Abstract
The development of antimicrobial resistance among gram-negative pathogens has been progressive and relentless. Pathogens of particular concern include extended-spectrum β-lactamase–producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa. Classic agents used to treat these pathogens have become outdated. Of the few new drugs available, many have already become targets for bacterial mechanisms of resistance. This review describes the current approach to infections due to these resistant organisms and elaborates on the available treatment options.
ESBL = extended-spectrum β-lactamase; IV = intravenously; KPC = Klebsiella pneumoniae carbapenemase; MDR = multidrug-resistant; MIC = minimum inhibitory concentration
Antimicrobial resistance has been shaping the field of infectious diseases since the discovery of penicillin. Many of the advances in antimicrobial drug development have resulted from efforts to combat ever-evolving mechanisms of resistance that render existing agents obsolete, thus prompting the search for new molecules that promise to be more effective and more resilient. Yet, the hope for a magic all-encompassing antimicrobial agent has long passed, and the number of new antimicrobial agents in the drug development pipeline is a small fraction of what it used to be.
Nowhere is the concept of antimicrobial resistance better portrayed than with the gram-negative bacilli, which have proven to be tough adversaries for clinicians and researchers alike. Of the 6 famous ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter species, Pseudomonas aeruginosa, and Enterobacter species) recognized as the most important emerging threats of this century, 4 are gram-negative bacilli (K pneumoniae, Acinetobacter species, P aeruginosa, and Enterobacter species).1 This review will address 3 major types of multidrug-resistant (MDR) gram-negative pathogens: extended-spectrum β-lactamase (ESBL)–producing Enterobacteriaceae, carbapenemase-producing Enterobacteriaceae, and MDR P aeruginosa. The resistance mechanisms exhibited by these organisms and the epidemiology of the infections they cause will be discussed. Existing and emerging therapeutic approaches to each type of organism will then be surveyed.
MECHANISMS OF RESISTANCE
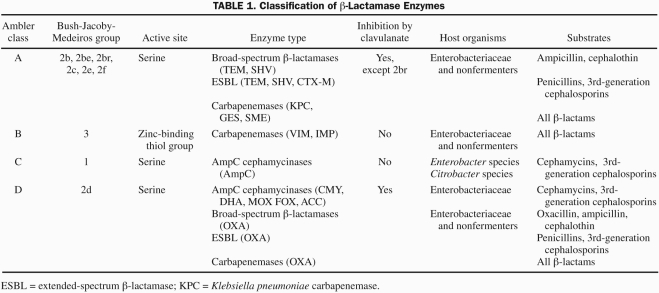
Production of β-lactamase is the most commonly encountered mechanism of resistance of bacterial pathogens to β-lactam antibiotics. Many enzymes have been described, encoded either by chromosomal genes or by genes located on movable elements such as plasmids and transposons. Classification schemes for β-lactamases are based on molecular structure (Ambler classification)2 or functional similarities (Bush-Jacoby-Medeiros classification)3 (Table 1). Extended-spectrum β-lactamase enzymes initially arose through point mutations in the genes encoding the classic TEM and SHV β-lactamases, thereby generating an array of enzymes with an expanded spectrum of activity.4 The potent hydrolytic activity of CTX-M enzymes against cefotaxime was later recognized. Unlike TEM, SHV, and CTX-M ESBL enzymes that are predominantly expressed by Enterobacteriaceae, the oxacillin-hydrolyzing enzymes have been mostly isolated from P aeruginosa, and some have evolved to exhibit the ESBL phenotype. In contrast to the plasmid-mediated ESBL enzymes, AmpC β-lactamases are predominantly chromosomally encoded.5 Their expression is mostly noted in Enterobacter species, Citrobacter species, and P aeruginosa. Although chromosomal AmpC enzymes are usually poorly expressed in Escherichia coli and Klebsiella species, plasmid-mediated AmpC enzymes can confer β-lactam resistance similar to Enterobacter isolates. Other less commonly encountered ESBL enzymes include PER-1, VEB-1, and BES-1.6
TABLE 1.
Classification of β-Lactamase Enzymes
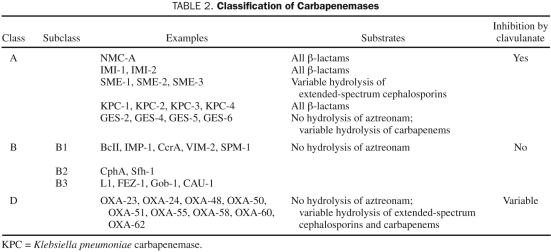
Carbapenemases are the β-lactamases with the widest spectrum of activity. In addition to hydrolyzing carbapenems, carbapenemases are active against most other members of the β-lactam family with few exceptions. The major drive behind the emergence of carbapenemases has been the widespread use of carbapenems both in the empirical and directed treatment of serious infections, which placed selection pressure on bacterial pathogens. On the basis of their molecular structure, carbapenemases belong to the A, B, or D classes of β-lactamase enzymes7 (Table 2). The plasmid-borne K pneumoniae carbapenemases (KPCs) are currently among the most prevalent and widely distributed carbapenemases. They are particularly difficult to detect by microbiology laboratories because many isolates have minimum inhibitory concentrations (MICs) against imipenem or meropenem that, albeit high, remain in the susceptible range.8,9 It has been observed through in vitro studies that ertapenem may be the most appropriate substrate for detection of KPC production.8 Other clinically important carbapenemases include the metallo-β-lactamases and the oxacillin-hydrolyzing carbapenemases. Besides β-lactamase production, P aeruginosa isolates can exhibit additional resistance mechanisms, such as aminoglycoside-modifying enzymes, efflux pumps, porin loss, and various target site modifications.10
TABLE 2.
Classification of Carbapenemases
EPIDEMIOLOGY
The medical literature abounds with studies illustrating the global increase in the burden of antimicrobial resistance among gram-negative pathogens.11,12 However, wide regional differences exist, accentuating the need to take into account the local epidemiology (at the level of the country, the region, the hospital, and at times the individual hospital unit) when making decisions about empirical therapy for serious infections.
The Study for Monitoring Antimicrobial Resistance Trends collected 6156 gram-negative isolates from patients with intra-abdominal infections in 28 countries during 2004. The overall rate of ESBL production was 17% among K pneumoniae and 10% among E coli isolates, with the highest rates being in isolates from Latin America, the Middle East, Africa, and Asia and the lowest being in Europe and the United States.13 These results were confirmed by the Tigecycline Evaluation and Surveillance Trial global surveillance database in 2007.14 Most notable in the epidemiology of ESBL-producing organisms is the recent worldwide dissemination of CTX-M–type β-lactamases,15 particularly the CTX-M-15 enzyme.16 In a recent multinational study, CTX-M enzymes were the most frequently identified ESBLs, accounting for 65% of all β-lactamases.17
Although chromosomally mediated carbapenemases have long been recognized in gram-negative bacilli, they were mostly species-specific with a limited potential for spread except in a clonal manner.7 Recent trends, however, have refocused attention on plasmid-mediated carbapenemases such as KPCs. Since the first report from North Carolina in the late 1990s,18 a multitude of studies have described the relatively rapid emergence of KPC enzymes.19 In addition to certain regions of the United States, hospital outbreaks due to KPC-bearing gram-negative pathogens have been reported from Europe,20 Asia,21 and South America.22 Other carbapenemases that have been associated with recent outbreaks include IMP and VIM metallo-β-lactamases.7 In addition, 2009 witnessed the emergence of the New Delhi metallo-β-lactamase in Enterobacteriaceae,23 which led to the hospitalization of many patients in India and Pakistan.
THERAPEUTIC APPROACHES
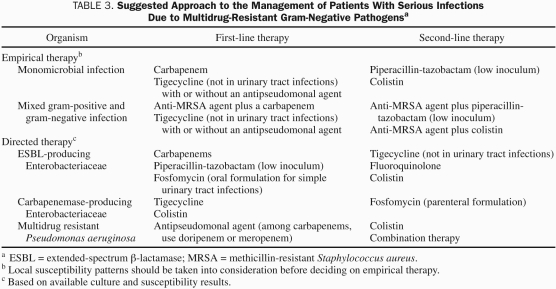
A summary of therapeutic approaches and challenges for 3 of the emerging gram-negative organisms of most concern follows (see also Table 3), including an inventory of existing antibiotic options for each organism.
TABLE 3.
Suggested Approach to the Management of Patients With Serious Infections Due to Multidrug-Resistant Gram-Negative Pathogensa
Extended-Spectrum β-Lactamase–Producing Enterobacteriaceae
The propensity of ESBL-producing organisms to be concomitantly resistant to other classes of antibiotics greatly limits the choice of antibiotics that can be used for treatment.24 The genes encoding for ESBL enzymes are located on large plasmids that can harbor resistance genes to fluoroquinolones, aminoglycosides, and trimethoprim-sulfamethoxazole.
Infections with ESBL-producing pathogens are usually suspected in patients who have recently received broad-spectrum antibiotics, particularly third-generation cephalosporins and quinolones.6 Other risk factors include age older than 60 years, comorbid conditions, recent hospital and intensive care unit admission, and invasive devices.
Antibiotic Options. Carbapenems. Carbapenems are considered first-line agents in treating infections caused by ESBL-producing organisms (imipenem at 500 mg intravenously [IV] every 6 hours up to 1 g IV every 8 hours in serious infections or meropenem at 1 g IV every 8 hours). However, no data from randomized controlled trials support their use for this purpose. Most of the evidence instead originates from case series and retrospective studies, which compile the responses and outcomes of patients with bacteremia receiving carbapenem therapy.25 In a multinational study of 85 patients with ESBL-producing K pneumoniae bacteremia, carbapenem use was an independent predictor of lower mortality rate compared with the use of other antibiotic agents that exhibited in vitro activity.26 This therapeutic advantage of carbapenems has been attributed to the high inoculum effect as well as high MICs of other agents that are close to the susceptibility breakpoints. More recent data have shown that ertapenem at 1 g/d may be used successfully for ESBL-associated bacteremia.27 When dosed at 500 mg IV every 8 hours, doripenem, the newest addition to the carbapenem class, exhibits an activity against ESBL-producing pathogens that is similar to that of imipenem and meropenem.28
Tigecycline. Tigecycline, the first member of the glycylcycline class of antibiotics, is approved by the US Food and Drug Administration for the treatment of complicated skin and skin structure infections, complicated intra-abdominal infections, and community-acquired pneumonia when appropriately dosed (100-mg loading dose IV followed by 50 mg IV every 12 hours). Tigecycline is active in vitro against Enterobacteriaceae, including ESBL-producing isolates.29 Clinical data, although promising, are still limited. Despite its excellent activity, one of the factors hindering the wider use of tigecycline for ESBL-associated infections is the fact that a large proportion of these infections are in the urinary tract, where tigecycline has a limited penetration.30 Although some case reports have reported a favorable outcome, tigecycline is not a suitable choice for the treatment of urinary tract infections. In addition, because of its rapid tissue distribution after intravenous infusion, concerns have been raised about using tigecycline for the treatment of primary bloodstream infections.30 In a recently published comparative study of tigecycline vs imipenemcilastatin in patients with hospital-acquired pneumonia, tigecycline fared worse in the subset of patients with ventilator-associated pneumonia.31 It is our opinion that dose escalation needs to be considered if tigecycline is used for ventilator-associated pneumonia with MDR organisms other than Pseudomonas species.
β-Lactam/β-Lactamase Inhibitor Combinations. Classic β-lactam inhibitors, such as sulbactam, clavulanate, and tazobactam, have variable inhibitory activity against ESBL enzymes12 (Table 1). Tazobactam, which appears to be the most potent of the 3, is active against some of the TEM, SHV, and CTX-M enzymes.32 In a Spanish study from 11 centers, the cure rate of patients with cystitis treated with amoxicillin-clavulanate was 93% with susceptible ESBL-producing isolates and 56% with isolates of intermediate susceptibility and resistance, suggesting that amoxicillin-clavulanate may be successful in the treatment of simple cystitis.33 Clinical data supporting the use of piperacillin-tazobactam are mounting.34 Because it achieves high concentrations in the urinary tract, piperacillin-tazobactam may be used successfully in the treatment of urinary tract infections35 and in other infections in which a low bacterial inoculum is expected.36 In a series of patients with infections due to ESBL-producing organisms, piperacillin-tazobactam was used successfully against susceptible isolates originating from the urinary tract as well as other sites.35 Although it was initially thought that piperacillin-tazobactam should be avoided in serious infections such as bacteremias, this notion is being challenged by emerging evidence showing that the use of piperacillin-tazobactam against susceptible isolates often results in a favorable outcome.37
Cephalosporins. Studies suggest that the use of cephalosporins, including cephamycins and cefepime, is associated with a worse outcome compared with the use of carbapenems, despite apparent in vitro susceptibility.38 Cephalosporins are therefore not recommended in patients with suspected or confirmed infections with ESBL-producing organisms.
Aminoglycosides, Fluoroquinolones, and Trimethoprim-Sulfamethoxazole. The high rates of concurrent resistance to these agents and the potential for emergence of resistance on treatment often preclude their use for empirical coverage. Some studies have observed a suboptimal response to quinolones vs carbapenems in ESBL-producing isolates with retained susceptibility to quinolones.25 Aminoglycosides, fluoroquinolones, and trimethoprim-sulfamethoxazole should be used with caution in serious infections even after documentation of in vitro activity. Clinical response should be closely monitored, and switching to carbapenems should be considered in patients who do not improve.
Colistin. Colistin has been successfully used to treat ESBL-associated infections in a few case reports.39,40 In the absence of formally adopted Clinical and Laboratory Standards Institute breakpoints for susceptibility of colistin against Enterobacteriaceae, susceptibility testing by E-test and disk diffusion methods has been proposed recently.41 Colistin use is discussed in more detail in the “Multidrug-Resistant P aeruginosa” section.
Fosfomycin. Fosfomycin inhibits bacterial cell wall synthesis, thereby exhibiting bactericidal activity against gram-positive and gram-negative pathogens.42 Fosfomycin is approved by the US Food and Drug Administration for the treatment of uncomplicated urinary tract infections at a single oral dose of 3 g. The emergence of resistance among gram-negative bacilli has sparked new interest in using fosfomycin to treat infections caused by MDR isolates. In vitro studies have shown that fosfomycin remains active against ESBL-producing E coli and K pneumoniae isolates.43 The drug appears to be useful in the oral treatment of ESBL-associated infections of the urinary tract, and initial clinical studies are promising.44 An intravenous formulation of fosfomycin, currently available in some European countries, could be useful in treating systemic infections.45
Other Agents. Also active in vitro against ESBL-producing organisms are the β-lactams temocillin46 and pivmecillinam,47 the carbapenems biapenem,48 faropenem,49 and tomopenem,50 and the urinary tract agent nitrofurantoin.51 More data are needed to support their use in the clinical setting.
Carbapenem-Resistant Enterobacteriaceae
Recognizing carbapenemase expression is the key to the appropriate management of infections caused by carbapenem-resistant Enterobacteriaceae. Unusually elevated MICs to carbapenems should arouse suspicion for a carbapenem-resistant isolate and preclude the use of carbapenems even if the MICs do not exceed the breakpoints for resistance. As with ESBL-producing organisms, carbapenemase-producing strains are likely to exhibit simultaneous resistance to aminoglycosides and fluoroquinolones.52
Antibiotic Options. Tigecycline. Isolates may show in vitro susceptibility to tigecycline,53 but clinical experience with carbapenem-resistant strains is limited. A recent review by Hirsch and Tam54 gathered data from 15 publications on the treatment of 55 patients with KPC-related infections. A favorable outcome was achieved in 5 of 7 patients treated with tigecycline. Despite tigecycline being one of the first-line agents for use in the setting of carbapenemase-producing isolates, it is worth noting that clinical failures have been reported in the literature, as exemplified by a brief report by Anthony et al,55 in which some patients with MDR gram-negative pathogens, including ESBL- and KPC-producing isolates, had a negative clinical and/or microbiological outcome with tigecycline. In addition, as discussed previously, primary bloodstream infections and urinary tract infections present a challenge for the use of tigecycline.
Colistin. Although colistin retained activity against carbapenemase-producing Enterobacteriaceae in initial studies,56 more recent data suggest that resistance to colistin is emerging, and outbreaks of colistin-resistant strains have been reported.57 In the review by Hirsch and Tam,54 monotherapy with polymyxins (n =7) was associated with poor response rates, whereas combination therapy (n= 11) gave more promising results.
Fosfomycin. The activity of fosfomycin was evaluated against 68 KPC-producing K pneumoniae isolates, 23 of which were nonsusceptible to tigecycline and/or colistin.58 The susceptibility rates were 93% for the overall group, 87% for the group nonsusceptible to tigecycline and/or colistin, and 83% (5 of 6 isolates) for the extremely drug-resistant subgroup that was nonsusceptible to tigecycline and/or colistin. Clinical correlation of this in vitro study is needed.
Rifampin. In vitro studies suggest that rifampin has a synergistic activity when used as part of a combination therapy regimen against carbapenemase-producing E coli and K pneumoniae.59 More clinical data are needed.
Agents Under Development. Agents under development include new β-lactamase inhibitors with activity against carbapenemases, such as MK-7655,60 NXL104,61 and 6-alkylidenepenam sulfones,62 and several bis-indole compounds,63 the mode of action of which is currently unidentified.
Multidrug-Resistant P aeruginosa
For the purpose of this review, we define MDR P aeruginosa as strains that are resistant to 2 or more classes of antibiotics. In recent years, the treatment of infections caused by P aeruginosa has become a challenging task for clinicians. The emergence of antimicrobial resistance has played a pivotal role in determining the approach to patients with Pseudomonas species infections. Central to this approach is the recognition that delayed therapy correlates with increased mortality even when a patient is considered clinically stable at the time of initial evaluation.64 Because the treatment of serious P aeruginosa infections is frequently empirical until the organism is isolated and susceptibility testing performed, high resistance rates raise the likelihood of administering inappropriate initial therapy, hence contributing to the observed high mortality rates.
Antibiotic Options. When used in the appropriate dosage, the following agents have shown reliable activity against Pseudomonas isolates.
Antipseudomonal Penicillins. Ticarcillin should be dosed at 3 g IV every 4 hours and piperacillin at 4 g IV every 4 hours.
β-Lactam/β-Lactamase Inhibitor Combinations. Ticarcillin-clavulanate should be dosed at 3 g of ticarcillin and 0.1 g of clavulanic acid IV every 4 hours, and piperacillin-tazobactam should be dosed at 4 g of piperacillin and 0.5 g of tazobactam IV every 6 hours or 3 g of piperacillin and 0.375 g of tazobactam IV every 4 hours.
Cephalosporins. Ceftazidime should be dosed at 2 g IV every 8 hours and cefepime at 2 g IV every 8 hours. Because of their good activity and narrow spectrum compared with carbapenems, cephalosporins are still considered treatments of choice if the isolate is susceptible.
Monobactams. Aztreonam should be dosed at 2 g IV every 8 hours. P aeruginosa isolates that produce metallo-β-lactamases may be susceptible to aztreonam, which demonstrates resistance to hydrolysis by class B β-lactamases65 (Table 2). Clinical correlation is needed.
Carbapenems. Imipenem should be dosed at 500 mg IV every 6 hours up to 1 g every 8 hours for serious infections, meropenem at 1 g IV every 8 hours, and doripenem at 500 mg IV every 8 hours. The various carbapenems have different levels of activity against Pseudomonas isolates. In vitro studies have shown that MICs were lowest with doripenem, followed by meropenem, then imipenem.66,67 However, doripenem, like other carbapenems, has minimal activity against metallo-β-lactamase–producing P aeruginosa strains.68 In contrast, imipenem has been associated with a higher risk of selecting for resistant Pseudomonas isolates compared with other carbapenems. Whether these in vitro differences among carbapenems translate into clinical outcome differences has not yet been determined. Carbapenems are usually used in the empirical treatment of suspected Pseudomonas species infections or when a polymicrobial infection is considered a possibility. In view of their broad spectrum of activity and the inherent risk of selecting for MDR organisms including P aeruginosa and Acinetobacter species, antibiotic therapy should be de-escalated when possible based on culture results.
Fluoroquinolones. Ciprofloxacin should be dosed at 400 mg IV every 8 hours or 750 mg orally every 12 hours, and levofloxacin should be dosed at 750 mg orally or IV daily. Although both ciprofloxacin and levofloxacin are active against P aeruginosa, levofloxacin use might be associated with a higher risk of isolation of quinolone-resistant P aeruginosa than ciprofloxacin.69
Colistin. Colistin base should be dosed daily at 2.5 to 5.0 mg/kg intramuscularly or IV in 2 to 4 divided doses. The increasing rates of MDR Pseudomonas isolates have prompted clinicians to turn to agents such as the polymyxins that had for a while fallen out of use due to their adverse effect profile.70 Studies have shown that, despite the risk for nephrotoxicity in patients receiving colistin, this drug may be useful as salvage therapy when therapeutic choices are seriously limited.71 More recently, compiled data seem to indicate that colistin use is associated with a lower than expected incidence of nephrotoxicity.72 This may be due to better fluid management and critical care services. Nonetheless, renal function should be well monitored during therapy and dose adjustment should be performed in patients with reduced creatinine clearance as follows: serum creatinine of 1.3 to 1.5 mg/dL, 2.5 to 3.8 mg/kg IV daily; serum creatinine of 1.6 to 2.5 mg/dL, 2.5 mg/kg IV daily; and serum creatinine of 2.6 to 4.0 mg/dL, 1.5 mg/kg IV daily.
It should be noted, however, that the ideal dose of colistin has not been evaluated in randomized clinical trials.73 In a recent retrospective analysis of 258 episodes of MDR gram-negative infections, 68 of which were caused by P aeruginosa, higher daily doses of colistin were independently associated with better survival regardless of the pathogen.74 The average daily dose of colistin that was used was 480±200 mg IV. The nephrotoxicity rate in this series was 10% and was independent of the dose used.
Other Antimicrobial Agents. Other antimicrobial agents possess activity against P aeruginosa but are generally not recommended as monotherapy because of their high propensity to induce resistance. Hence, they are mostly used in combination with other antipseudomonal agents, such as aminoglycosides (amikacin at 5.0-7.5 mg/kg of ideal body weight IV every 8 hours, gentamicin and tobramycin at 1.0-2.5 mg/kg of ideal body weight IV every 8-12 hours) and rifampin (at 600 mg orally or IV once daily, particularly in cases of P aeruginosa bacteremia refractory to standard treatment).75
Combination Therapy. The use of combination therapy in Pseudomonas species infections has been a controversial issue among specialists in infectious diseases. Whereas counterarguments include the additional costs and increased risk of adverse effects inherent in the concurrent use of multiple agents, proponents of combination therapy cite the potential for synergistic efficacy as well as the potential benefit of reducing the risk of emergence of resistance. Another rationale is to ensure an initial broad spectrum of activity when the risk of MDR isolates is high by using drugs with different mechanisms of action and/or resistance.
The results of clinical studies on the value of combination therapy in the treatment of P aeruginosa have been conflicting. Although older studies showed that combination therapy was more effective at reducing mortality rates in patients with Pseudomonas bacteremia than monotherapy,76 these results could not be corroborated by other authors.77 At least 2 meta-analyses have been published without resolving the question of whether the benefits of combination therapy outweigh the risks. The first meta-analysis evaluated 64 randomized trials comparing β-lactam monotherapy with combination therapy (a β-lactam and an aminolycoside) in more than 7500 immunocompetent patients with severe infections, 426 of whom were infected with P aeruginosa.78 Combination therapy offered no survival advantage but was associated with a higher risk of nephrotoxicity than was monotherapy. A second meta-analysis evaluated 17 studies, only 2 of which were randomized trials, in patients with gram-negative bacteremia.79 Mortality rates were significantly reduced in the P aeruginosa subgroup but not in the overall population.
Data from in vitro studies and clinical trials regarding the prevention of resistance emergence during treatment of P aeruginosa infections with combination therapy are scarce and inconclusive.80 For example, one study suggested that the addition of levofloxacin to imipenem might hamper the emergence of resistance.81 In another study, addition of an aminoglycoside to various β-lactam antibiotics did not alter the risk of selection for resistant isolates.82
The most used drug combination for Pseudomonas species infections is an aminoglycoside with a β-lactam.83 In a more recent study, the checkerboard technique was used to test for synergistic activity of various combinations of anti-pseudomonal agents (ceftazidime-tobramycin, piperacillin-tazobactam-tobramycin, imipenem-tobramycin, imipenem-isepamycin, imipenem-ciprofloxacin, and ciprofloxacin-tobramycin).84 Ceftazidime-tobramycin and piperacillin-tazobactam-tobramycin combinations were associated with the highest ratios of synergy. Antagonism was not observed in any of the combinations. In addition, on the basis of in vitro findings, the following drug combinations have been found to provide enhanced activity against highly resistant P aeruginosa: a fluoroquinolone with either ceftazidime or cefepime,85 ticarcillin with tobramycin and rifampin,86 polymyxin B with rifampin,87 ceftazidime with colistin,88 clarithromycin with tobramycin,89 and colistin with rifampin.90
These novel combinations are not meant for routine use and should be restricted to the treatment of MDR isolates because they include agents that, when used alone, may be inactive or unreliable for the treatment of Pseudomonas species infections. Clinical data to support the use of these regimens are not yet available. In addition to the checker-board technique, the E-test is another useful tool for determining MICs and testing antimicrobial combinations that can provide clinicians with potential treatment options. A novel parameter, the susceptible breakpoint index, allows ranking of the antimicrobial combinations by order of expected activity.91
Empirical therapy with 2 antipseudomonal agents may be considered when the perceived risk of antimicrobial resistance is substantial or in the setting of neutropenic fever, severe sepsis or septic shock, or serious infections such as pneumonia, endocarditis, and meningitis. Once susceptibility results become available, treatment with 1 active agent is acceptable.
Inhaled Antibiotics. Intermittent aerosolization of antibiotics into the respiratory tract has been used in patients with P aeruginosa pneumonia, particularly in the setting of cystic fibrosis. This mode of delivery is used to attain high drug levels locally in the respiratory tract without increasing systemic adverse effects. Several agents have been used as inhaled therapy, including tobramycin, colistin, and β-lactams.
Tobramycin is the inhaled antibiotic that has been the most widely used in the treatment of P aeruginosa pneumonia. The supporting evidence comes from studies that showed increased bacterial eradication with inhaled tobramycin.92,93 However, clinical outcomes were not always consistent in different patient populations. For example, in one study, inhaled tobramycin was associated with improved pulmonary function and with weight gain in adolescent patients with cystic fibrosis during a 2-year period of long-term, intermittent use.94 In contrast, the overall clinical outcome in intubated adult patients with gram-negative pneumonia did not change with inhaled tobramycin administration despite confirmed bacterial eradication.92
Inhaled colistin has also been used successfully in the management of MDR P aeruginosa pneumonia that does not improve with IV administered therapy. In one study from Singapore, nebulized colistin was used alone in the treatment of 21 patients with pneumonia due to MDR Acinetobacter baumannii and P aeruginosa.95 Overall clinical and microbiological response rates were 57% and 86%, respectively, and nephrotoxicity was not observed.
Despite these results, more data on clinical efficacy are needed, specifically regarding patient outcomes. At this point, the routine use of inhaled antibiotics is not recommended for P aeruginosa pneumonia.
Agents Under Development. A number of antimicrobial agents with antipseudomonal activity are currently in various phases of development. However, clinical data regarding efficacy are still lacking.
Drugs in Phase 2 Trials. The following drugs are currently in phase 2 trials: sitafloxacin (a quinolone with better activity against gyrA or parC mutants than ciprofloxacin),96 KB001 (a high-affinity antibody fragment that reduces the toxicity and pathogenicity of P aeruginosa),97 CXA-101 (a novel cephalosporin with potent activity against MDR strains),98 and ceftazidime/NXL104 (a cephalosporin/β-lactamase inhibitor combination meant to restore the in vitro activity of ceftazidime against class A, C, and some class D β-lactamase–producing strains).99
Drugs in Phase 1 Trials. BLI-489/piperacillin (another β-lactamase inhibitor combination)100 and CB-182,804 (a lipopeptide with apparent bactericidal activity against MDR strains) are currently in phase 1 trials (more information on CB-182,804 available at http://www.cubist.com/products/gram-negative.php).
Experimental Agents. These agents have not undergone any clinical trials and include new β-lactams, new β-lactamase inhibitors, peptides, efflux inhibitors, and virulence modulators.101
Extended-Infusion Strategy for β-Lactams
Because the killing activity of β-lactams is time-dependent, a positive correlation exists between their efficacy and the amount of time the drug concentration exceeds the MIC value during the dosing interval. To optimize dosing strategies to achieve better bacterial killing, studies have evaluated the role of administering β-lactams in extended infusions with encouraging results. Lodise et al102 compared the outcome of patients with P aeruginosa infections treated with piperacillin-tazobactam in 2 dosage regimens (3.375 g IV for 30 minutes every 4-6 hours vs 3.375 g IV for 4 hours every 8 hours). Patients with Acute Physiology And Chronic Health Evaluation (APACHE) II scores of 17 or greater who received extended-infusion therapy had lower mortality rates (12.2% vs. 31.6%; P=.04) and shorter hospital stays compared with those who received intermittent-infusion therapy (21 days vs 38 days; P=.02). More recently, 3 immunocompromised patients with MDR P aeruginosa infections were treated successfully with continuous infusions of β-lactam antibiotics (ceftazidime in 2 patients and aztreonam in the third patient).103
Carbapenems have also been evaluated in extended-infusion regimens. Using a Monte Carlo simulation, lengthening meropenem infusions from 30 minutes to 3 hours was found to be advantageous with isolates of P aeruginosa and Acinetobacter species with intermediate resistance.104 This benefit was not observed with Enterobacteriaceae, which usually exhibit low MICs, and with resistant isolates having very high MICs. Subsequently, doripenem was used in clinical trials in extended infusions and at lower doses compared with other carbapenems with equivalent efficacy results (doripenem infused at 500 mg during a 4-hour period every 8 hours vs imipenem infused at 500 mg during a 30-minute period every 6 hours or at 1 g during a 60-minute period every 8 hours and meropenem infused at 1 g as a 3- to 5-minute bolus every 8 hours).105,106 When used at higher doses in a murine model (1 g every 8 hours), an extended infusion of doripenem during a 4-hour period achieved a static antibacterial effect on KPC-producing isolates.107
Extended-infusion strategy therefore appears to be a valuable approach in certain settings and deserves further study. The effect of this dosing regimen on the potential for selection of resistant mutants is yet to be determined.
CONCLUSION
The treatment of infections caused by MDR pathogens is complicated. Treatment options are currently limited, and it will be some time before more investigational agents become available for clinical use, if ever. Meanwhile, prevention strategies should go hand in hand with antimicrobial treatment. The importance of antimicrobial stewardship and infection control policies cannot be discounted in the fight against the worldwide emergence and spread of MDR pathogens.
Supplementary Material
On completion of this article, readers should be able to (1) recognize the burden of multidrug-resistant gram-negative organisms in causing health care–associated infections and their effect on patient outcome, (2) recognize the various mechanisms leading to resistance, and (3) identify the current approach and the choice of empirical and directed therapy in the management of infections with these multidrug-resistant pathogens.
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
The contributions to the Symposium on Antimicrobial Therapy are now a CME activity. For CME credit, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1. Peterson LR. Bad bugs, no drugs: no ESCAPE revisited. Clin Infect Dis. 2009;49:992-993 [DOI] [PubMed] [Google Scholar]
- 2. Ambler RP. The structure of β-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980;289:321-331 [DOI] [PubMed] [Google Scholar]
- 3. Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211-1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Page MG. Extended-spectrum β-lactamases: structure and kinetic mechanism. Clin Microbiol Infect. 2008;14(suppl 1):63-74 [DOI] [PubMed] [Google Scholar]
- 5. Jacoby GA. AmpC β-lactamases. Clin Microbiol Rev. 2009;22:161-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev. 2005;18:657-686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Queenan AM, Bush K. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev. 2007;20:440-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bratu S, Mooty M, Nichani S, et al. Emergence of KPC-possessing Klebsiella pneumoniae in Brooklyn, New York: epidemiology and recommendations for detection. Antimicrob Agents Chemother. 2005;49:3018-3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tenover FC, Kalsi RK, Williams PP, et al. Carbapenem resistance in Klebsiella pneumoniae not detected by automated susceptibility testing. Emerg Infect Dis. 2006;12:1209-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zavascki AP, Carvalhaes CG, Picao RC, Gales AC. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8:71-93 [DOI] [PubMed] [Google Scholar]
- 11. Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century: a clinical super-challenge. N Engl J Med. 2009;360:439-443 [DOI] [PubMed] [Google Scholar]
- 12. Pfeifer Y, Cullik A, Witte W. Resistance to cephalosporins and carbapenems in gram-negative bacterial pathogens. Int J Med MicroBiol. 2010;300:371-379 [DOI] [PubMed] [Google Scholar]
- 13. Rossi F, Baquero F, Hsueh PR, et al. In vitro susceptibilities of aerobic and facultatively anaerobic gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: 2004 results from SMART (Study for Monitoring Antimicrobial Resistance Trends). J Antimicrob Chemother. 2006;58:205-210 [DOI] [PubMed] [Google Scholar]
- 14. Reinert RR, Low DE, Rossi F, Zhang X, Wattal C, Dowzicky MJ. Antimicrobial susceptibility among organisms from the Asia/Pacific Rim, Europe and Latin and North America collected as part of TEST and the in vitro activity of tigecycline. J Antimicrob Chemother. 2007;60:1018-1029 [DOI] [PubMed] [Google Scholar]
- 15. Bonnet R. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother. 2004;48:1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Livermore DM, Canton R, Gniadkowski M, et al. CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother. 2007;59:165-174 [DOI] [PubMed] [Google Scholar]
- 17. Ben-Ami R, Rodriguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum β-lactamase-producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682-690 [DOI] [PubMed] [Google Scholar]
- 18. Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45:1151-1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bratu S, Landman D, Haag R, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med. 2005;165:1430-1435 [DOI] [PubMed] [Google Scholar]
- 20. Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother. 2005;49:4423-4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei ZQ, Du XX, Yu YS, Shen P, Chen YG, Li LJ. Plasmid-mediated KPC-2 in a Klebsiella pneumoniae isolate from China. Antimicrob Agents Chemother. 2007;51:763-765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Villegas MV, Lolans K, Correa A, et al. First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob Agents Chemother. 2006;50:2880-2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pitout JD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159-166 [DOI] [PubMed] [Google Scholar]
- 25. Endimiani A, Luzzaro F, Perilli M, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum β-lactamase: treatment outcome of patients receiving imipenem or ciprofloxacin. Clin Infect Dis. 2004;38:243-251 [DOI] [PubMed] [Google Scholar]
- 26. Paterson DL, Ko WC, Von Gottberg A, et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum β-lactamases. Clin Infect Dis. 2004;39:31-37 [DOI] [PubMed] [Google Scholar]
- 27. Lye DC, Wijaya L, Chan J, Teng CP, Leo YS. Ertapenem for treatment of extended-spectrum β-lactamase-producing and multidrug-resistant gram-negative bacteraemia. Ann Acad Med Singapore. 2008;37:831-834 [PubMed] [Google Scholar]
- 28. Paterson DL, Depestel DD. Doripenem. Clin Infect Dis. 2009;49:291-298 [DOI] [PubMed] [Google Scholar]
- 29. Kelesidis T, Karageorgopoulos DE, Kelesidis I, Falagas ME. Tigecycline for the treatment of multidrug-resistant Enterobacteriaceae: a systematic review of the evidence from microbiological and clinical studies. J Antimicrob Chemother. 2008;62:895-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Falagas ME, Karageorgopoulos DE, Dimopoulos G. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of tigecycline. Curr Drug Metab. 2009;10:13-21 [DOI] [PubMed] [Google Scholar]
- 31. Freire AT, Melnyk V, Kim MJ, et al. 311 Study Group Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis. 2010;68:140-151 [DOI] [PubMed] [Google Scholar]
- 32. Payne DJ, Cramp R, Winstanley DJ, Knowles DJ. Comparative activities of clavulanic acid, sulbactam, and tazobactam against clinically important β-lactamases. Antimicrob Agents Chemother. 1994;38:767-772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Arch Intern Med. 2008;168:1897-1902 [DOI] [PubMed] [Google Scholar]
- 34. Peterson LR. Antibiotic policy and prescribing strategies for therapy of extended-spectrum β-lactamase-producing Enterobacteriaceae: the role of piperacillin-tazobactam. Clin Microbiol Infect. 2008;14(suppl 1):181-184 [DOI] [PubMed] [Google Scholar]
- 35. Gavin PJ, Suseno MT, Thomson RB, Jr, et al. Clinical correlation of the CLSI susceptibility breakpoint for piperacillin-tazobactam against extended-spectrum-β-lactamase-producing Escherichia coli and Klebsiella species. Antimicrob Agents Chemother. 2006;50:2244-2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thomson KS, Moland ES. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2001;45:3548-3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rodriguez-Bano J, Navarro MD, Romero L, et al. Bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli in the CTX-M era: a new clinical challenge. Clin Infect Dis. 2006;43:1407-1414 [DOI] [PubMed] [Google Scholar]
- 38. Zanetti G, Bally F, Greub G, et al. Cefepime versus imipenem-cilastatin for treatment of nosocomial pneumonia in intensive care unit patients: a multicenter, evaluator-blind, prospective, randomized study. Antimicrob Agents Chemother. 2003;47:3442-3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parchuri S, Mohan S, Cunha BA. Extended spectrum β-lactamase-producing Klebsiella pneumoniae chronic ambulatory peritoneal dialysis peritonitis treated successfully with polymyxin B. Heart Lung. 2005;34:360-363 [DOI] [PubMed] [Google Scholar]
- 40. Segal-Maurer S, Mariano N, Qavi A, Urban C, Rahal JJ., Jr Successful treatment of ceftazidime-resistant Klebsiella pneumoniae ventriculitis with intravenous meropenem and intraventricular polymyxin B: case report and review. Clin Infect Dis. 1999;28:1134-1138 [DOI] [PubMed] [Google Scholar]
- 41. Galani I, Kontopidou F, Souli M, et al. Colistin susceptibility testing by Etest and disk diffusion methods. Int J Antimicrob Agents. 2008;31:434-439 [DOI] [PubMed] [Google Scholar]
- 42. Reeves DS. Fosfomycin trometamol. J Antimicrob Chemother. 1994;34:853-858 [DOI] [PubMed] [Google Scholar]
- 43. Falagas ME, Kanellopoulou MD, Karageorgopoulos DE, et al. Antimicrobial susceptibility of multidrug-resistant gram negative bacteria to fosfomycin. Eur J Clin Microbiol Infect Dis. 2008;27:439-443 [DOI] [PubMed] [Google Scholar]
- 44. Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum β-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis. 2010;10:43-50 [DOI] [PubMed] [Google Scholar]
- 45. Popovic M, Steinort D, Pillai S, Joukhadar C. Fosfomycin: an old, new friend? Eur J Clin Microbiol Infect Dis. 2010;29:127-142 [DOI] [PubMed] [Google Scholar]
- 46. Glupczynski Y, Huang TD, Berhin C, et al. In vitro activity of temocillin against prevalent extended-spectrum β-lactamases producing Enterobacteriaceae from Belgian intensive care units. Eur J Clin Microbiol Infect Dis. 2007;26:777-783 [DOI] [PubMed] [Google Scholar]
- 47. Nicolle LE, Mulvey MR. Successful treatment of CTX-M ESBL producing Escherichia coli relapsing pyelonephritis with long term pivmecillinam. Scand J Infect Dis. 2007;39:748-749 [DOI] [PubMed] [Google Scholar]
- 48. Jia B, Lu P, Huang W, et al. A multicenter, randomized controlled clinical study on biapenem and imipenem/cilastatin injection in the treatment of respiratory and urinary tract infections. Chemotherapy. 2010;56:285-290 [DOI] [PubMed] [Google Scholar]
- 49. Mushtaq S, Hope R, Warner M, Livermore DM. Activity of faropenem against cephalosporin-resistant Enterobacteriaceae. J Antimicrob Chemother. 2007;59:1025-1030 [DOI] [PubMed] [Google Scholar]
- 50. Koga T, Abe T, Inoue H, et al. In vitro and in vivo antibacterial activities of CS-023 (RO4908463), a novel parenteral carbapenem. Antimicrob Agents Chemother. 2005;49:3239-3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garau J. Other antimicrobials of interest in the era of extended-spectrum β-lactamases: fosfomycin, nitrofurantoin and tigecycline. Clin Microbiol Infect. 2008;14(suppl 1):198-202 [DOI] [PubMed] [Google Scholar]
- 52. Bratu S, Tolaney P, Karumudi U, et al. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J Antimicrob Chemother. 2005;56:128-132 [DOI] [PubMed] [Google Scholar]
- 53. Castanheira M, Sader HS, Deshpande LM, Fritsche TR, Jones RN. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-β-lactamase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother. 2008;52:570-573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hirsch EB, Tam VH. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother. 2010;65:1119-1125 [DOI] [PubMed] [Google Scholar]
- 55. Anthony KB, Fishman NO, Linkin DR, Gasink LB, Edelstein PH, Lautenbach E. Clinical and microbiological outcomes of serious infections with multidrug-resistant gram-negative organisms treated with tigecycline. Clin Infect Dis. 2008;46:567-570 [DOI] [PubMed] [Google Scholar]
- 56. Samra Z, Ofir O, Lishtzinsky Y, Madar-Shapiro L, Bishara J. Outbreak of carbapenem-resistant Klebsiella pneumoniae producing KPC-3 in a tertiary medical centre in Israel. Int J Antimicrob Agents. 2007;30:525-529 [DOI] [PubMed] [Google Scholar]
- 57. Kontopoulou K, Protonotariou E, Vasilakos K, et al. Hospital outbreak caused by Klebsiella pneumoniae producing KPC-2 β-lactamase resistant to colistin. J Hosp Infect. 2010;76:70-73 [DOI] [PubMed] [Google Scholar]
- 58. Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother. 2010;54:526-529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Urban C, Mariano N, Rahal JJ. In vitro double and triple bactericidal activities of doripenem, polymyxin B, and rifampin against multidrug-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli. Antimicrob Agents Chemother. 2010;54:2732-2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Melchers R, Mavridou E, Van Mil A, Motyl MR, Mouton JW. In vitro activity of imipenem alone and in combination with MK-7655: a new β-lactamase inhibitor [poster F1-2138]. Poster presented at: 50th Interscience Conference on Antimicrobial Agents and Chemotherapy; Boston, MA; September 12-15, 2010 [Google Scholar]
- 61. Livermore DM. Spectrum and potential of NXL104 as a β-lactamase inhibitor [poster 1849]. Presented at: 50th Interscience Conference on Antimicrobial Agents and Chemotherapy; Boston, MA; September 12-15, 2010 [Google Scholar]
- 62. Sheri A, Pagadala SR, Young K, et al. Optimization of a carbapenem/β-lactamase inhibitor combination against highly resistant gram-negative microorganisms [poster F1-1496]. Presented at: 50th Interscience Conference on Antimicrobial Agents and Chemotherapy; Boston, MA; September 12-15, 2010 [Google Scholar]
- 63. Jacobs MR, Bajaksouzian S, Butler MM, et al. Activity of novel bis-in-dole agents against carbapenemase-producing Klebsiella pneumoniae [poster F1-1632]. Presented at: 50th Interscience Conference on Antimicrobial Agents and Chemotherapy; Boston, MA; September 12-15, 2010 [Google Scholar]
- 64. Tam VH, Rogers CA, Chang KT, Weston JS, Caeiro JP, Garey KW. Impact of multidrug-resistant Pseudomonas aeruginosa bacteremia on patient outcomes. Antimicrob Agents Chemother. 2010;54:3717-3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bellais S, Mimoz O, Leotard S, Jacolot A, Petitjean O, Nordmann P. Efficacy of β-lactams for treating experimentally induced pneumonia due to a carbapenem-hydrolyzing metallo-β-lactamase-producing strain of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2002;46:2032-2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gimeno C, Canton R, Garcia A, Gobernado M. Comparative activity of doripenem, meropenem, and imipenem in recent clinical isolates obtained during the COMPACT-Spain epidemiological surveillance study [in Spanish]. Rev Esp Quimioter. 2010;23:144-152 [PubMed] [Google Scholar]
- 67. Pillar CM, Torres MK, Brown NP, Shah D, Sahm DF. In vitro activity of doripenem, a carbapenem for the treatment of challenging infections caused by gram-negative bacteria, against recent clinical isolates from the United States. Antimicrob Agents Chemother. 2008;52:4388-4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mushtaq S, Ge Y, Livermore DM. Doripenem versus Pseudomonas aeruginosa in vitro: activity against characterized isolates, mutants, and transcon jugants and resistance selection potential. Antimicrob Agents Chemother. 2004;48:3086-3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kaye KS, Kanafani ZA, Dodds AE, Engemann JJ, Weber SG, Carmeli Y. Differential effects of levofloxacin and ciprofloxacin on the risk for isolation of quinolone-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50:2192-2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kallel H, Hergafi L, Bahloul M, et al. Safety and efficacy of colistin compared with imipenem in the treatment of ventilator-associated pneumonia: a matched case-control study. Intensive Care Med. 2007;33:1162-1167 [DOI] [PubMed] [Google Scholar]
- 71. Linden PK, Kusne S, Coley K, Fontes P, Kramer DJ, Paterson D. Use of parenteral colistin for the treatment of serious infection due to antimicrobial-resistant Pseudomonas aeruginosa. Clin Infect Dis. 2003;37:e154-e160 [DOI] [PubMed] [Google Scholar]
- 72. Falagas ME, Kasiakou SK. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care. 2006;10:R27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Daikos GL, Skiada A, Pavleas J, et al. Serum bactericidal activity of three different dosing regimens of colistin with implications for optimum clinical use. J Chemother. 2010;22:175-178 [DOI] [PubMed] [Google Scholar]
- 74. Falagas ME, Rafailidis PI, Ioannidou E, et al. Colistin therapy for microbiologically documented multidrug-resistant gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents. 2010;35:194-199 [DOI] [PubMed] [Google Scholar]
- 75. Korvick JA, Peacock JE, Jr, Muder RR, Wheeler RR, Yu VL. Addition of rifampin to combination antibiotic therapy for Pseudomonas aeruginosa bacteremia: prospective trial using the Zelen protocol. Antimicrob Agents Chemother. 1992;36:620-625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med. 1989;87:540-546 [DOI] [PubMed] [Google Scholar]
- 77. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2003;47:2756-2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus β lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and meta-analysis of randomised trials [published correction appears in BMJ. 2004;328(7444):884] BMJ. 2004;328:668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in gram-negative bacteraemia? a meta-analysis. Lancet Infect Dis. 2004;4:519-527 [DOI] [PubMed] [Google Scholar]
- 80. Drago L, De Vecchi E, Nicola L, Tocalli L, Gismondo MR. In vitro selection of resistance in Pseudomonas aeruginosa and Acinetobacter spp. by levofloxacin and ciprofloxacin alone and in combination with β-lactams and amikacin. J Antimicrob Chemother. 2005;56:353-359 [DOI] [PubMed] [Google Scholar]
- 81. Lister PD, Wolter DJ. Levofloxacin-imipenem combination prevents the emergence of resistance among clinical isolates of Pseudomonas aeruginosa. Clin Infect Dis. 2005;40(suppl 2):S105-S114 [DOI] [PubMed] [Google Scholar]
- 82. Carmeli Y, Troillet N, Eliopoulos GM, Samore MH. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob Agents Chemother. 1999;43:1379-1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dubois V, Arpin C, Melon M, et al. Nosocomial outbreak due to a multi-resistant strain of Pseudomonas aeruginosa P12: efficacy of cefepime-amikacin therapy and analysis of β-lactam resistance. J Clin MicroBiol. 2001;39:2072-2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dundar D, Otkun M. In-vitro efficacy of synergistic antibiotic combinations in multidrug resistant Pseudomonas aeruginosa strains. Yonsei Med J. 2010;51:111-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fish DN, Choi MK, Jung R. Synergic activity of cephalosporins plus fluoroquinolones against Pseudomonas aeruginosa with resistance to one or both drugs. J Antimicrob Chemother. 2002;50:1045-1049 [DOI] [PubMed] [Google Scholar]
- 86. Zuravleff JJ, Yu VL, Yee RB. Ticarcillin-tobramycin-rifampin: in vitro synergy of the triplet combination against Pseudomonas aeruginosa. J Lab Clin Med. 1983;101:896-902 [PubMed] [Google Scholar]
- 87. Urena MT, Barasoain I, Espinosa M, Garcia E, Portoles A. Evaluation of different antibiotic actions combined with rifampicin: in vitro synergism against Pseudomonas and Proteus. Chemotherapy. 1975;21:82-89 [DOI] [PubMed] [Google Scholar]
- 88. Gunderson BW, Ibrahim KH, Hovde LB, Fromm TL, Reed MD, Rotschafer JC. Synergistic activity of colistin and ceftazidime against multi-antibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 2003;47:905-909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Saiman L, Chen Y, Gabriel PS, Knirsch C. Synergistic activities of macrolide antibiotics against Pseudomonas aeruginosa, Burkholderia cepacia, Stenotrophomonas maltophilia, and Alcaligenes xylosoxidans isolated from patients with cystic fibrosis. Antimicrob Agents Chemother. 2002;46:1105-1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Timurkaynak F, Can F, Azap OK, Demirbilek M, Arslan H, Karaman SO. In vitro activities of non-traditional antimicrobials alone or in combination against multidrug-resistant strains of Pseudomonas aeruginosa and Acinetobacter baumannii isolated from intensive care units. Int J Antimicrob Agents. 2006;27:224-228 [DOI] [PubMed] [Google Scholar]
- 91. Milne KE, Gould IM. Combination testing of multidrug-resistant cystic fibrosis isolates of Pseudomonas aeruginosa: use of a new parameter, the susceptible breakpoint index. J Antimicrob Chemother. 2010;65:82-90 [DOI] [PubMed] [Google Scholar]
- 92. Brown RB, Kruse JA, Counts GW, Russell JA, Christou NV, Sands ML, The Endotracheal Tobramycin Study Group Double-blind study of endotracheal tobramycin in the treatment of gram-negative bacterial pneumonia. Antimicrob Agents Chemother. 1990;34:269-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ratjen F, Doring G, Nikolaizik WH. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Lancet. 2001;358:983-984 [DOI] [PubMed] [Google Scholar]
- 94. Moss RB. Long-term benefits of inhaled tobramycin in adolescent patients with cystic fibrosis. Chest. 2002;121:55-63 [DOI] [PubMed] [Google Scholar]
- 95. Kwa AL, Loh C, Low JG, Kurup A, Tam VH. Nebulized colistin in the treatment of pneumonia due to multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis. 2005;41:754-757 [DOI] [PubMed] [Google Scholar]
- 96. Feldman C, White H, O'Grady J, Flitcroft A, Briggs A, Richards G. An open, randomised, multi-centre study comparing the safety and efficacy of sitafloxacin and imipenem/cilastatin in the intravenous treatment of hospitalised patients with pneumonia. Int J Antimicrob Agents. 2001;17:177-188 [DOI] [PubMed] [Google Scholar]
- 97. Baer M, Sawa T, Flynn P, et al. An engineered human antibody fab fragment specific for Pseudomonas aeruginosa PcrV antigen has potent antibacterial activity. Infect Immun. 2009;77:1083-1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Takeda S, Nakai T, Wakai Y, Ikeda F, Hatano K. In vitro and in vivo activities of a new cephalosporin, FR264205, against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2007;51:826-830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mushtaq S, Warner M, Livermore DM. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother. 2010;65(11)2376-2381 [DOI] [PubMed] [Google Scholar]
- 100. Venkatesan AM, Agarwal A, Abe T, et al. Novel imidazole substituted 6-methylidene-penems as broad spectrum β-lactamase inhibitors. Bioorg Med Chem. 2004;12:5807-5817 [DOI] [PubMed] [Google Scholar]
- 101. Page MG, Heim J. Prospects for the next anti-Pseudomonas drug. Curr Opin Pharmacol. 2009;9:558-565 [DOI] [PubMed] [Google Scholar]
- 102. Lodise TP, Jr, Lomaestro B, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis. 2007;44:357-363 [DOI] [PubMed] [Google Scholar]
- 103. Moriyama B, Henning SA, Childs R, et al. High-dose continuous infusion β-lactam antibiotics for the treatment of resistant Pseudomonas aeruginosa infections in immunocompromised patients. Ann Pharmacother. 2010;44:929-935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kuti JL, Dandekar PK, Nightingale CH, Nicolau DP. Use of Monte Carlo simulation to design an optimized pharmacodynamic dosing strategy for meropenem. J Clin Pharmacol. 2003;43:1116-1123 [DOI] [PubMed] [Google Scholar]
- 105. Chastre J, Wunderink R, Prokocimer P, Lee M, Kaniga K, Friedland I. Efficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator-associated pneumonia: a multicenter, randomized study. Crit Care Med. 2008;36:1089-1096 [DOI] [PubMed] [Google Scholar]
- 106. Malafaia O, Umeh O, Jiang J. Doripenem versus meropenem for the treatment of complicated intra-abdominal infections [poster L-1564b]. Presented at: 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA; September 27-30, 2006 [Google Scholar]
- 107. Bulik CC, Nicolau DP. In vivo efficacy of 1g human simulated prolonged infusion doripenem (DOR) against carbapenemase producing Klebsiella pneumoniae (KPC) [poster A1-014]. Presented at: 50th Interscience Conference on Antimicrobial Agents and Chemotherapy; Boston, MA; September 12-15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.