunoreactivity according to genotype and age category and cumulative immunoreactivity according to genotype and sex for the Biosite BNP assay (A and B, respectively), the Shionogi BNP assay (C and D, respectively), and the Roche NT-proBNP assay (E and F, respectively). Please note the different scales. BNP = brain-type natriuretic peptide; IR = immunoreactivity; NT-proBNP = amino-terminal proBNP.
TABLE 2.
Genotyping Assay Results, Stratified by Age and Sexa,b
TABLE 3.
Multivariate Analysis With Respective Assay Results as Dependent Variable
Effect of BNP Genotype on Diagnostic Test Characteristics
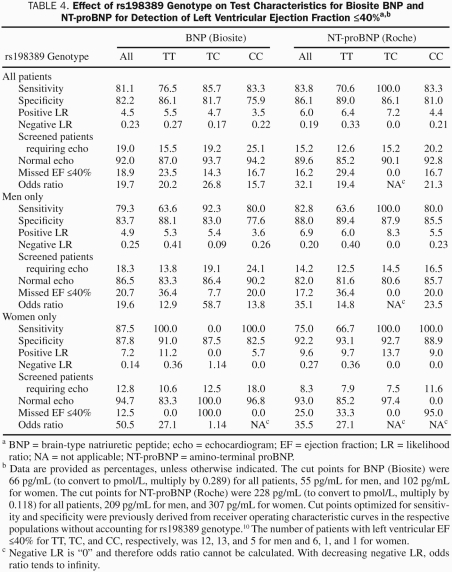
Table 4 shows the test characteristics for the detection of ejection fraction less than or equal to 40% for the Biosite BNP and NT-proBNP assays for individual genotypes for all patients combined, as well as for men and women separately, when cutoffs were derived from receiver operating characteristic curves generated without accounting for genotype.10 Sensitivity was generally lowest for the TT genotype and highest with the TC genotype, and the difference between the two could be up to 36.4%. As already discussed, given that the number of patients with normal ejection fraction was much higher than the number with reduced ejection fraction, specificity is derived from a much higher number than sensitivity, making its estimate more robust. As expected, specificity decreased consistently with increasing number of C alleles, being as much as 10.5% lower with the CC than with the TT genotype. The C allele was correspondingly associated with a higher percentage of patients needing an imaging study for verification and then having negative findings on that study. Given that the TC genotype represented the largest proportion of the total population and was associated with intermediate BNP levels, one would expect that the cut point, which was chosen on the basis of the maximization of sensitivity and specificity in the total population, would lead to the overall best test characteristics in the TC genotype. Indeed, in most cases the odds ratio was maximal in the TC genotype, except for women, in whom the low number of affected individuals makes the estimate the least robust.
TABLE 4.
Effect of rs198389 Genotype on Test Characteristics for Biosite BNP and NT-proBNP for Detection of Left Ventricular Ejection Fraction ≤40%a,b
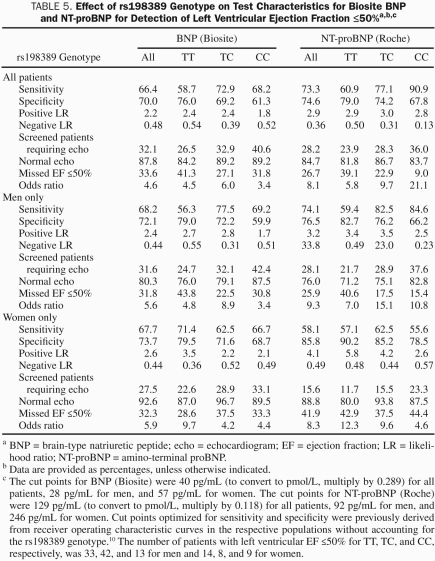
Table 5 shows the same test characteristics for the detection of an ejection fraction less than or equal to 50%, which demonstrated a pattern similar to that described for the detection of an ejection fraction less than or equal to 40%. However, as previously reported, test characteristics are, in general, worse for the detection of ejection fraction of 50% or less compared with 40% or less.10
TABLE 5.
Effect of rs198389 Genotype on Test Characteristics for Biosite BNP and NT-proBNP for Detection of Left Ventricular Ejection Fraction ≤50%a,b,c
DISCUSSION
Our study shows that rs198389 is common in the general adult population and that the C allele is associated with higher immunoreactivity of circulating BNP forms as measured by 3 widely used clinical assays. Despite this increase in this pleiotropic hormone, no differences in cardiovascular disease prevalence or overall survival were observed. Consequently, rs198389 can be expected to confound the association of BNP and NT-proBNP assays with cardiovascular disease and prognosis. Indeed, we demonstrate for 2 assays (Biosite BNP and Roche NT-proBNP) that genotype affects the test characteristics for the detection of LV systolic dysfunction in the general community and that consideration of genotype may improve interpretation of BNP assays.
We defined for the first time the prevalence of rs198389 in a random sample of the US general community population. Minor allele frequency (42.3%) was similar to frequencies reported in France, England, and Detroit but much higher than that in Japan (14.0%).16-18,22 We found that the C allele independently predicted higher BNP-IR and NT-proBNP immunoreactivity as measured by 3 commonly used assay systems. Therefore, in addition to age and sex, genotype is an important determinant of plasma BNP and NT-proBNP levels in the general community and explains some of the variability in plasma BNP concentrations that have been reported.23 It is interesting to note that this genetic elevation of BNP did not have any detectable effect on cardiovascular parameters. The natriuretic and vasodilating actions of BNP could have resulted in lower blood pressure and lower prevalence of hypertension. Its antihypertrophic and antifibrotic actions could have affected LV mass and dimensions. Thus, BNP levels may be elevated enough to affect assay interpretation but are insufficient to affect phenotype or are opposed by counter-regulatory mechanisms. Also, it is unknown to what degree the elevated BNP-IR represents bioactive forms of BNP such as BNP1-32. Indeed, proBNP1-108, the less bioactive prohormone of BNP, which is recognized by both BNP and NT-proBNP assays, was significantly elevated with every additional C allele.24 Of note, Newton-Cheh et al15 reported 2 SNPs (rs5068 and rs198358) at the NPPA/NPPB locus that were associated with increased levels of both ANP and BNP as well as decreased blood pressure and prevalence of hypertension. Interestingly, rs198389 in our study affected BNP but not ANP levels and did not affect plasma levels of cGMP, the second messenger of ANP and BNP. The relevance of this finding is currently unclear. It remains to be investigated whether rs198389 has a disease-modifying effect in clinical situations that are usually associated with higher BNP levels, such as HF. Indeed, given that BNP elevation in HF is considered both a compensatory mechanism and a marker of disease severity, BNP increases due to rs198389 could theoretically improve outcomes while paradoxically suggesting a poorer prognosis.
Given the association of BNP with increased cardiac stress, BNP assays have been evaluated for a number of clinical purposes, such as aiding in the diagnosis of HF in patients with dyspnea in the emergency department and screening populations for asymptomatic LV dysfunction. Epidemiological studies have shown that about 50% of patients with LV systolic dysfunction are asymptomatic and that these asymptomatic patients are at increased risk of developing overt HF.19,25,26 Importantly, patients with asymptomatic LV dysfunction benefit from treatment, which provides a powerful rationale to screen for this condition, and BNP has been evaluated for this purpose.6,7,9,10,27-30 Because rs198389 affected BNP levels but was not associated with cardiovascular morbidity and mortality or with phenotype, we hypothesized that rs198389 confounds test characteristics of BNP assays that could be used to identify LV dysfunction in the general community. We used genotype-unadjusted cut points to illustrate the effect of rs198389 on diagnostic test characteristics. As hypothesized, specificity consistently decreased with additional C alleles, whereas sensitivity was usually lowest with the TT genotype and highest with the TC genotype. As indicated, given the much higher number of patients with normal LV ejection fraction, our estimate of specificity is more robust. Also, the cumulative frequencies shown in Figure 2 imply that, even if other cut points were chosen, specificity would be affected in a substantial way over a wide range of possible cut points. Our results suggest that adjusting diagnostic cut points not only for age and sex but also for genotype improves the test characteristics for the detection of LV dysfunction, which could make screening for asymptomatic individuals more cost-effective.9 However, because of the limited number of patients in our study with reduced LV ejection fraction, we cannot provide a good estimate of the effect of rs198389 on sensitivity and related parameters.
Measurement of BNP or NT-proBNP is widely used in clinical practice to aid in the diagnosis of HF and increasingly to identify at-risk patients. Therefore, one could speculate that our findings may have implications for individualized medicine because genotype informs the interpretation of a biomarker. Genotyping for rs198389 could be done independently or as part of a broader genotyping strategy including other SNPs known to affect biomarker levels or disease susceptibility. Elevated BNP levels in the setting of acute decompensated HF are often dramatic, so the relatively small influence of genotype would likely not be a significant confounder in this setting. However, genotype-adjusted cut points may be useful in the setting of asymptomatic LV dysfunction or in compensated chronic LV dysfunction, in which some patients fall in a diagnostic gray zone. They might be of particular utility for studies that use BNP target levels to guide therapy.31-33 Also, a BNP level within the normal range for the general population but in the upper tertile or quartile of that normal range has been reported to be associated with increased mortality and risk of adverse cardiovascular outcomes.11,12 Adjusting cut points for genotype in this prognostic scenario may be helpful. Of note, genetic confounding has also been reported for other biomarkers, such as C-reactive protein and prostate-specific antigen.34,35
As previously mentioned, an important limitation of our study is that in our general population sample we have a limited number of patients with reduced LV ejection fraction. This precludes us from evaluating how the rs198389 genotype affects sensitivity. Another limitation is that our population sample was from Olmsted County, Minnesota, and almost exclusively white. The effect of rs198389 on BNP levels in other populations has been demonstrated, but the effect on diagnostic test characteristics in other populations may differ.16-18 It should also be noted that the realization that a biomarker is confounded by genetic variation does not necessarily decrease its utility when utility was established without considering genotype; however, accounting for genotype may improve its diagnostic or prognostic utility. Further studies are required to test this in the case of rs198389 and BNP assays.
CONCLUSION
We found that the C allele of the functional BNP rs198389 polymorphism was common in this general US community population and was associated with an increase in BNP-IR. To our knowledge, we demonstrated for the first time that the C allele altered diagnostic test characteristics. Given the widespread use of BNP as a diagnostic biomarker, these findings have potential implications for individualized medicine.
Footnotes
This study was funded by grants HL07111 (G.B.), HL76611, HL36634, and HL55502 from the National Institutes of Health.
REFERENCES
- 1. Saito Y, Nakao K, Itoh H, et al. Brain natriuretic peptide is a novel cardiac hormone. Biochem Biophys Res Commun. 1989;158(2)360-368 [DOI] [PubMed] [Google Scholar]
- 2. Kambayashi Y, Nakao K, Mukoyama M, et al. Isolation and sequence determination of human brain natriuretic peptide in human atrium. FEBS Lett. 1990;259(2)341-345 [DOI] [PubMed] [Google Scholar]
- 3. Mukoyama M, Nakao K, Hosoda K, et al. Brain natriuretic peptide as a novel cardiac hormone in humans: evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87(4)1402-1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3)161-167 [DOI] [PubMed] [Google Scholar]
- 5. Mueller C, Scholer A, Laule-Kilian K, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350(7):647-654 [DOI] [PubMed] [Google Scholar]
- 6. McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet. 1998;351(9095)9-13 [DOI] [PubMed] [Google Scholar]
- 7. Vasan RS, Benjamin EJ, Larson MG, et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288(10)1252-1259 [DOI] [PubMed] [Google Scholar]
- 8. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40(5)976-982 [DOI] [PubMed] [Google Scholar]
- 9. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: a community-based study. Circulation. 2004;109(25)3176-3181 [DOI] [PubMed] [Google Scholar]
- 10. Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47(2)345-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655-663 [DOI] [PubMed] [Google Scholar]
- 12. McKie PM, Rodeheffer RJ, Cataliotti A, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide: biomarkers for mortality in a large community-based cohort free of heart failure. Hypertension. 2006;47(5)874-880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol. 2002;90(3)254-258 [DOI] [PubMed] [Google Scholar]
- 14. Arden KC, Viars CS, Weiss S, Argentin S, Nemer M. Localization of the human B-type natriuretic peptide precursor (NPPB) gene to chromosome 1p36. Genomics. 1995;26(2)385-389 [DOI] [PubMed] [Google Scholar]
- 15. Newton-Cheh C, Larson MG, Vasan RS, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41(3)348-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meirhaeghe A, Sandhu MS, McCarthy MI, et al. Association between the T-381C polymorphism of the brain natriuretic peptide gene and risk of type 2 diabetes in human populations. Hum Mol Genet. 2007;16(11)1343-1350 [DOI] [PubMed] [Google Scholar]
- 17. Takeishi Y, Toriyama S, Takabatake N, et al. Linkage disequilibrium analyses of natriuretic peptide precursor B locus reveal risk haplotype conferring high plasma BNP levels. Biochem Biophys Res Commun. 2007;362(2):480-484 [DOI] [PubMed] [Google Scholar]
- 18. Lanfear DE, Stolker JM, Marsh S, Rich MW, McLeod HL. Genetic variation in the B-type natiuretic peptide pathway affects BNP levels. Cardiovasc Drugs Ther. 2007;21(1)55-62 [DOI] [PubMed] [Google Scholar]
- 19. Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2)194-202 [DOI] [PubMed] [Google Scholar]
- 20. Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115(12)1563-1570 [DOI] [PubMed] [Google Scholar]
- 21. Giuliani I, Rieunier F, Larue C, et al. Assay for measurement of intact B-type natriuretic peptide prohormone in blood. Clin Chem. 2006;52(6):1054-1061 [DOI] [PubMed] [Google Scholar]
- 22. Kajita M, Ezura Y, Iwasaki H, et al. Association of the -381T/C promoter variation of the brain natriuretic peptide gene with low bone-mineral density and rapid postmenopausal bone loss. J Hum Genet. 2003;48(2)77-81 [DOI] [PubMed] [Google Scholar]
- 23. Wang TJ, Larson MG, Levy D, et al. Heritability and genetic linkage of plasma natriuretic peptide levels. Circulation. 2003;108(1)13-16 [DOI] [PubMed] [Google Scholar]
- 24. Heublein DM, Huntley BK, Boerrigter G, et al. Immunoreactivity and guanosine 3′,5′-cyclic monophosphate activating actions of various molecular forms of human B-type natriuretic peptide. Hypertension. 2007;49(5):1114-1119 [DOI] [PubMed] [Google Scholar]
- 25. McDonagh TA, Morrison CE, Lawrence A, et al. Symptomatic and asymptomatic left-ventricular systolic dysfunction in an urban population. Lancet. 1997;350(9081)829-833 [DOI] [PubMed] [Google Scholar]
- 26. Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108(8)977-982 [DOI] [PubMed] [Google Scholar]
- 27. Hedberg P, Lonnberg I, Jonason T, Nilsson G, Pehrsson K, Ringqvist I. Electrocardiogram and B-type natriuretic peptide as screening tools for left ventricular systolic dysfunction in a population-based sample of 75-year-old men and women. Am Heart J. 2004;148(3)524-529 [DOI] [PubMed] [Google Scholar]
- 28. Clerico A, Fontana M, Zyw L, Passino C, Emdin M. Comparison of the diagnostic accuracy of brain natriuretic peptide (BNP) and the N-terminal part of the propeptide of BNP immunoassays in chronic and acute heart failure: a systematic review. Clin Chem. 2007;53(5)813-822 [DOI] [PubMed] [Google Scholar]
- 29. Ewald B, Ewald D, Thakkinstian A, Attia J. Meta-analysis of B type natriuretic peptide and N-terminal pro B natriuretic peptide in the diagnosis of clinical heart failure and population screening for left ventricular systolic dysfunction. Intern Med J. 2008;38(2)101-113 [DOI] [PubMed] [Google Scholar]
- 30. Battaglia M, Pewsner D, Juni P, Egger M, Bucher HC, Bachmann LM. Accuracy of B-type natriuretic peptide tests to exclude congestive heart failure: systematic review of test accuracy studies. Arch Intern Med. 2006;166(10):1073-1080 [DOI] [PubMed] [Google Scholar]
- 31. Jourdain P, Jondeau G, Funck F, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007;49(16)1733-1739 [DOI] [PubMed] [Google Scholar]
- 32. Pfisterer M, Buser P, Rickli H, et al. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA. 2009;301(4)383-392 [DOI] [PubMed] [Google Scholar]
- 33. Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro-B-type natriuretic peptide-guided treatment for chronic heart failure: results from the BATTLESCARRED (NT-proBNP-Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol. 2009;55(1)53-60 [DOI] [PubMed] [Google Scholar]
- 34. Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359(18)1897-1908 [DOI] [PubMed] [Google Scholar]
- 35. Gudmundsson J, Besenbacher S, Sulem P, et al. Genetic correction of PSA values using sequence variants associated with PSA yevels. Sci Transl Med. 2010;2(62):62ra92 [DOI] [PMC free article] [PubMed] [Google Scholar]