Abstract
P-bodies are dynamic aggregates of RNA and proteins involved in several post-transcriptional regulation processes. P-bodies have been shown to play important roles in regulating viral infection, whereas their interplay with bacterial pathogens, specifically intracellular bacteria that extensively manipulate host cell pathways, remains unknown. Here, we report that Salmonella infection induces P-body disassembly in a cell type-specific manner, and independently of previously characterized pathways such as inhibition of host cell RNA synthesis or microRNA-mediated gene silencing. We show that the Salmonella-induced P-body disassembly depends on the activation of the SPI-2 encoded type 3 secretion system, and that the secreted effector protein SpvB plays a major role in this process. P-body disruption is also induced by the related pathogen, Shigella flexneri, arguing that this might be a new mechanism by which intracellular bacterial pathogens subvert host cell function.
Introduction
A significant fraction of cellular messenger RNAs are not translated immediately after their export from the nucleus to the cytoplasm, being subjected to quality control and regulatory mechanisms that lead to mRNA degradation or translation repression. These different fates of transcripts have been associated with specific subcellular localizations: mRNAs being actively translated are associated with polysomes; mRNAs targeted for degradation or translation repression accumulate in P-bodies (a.k.a. mRNA processing bodies or GW bodies); and, in response to stress conditions, mRNAs stalled in translation initiation accumulate in stress granules.
P-bodies (henceforth, PBs) are dynamic cytoplasmic structures containing small regulatory and messenger RNAs, as well as proteins associated with post-transcriptional regulations such as mRNA decay, translational repression, mRNA surveillance and RNA-mediated gene silencing [1], [2]. PB formation is at least partially, a consequence of RNA silencing and decay, though not necessarily essential for these pathways [3], [4], [5], [6]. Stress granules (SGs) are a different type of cytoplasmic structures, composed of dynamic aggregates of RNA binding proteins, a subset of translation initiation factors, 40S ribosomal subunit and mRNAs. In contrast to the constitutive presence of PBs, SG form upon different environmental stresses that inhibit translation initiation, for instance, heat-shock, oxidative stress, UV irradiation or energy starvation [1], [7], [8]. PBs and SGs are often detected docked to each other, and it has been suggested that mRNAs can move between these two compartments [9].
There has been accumulating evidence to implicate PBs, SGs, and their protein components in the regulation of RNA metabolism during viral infections, with clear consequences for the life cycle of various viruses [10]. For example, PB components were found to be required for Brome mosaic virus and Hepatitis C virus replication [11], [12], [13], [14], whereas disruption of PBs was shown to enhance HIV-1 production and infectivity [15]. Recently, poliovirus infection was shown to induce PB disruption during the mid-phase of the infection cycle [16]. Additionally, West Nile and Dengue viruses hamper PB assembly later during infection, although both the mechanism and outcome for infection are yet unknown [17]. Viral infection has also been shown to affect the assembly of SGs, either positively by polioviruses and reoviruses [18], [19], or negatively by rotavirus and flaviviruses [17], [20]. A repression of SG formation might facilitate the translation of viral mRNAs; some SG components can limit viral infection, suggesting that formation of SGs might be part of the host antiviral response [10].
Similar to viruses, intracellular bacterial pathogens extensively manipulate host cellular pathways to ensure their survival and replication, impacting on host cell cytoskeleton, signal transduction pathways, membrane trafficking and pro-inflammatory responses, to name a few [21], [22], [23]. Unlike for viral pathogens, however, effects of bacterial pathogens on PBs and SGs have not been addressed. In this paper we investigate this matter with the bacterium Salmonella enterica serovar Typhimurium (hereafter, Salmonella), a Gram-negative model pathogen that targets a variety of eukaryotic hosts to cause different diseases ranging from gastroenteritis to typhoid fever [24], [25]. Salmonella can infect nonphagocytic cells, in particular epithelial cells, by actively promoting its own internalization and its intracellular survival. These processes are primarily mediated by two type 3 secretion systems (T3SS) that are encoded on the Salmonella pathogenicity island 1 and 2 (SPI-1 and SPI-2). The T3SS inject bacterial effector proteins directly into the host cell cytoplasm, to facilitate bacterial invasion (SPI-1), and intracellular replication (SPI-2) within the Salmonella-containing vacuole (SCV) [21], [22], [23].
We report here that Salmonella infection induces a novel type of PB disassembly in epithelial cells that is unrelated to previously determined mechanisms such as the inhibition of host cell transcription or microRNA-mediated gene silencing. Using a collection of bacterial effector mutants, we determined that PB disassembly is dependent on the activity of the SPI-2 encoded T3SS, and identified the secreted effector protein, SpvB, as a mediator of this process. Finally, we demonstrate that the effect of bacterial infection on PB integrity is not limited to Salmonella, and that Shigella flexneri also induces PB disassembly.
Results
Salmonella infection induces PB disassembly
The interplay between viruses and RNA granules, specifically PBs and SGs, has been characterized, whereas effects of bacterial infections on these granules have not been studied. To test whether bacterial infection impacts on PBs, HeLa cells – an epithelial cell line widely used for Salmonella studies – were infected with GFP-expressing bacteria for twenty hours, and then processed for immunofluorescence using antibodies which recognize the PB marker DDX6 (a.k.a. Rck/p54). Visual inspection of the immunofluorescence images indicated similar PB numbers in mock-treated cells, and in those cells of the infected samples that had not internalized the bacteria; whereas the subpopulation of host cells invaded by Salmonella showed reduction in PB number (Figure 1A).
Figure 1. Salmonella infection induces PB disassembly, but not SG formation.
(A) HeLa cells were mock-treated or infected for 20 hours with Salmonella expressing GFP, and either left untreated (control) or treated with puromycin for 1 hour. PBs were detected by staining the cells with anti-DDX6 antibody (red channel). Scale bar, 10 µm. The region highlighted by a white square is enlarged on the rightmost panel. (B) The average number of PBs per cell in mock-treated cells and Salmonella infected cells was calculated as described in Materials and Methods. Salmonella infected cells were divided into two populations: cells in which the bacteria were not internalized (Salmonella −) and cells in which the bacteria were internalized (Salmonella +). Mean values ± standard deviations from at least three independent experiments are shown. (C) SG formation was evaluated in HeLa cells mock-treated or infected for 20 hours with Salmonella, either left at 37°C (control) or after heat-shock for 1 hour at 46°C. Cells were stained with anti-TIAR antibody (red channel).
For quantification of PB number per cell in the different cell populations (mock-treated, Salmonella negative and Salmonella positive), we used automated image acquisition and analysis (see methods and Figure S1) to both distinguish between Salmonella positive and negative cells, and count PBs per cell in a systematic and unbiased way, for a significant number of cells (∼1,000 cells per experimental condition). Figure 1B shows that the average number of PBs per cell was reduced by approximately twofold in cells containing Salmonella, as compared to mock-treated (p<0.001) or non-invaded cells (p<0.01). Similar results were obtained using anti-GW182 patient autoimmune serum (IC-6 serum), which recognizes the two well characterized PB proteins, GW182 and Ge-1 (Figure S2A; [26]). Thus, successful bacterial infection negatively impacts on the number of PB per cell.
To determine whether infection impairs the formation of new PBs, or induces the disassembly of existing PBs, we used the protein synthesis inhibitor puromycin. Puromycin is well-established to increase both PB size and number, by causing premature polypeptide chain termination and polysome disassembly [27]; this in turn increases the pool of non-translating mRNPs which are essential for PB assembly [3], [28], [29]. HeLa cells were infected for twenty hours, followed by one hour of puromycin treatment. Puromycin invariably stimulated PB formation in all three investigated samples (Figure 1A-B), arguing that infection is inducing PB disassembly rather than inhibiting their formation.
To determine whether Salmonella induces PB disassembly in other cell types as well, we infected A431 cells, a human epithelial cell line, and RAW 264.7 cells, a murine cell line with macrophage-like characteristics. Intriguingly, PB dispersion was also observed in A431 cells, while infection had no impact on PB integrity in RAW 264.7 cells (Figure S2B). Overall, these results show that Salmonella infection causes PB disassembly, in a cell-type specific manner.
Considering that PBs and SGs share some protein and mRNA components and that both are involved in regulating cytoplasmic degradation and/or storage of mRNAs, we also tested Salmonella effects on SG assembly and integrity. In mock-treated cells, the RNA binding protein TIAR is distributed throughout the cell, and it partially redistributes to cytoplasmic SGs upon heat-shock (Figure 1C), as reported previously [30]. However, we did not observe relocalization of the TIAR protein at early or late times post-infection (2, 4 and 20 hours p.i.; Figure 1C and Figure S2C), indicating that Salmonella infection does not induce SG formation. In support of this observation, the localization of Ataxin-2 – another well characterized SG marker [31] – did not change either (data not shown). Likewise, Salmonella failed to inhibit SG formation induced by heat-shock (Figure 1C). Thus, interference of Salmonella with the formation of mRNA granules is specific to PBs.
RNA synthesis and microRNA activity remain intact upon Salmonella infection
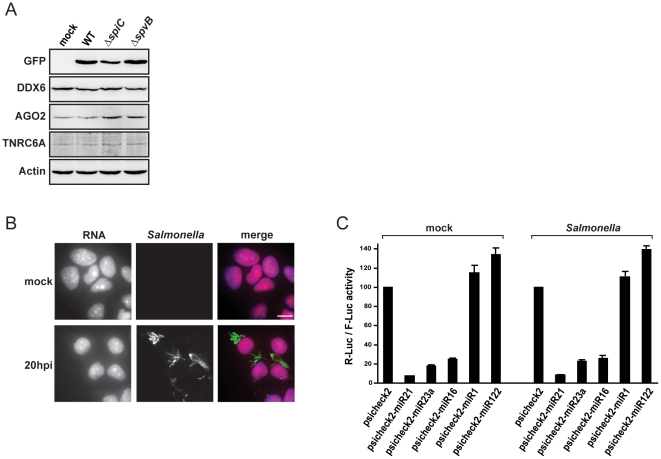
Given that Salmonella causes dramatic gene expression changes in infected host cells, we asked whether PB disassembly might simply result from reduced levels of cellular proteins that are essential for PB integrity. Specifically, we determined protein levels of DDX6, a DExH/D-box RNA helicase with multiple roles in mRNA metabolism, and of AGO2 and TNRC6A, two key proteins of the microRNA pathway. However, Western-blot analysis of extracts of Salmonella infected HeLa cells revealed no expression changes of the DDX6, TNRC6A and AGO2 proteins, as compared to mock-treated cells (Figure 2A).
Figure 2. Salmonella infection does not interfere with host cell RNA synthesis and microRNA silencing pathways.
(A) Western-blot analysis of DDX6, AGO2 and GW182 protein levels in HeLa cells mock-treated or infected for 20 hours with Salmonella WT or ΔSpiC and ΔSpvB mutant strains. GFP signal is shown to confirm the presence of intracellular bacteria. (B) Analysis of host cell RNA synthesis in mock-treated and Salmonella infected cells (20 hours p.i.), through the incorporation of EU in newly transcribed RNA, followed by the detection using Alexa Fluor 594 azide (red channel), as described in Materials and Methods. Salmonella was stained with anti-LPS antibody (green channel). Scale bar, 10 µm. (C) HeLa cells were transfected with the psiCHECK2 empty plasmid or with psiCHECK2 plasmids carrying binding sites for the indicated microRNAs. Twenty-four hours after transfection, cells were mock-treated or infected with Salmonella. Luciferase activities were measured 20 hours after infection. Renilla luciferase (R-Luc) activity was normalized to that of the Firefly control (F-Luc) and normalized R-Luc activities of cells transfected with the empty vector were set to 100. Error bars represent standard deviations from three independent experiments.
PB maintenance is well-established to depend on the presence of mRNPs that are committed to, or are undergoing, degradation. Therefore, we tested if Salmonella infection inhibits RNA synthesis in the host, which then would cause PB disassembly due to a reduction of the pool of non-translating cytoplasmic mRNAs. We assayed incorporation of 5-ethynyluridine (EU) into newly transcribed RNA, which is then detected using fluorescent azides [32]. Both mock and Salmonella infected cells (20 hours p.i.) show a comparably strong nuclear and cytoplasmic RNA staining (Figure 2B), arguing that infection does not abrogate host cell RNA synthesis. In contrast, control treatment of cells with actinomycin D, an inhibitor of RNA polymerase, did yield the expected strong decrease of EU staining throughout the cell (data not shown and [32]).
In addition to RNA synthesis, an intact microRNA pathway is important for PB maintenance, and was thus considered as another potential target of infection. To test whether Salmonella decreased global microRNA activity, HeLa cells were transfected with reporter vectors containing microRNA binding site downstream of a renilla luciferase ORF, in a vector that also expresses firefly luciferase as internal reference. Three reporter plasmids contained microRNA binding sites for miR-21, miR-23a or miR-16, all of which are highly expressed in HeLa cells yet not regulated by Salmonella infection (Schulte et al., in revision). Figure 2C shows that the reporters were strongly down-regulated in HeLa cells (between 4-fold and 13-fold repression), but invariably so in both mock-treated and Salmonella-infected cells, arguing that the bacteria do not generally impair microRNA activity. As additional controls, reporters containing binding sites for microRNAs that are not expressed in HeLa cells (miR-1 and miR-122) showed neither basal nor Salmonella-specific regulations. Altogether, these results argued against the possibilities that Salmonella induces PB disassembly via interference with general functions such as mRNA synthesis or microRNA-mediated gene silencing.
The SPI-2 T3SS is required for P-body disassembly
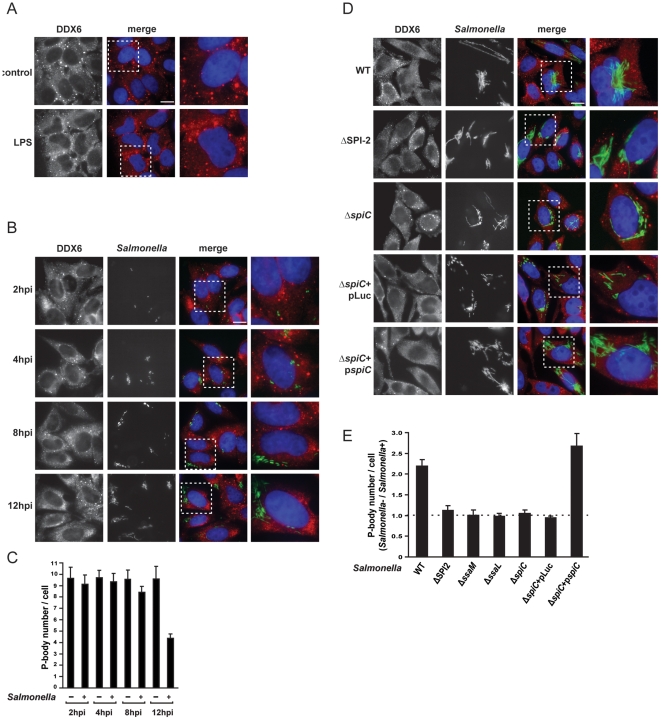
We considered the possibility that the observed PB disassembly could be associated with sensing of bacterial lipopolysaccharide (LPS) during the infection process. However, this was ruled out by the observation that incubation of HeLa cells with purified Salmonella LPS did not affect PB integrity (Figure 3A).
Figure 3. Salmonella induced PB disruption is dependent on the activation of the SPI-2 T3SS.
(A) HeLa cells were mock-treated or treated with LPS (1 µg/ml) for 20 hours. Cells were stained with anti-DDX6 antibody. Scale bar, 10 µm. The region highlighted by a white square is enlarged on the rightmost panel. (B) HeLa cells were infected with Salmonella for 2, 4, 8 and 12 hours as indicated, and then processed for PB detection using anti-DDX6 antibody. (C) Average number of PBs per cell in the population of cells in which Salmonella was not internalized (Salmonella −) and in cells in which the bacteria were internalized (Salmonella +), at the indicated times post-infection. Mean values ± standard deviations from at least three independent experiments are shown. (D) HeLa cells were infected with wild-type Salmonella or with the indicated mutant strains and collected at 20 hours p.i. PBs were detected using anti-DDX6 antibody (red channel). (E) Ratio of the average number of PBs per cell between the cell population in which bacteria where not internalized (Salmonella −) and the infected cells (Salmonella +) for wild-type and mutant Salmonella strains. Mean values ± standard deviations from at least three independent experiments are shown.
More generally, Salmonella infection is a sequential process, initiated by Salmonella inducing its own uptake into host cells via the effects of SPI-1 effector proteins, and followed by intracellular manipulation of host cell functions by the SPI-2 T3SS. A detailed analysis of the kinetics of PB disassembly upon Salmonella infection revealed that PB integrity is not affected at early and intermediate times of infection (2, 4 or 8 hours p.i.; Figure 3B–C) when the SPI-1 T3SS plays a major role. PB disassembly became apparent only at later times post-infection (12 and 20 hours p.i.; Figure 3B–C), indicating that it might be associated with the later activity of the SPI-2 T3SS which is highly expressed after bacterial enclosure in the SCV. In favour of this hypothesis, infection of HeLa cells with a Salmonella mutant deleted for the SPI-2 region (ΔSPI-2) was observed not to induce PB dispersion. Indeed, quantification revealed a ratio of ∼1.0 between the average number of PBs in cells without internalized bacteria (Salmonella−) and those successfully infected with the ΔSPI-2 mutant (Salmonella+); in other words, the same number of PBs per cell in either cell population (Figure 3E). In contrast, infection with wild-type Salmonella resulted in a ratio of 2.2 (Figure 1A, 3E).
The SpiC/SsaM/SsaL complex is a key component of functional SPI-2 T3SS, and regulates the secretion of both effector and translocon proteins [33], [34], the latter of which form a pore in the host membrane vacuole. To confirm the relationship between SPI-2 dependent secretion and PB disassembly, HeLa cells were infected with Salmonella ΔssaM, ΔssaL or ΔspiC mutants. Similarly to the ΔSPI-2 strain, infection with any of these three mutants failed to impact on PB integrity (Figure 3D and Figure S3). As expected, complementation of the ΔspiC mutant with a plasmid containing the wild-type spiC allele (ΔspiC+pspiC) rescued the PB disassembly phenotype (Figure 3D–E). Collectively, this data implicates the activity of the SPI-2 T3SS in PB dispersion.
Salmonella effector protein SpvB is involved in PB disassembly during infection
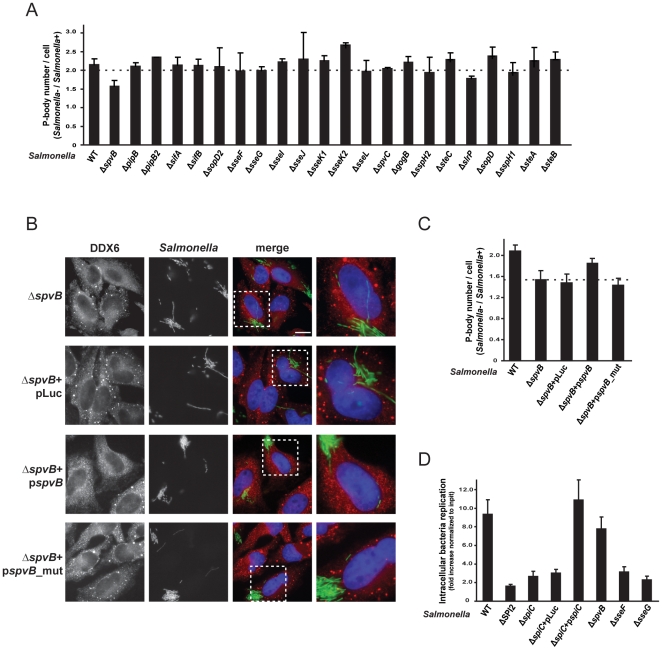
There are over twenty Salmonella proteins that are translocated by the SPI-2 T3SS into the host cell cytoplasm, and many of them act in concert to subvert the host cell cytoskeleton, signal transduction pathways, membrane trafficking and pro-inflammatory responses [21], [22], [23]. To determine which of these proteins might promote PB dispersion, we generated a collection of effector gene mutants and screened for their effect on PB integrity. Most of the tested mutants induced PB disassembly to the same extent as did wild-type Salmonella (Figure 4A and Figure S4). However, a ΔspvB mutant showed significantly lower PB disruption (Figure 4A–C), resulting in a Sa lmonel la−/Salmonella+ PB ratio of approximately 1.5 (p?0.001). Importantly, the ΔspvB strain replicates almost as efficiently as the wild-type strain (Figure 4D), arguing against the possibility that a lower replication rate impairs its ability to cause PB disruption. Along the same line, we observed Salmonella mutants which infect HeLa cells with efficiency comparable to that of the ΔspiC mutant but still induce PB disassembly (e.g. ΔsseG and sseF; Figure 4D), reinforcing the notion that PB dispersion is not generally coupled to bacterial replication or load. Complementation of the ΔspvB mutant with a plasmid carrying the spvB gene (ΔspvB+pspvB) restored PB disassembly, whereas the parental cloning vector (ΔspvB+pLuc) did not (Figure 4B–C).
Figure 4. SpvB effector protein is involved in PB disassembly induced by Salmonella infection.
(A) HeLa cells were infected with wild-type or single mutants of all Salmonella effector proteins. The ratio of the average number of PBs per cell, between the cell population in which bacteria where not internalized (Salmonella −) and the infected cells (Salmonella +), was calculated. Mean values ± standard deviations from at least three independent experiments are shown. Representative images of cells infected with the different mutants are shown in Figure S4. (B) HeLa cells were infected with wild-type Salmonella or with the indicated mutant strains. Twenty-hours post-infection cells were processed for immunofluorescence and PBs were detected using anti-DDX6 antibody (red channel). Scale bar, 10 µm. The region highlighted by a white square is enlarged on the rightmost panel. (C) Ratio of the average number of PBs per cell between the cell population in which bacteria where not internalized (Salmonella −) and the infected cells (Salmonella +) for wild-type and mutant Salmonella strains. (D) Intracellular replication assays were performed in HeLa cells with the indicated strains. Values indicate the fold increase, calculated as the ratio between the intracellular bacteria at 20 hours p.i. and the input bacteria used for infection. Error bars represent standard deviations from three independent experiments.
The only previously known function of the Salmonella SpvB protein is an ADP-ribosyl transferase activity on monomeric actin as the primary substrate, blocking actin polymerization to F-actin filaments during infection [35], [36], [37], [38], [39]. This catalytic activity is abrogated by mutation of two conserved glutamate residues to aspartates (E538D and E540D) of SpvB [35]. Similarly, we observed that mutation of these residues in our spvB complementation plasmid rendered it unable to complement the ΔspvB strain for PB disassembly (Figure 4B and C), indicating that Salmonella infection might interfere with PB integrity by perturbing the actin cytoskeleton. However, treatment of HeLa cells with cytochalasin D, a potent inhibitor of actin polymerization, had no effect on PB integrity (data not shown), arguing that SpvB targets PB integrity in a process unrelated to its actin depolymerizing activity.
PB disassembly induced by bacterial infection is not restricted to Salmonella
To establish whether PB disassembly could be a more general characteristic of bacterial pathogens, we analyzed the integrity of PBs in cells upon invasion by Shigella flexneri, an enteroinvasive pathogen that like Salmonella has epithelial cells as primary target [40]. Similar to Salmonella, Shigella also dispersed PBs after internalization (Figure 5). This was most evident at intermediate and late times post-infection (6, 12 and 20 hours p.i.; Figure 5 and data not shown). Thus, PB disassembly induced by bacterial infection is not restricted to Salmonella, indicating that this phenomenon likely has biological significance to the life cycle of different bacteria.
Figure 5. Shigella infection also induces PB disassembly.
HeLa cells were mock-treated or infected with Shigella for 12 and 20 hours. PBs and Shigella were detected using anti-GW182 (green channel) and anti-Shigella (red channel) antibodies, respectively. Scale bar, 10 µm. The region highlighted by a white square is enlarged on the rightmost panel.
Discussion
Bacterial pathogens are endowed with extraordinarily abilities to subvert host cell functions as they initiate and establish an infection. Extensive work on the model pathogen Salmonella has revealed a plethora of protein-based signaling cascades and structural functions as targets of T3SS-secreted virulence factors, yet whether and how bacteria interfere with RNA-related processes in their hosts has only begun to be addressed [41]. The present study is the first to address the effect of bacterial infection on P-body integrity. Our results show that Salmonella infection induces PB disassembly in epithelial cells, and implicates the SPI-2 encoded T3SS and the associated SpvB effector as one factor in this process. This effect is not restricted to a single bacterial pathogen, since Shigella infection also induces PB disruption.
Previous reports have demonstrated that although PBs are not required for silencing, PB formation can be prevented by blocking the microRNA pathway, either at the level of microRNA biogenesis (e.g., by depleting Dicer or Drosha) or at the silencing step (e.g., by AGO or GW182 knockdown; [3], [4], [42]). Using deep sequencing of the small RNA population of cells infected with Salmonella, we have shown that bacterial infection induces the regulation of a limited number of microRNAs (Schulte et al., in revision). Specifically, we found that most microRNAs of HeLa cells were unaffected by Salmonella, with only the let-7 family of microRNAs being twofold downregulated. Consistent with a limited effect on microRNA pathways, reporter vectors containing microRNA binding sites were here observed to be invariably regulated during Salmonella infection. A recent study in human macrophage-like THP1 cells revealed that LPS stimulation increases of the number of PBs per cell, and attributed this effect to increased miR-146 expression [43]. However, stimulation of HeLa cells with LPS does not affect PB number or miR-146 levels (Schulte et al., in revision), likely due to the fact that the LPS sensing components TLR2 and the TLR4 co-factor MD-2 are not expressed in HeLa cells [44], [45]. Collectively, these results argue that the Salmonella-induced PB disruption is not a simple consequence of impaired microRNA biogenesis and activity.
Interference with key components of PBs, either proteins or RNA, can also result in PB dispersion. We provide evidence that the PB disassembly upon bacterial infection is unrelated with an effect on the general mRNA synthesis. Additionally, we show that the levels of crucial PB proteins (DDX6, AGO2 and TNRC6A) remain unchanged during infection. Likewise, PB formation remains intact in infected cells treated with the protein synthesis inhibitor puromycin, arguing that Salmonella infection does not block PB formation, but rather disassembles existing PBs. This again supports the notion that essential PB biogenesis components are not directly targeted by Salmonella infection. Intriguingly, however, we observe that the relative increase in PB formation after puromycin treatment is strongest in Salmonella-containing cells, as if the infection limits the pool of translationally repressed cytoplasmic mRNAs required for PB formation.
Analysis of PB integrity over the course of infection suggests that PB disruption occurs late, and argues that bacterial internalization, per se, is not sufficient for the observed phenomenon. Moreover, our results demonstrate that the Salmonella SPI-2 T3SS is essential for PB dispersion. That is, there was no PB disassembly in cells infected with Salmonella mutants deprived of either the complete SPI-2 island or individual components of the SpiC/SsaM/SsaL complex required for SPI-2 dependent translocon assembly and effector secretion [33], [34]. Although a matter of controversy, the SpiC protein was also reported as being translocated by the SPI-2 T3SS, and to interfere with endosomal trafficking [46]. However, given that overexpression of SpiC in HeLa cells had no effect on PB integrity (data not shown), SpiC function as a secreted effector protein is unlikely involved in PB dispersion.
Our candidate approach has identified the SPI-2 effector SpvB as a potential mediator of PB disruption; of all mutants tested, the Salmonella ΔspvB strain was consistently impaired in PB disassembly, relative to the wild-type strain. The plasmid-encoded SpvB protein has been known to induce depolymerization of the actin cytoskeleton at late stages of infection via its ADP-ribosyl-transferase activity [35], [36], [37], [38], [39]. At first glance, our result showing that a catalytically inactive ADP-ribosyl-transferase mutant of SpvB is also unable to disperse PBs lends itself for the straight-forward conclusion that SpvB's actin-depolymerizing activity critically contributes to PB dispersion by Salmonella. However, while previous studies showed that microtubule disruption increases in the average number of PB per cell, interference with the actin cytoskeleton has little effects on PB assembly [47], [48]. Moreover, cytochalasin D – an inhibitor of actin polymerization – was here found to have no impact on PB disruption. In other words, although the same region of SpvB is critical for both processes, PB disruption cannot be a simple consequence of SpvB-mediated actin depolymerization. Interestingly, the N-terminus of SpvB has homology with the TcaC protein of Photorhabdus luminescens, a secreted insecticidal toxin that acts through an unknown mechanism and causes cytopathology [49]. Thus, is the observed PB disruption a consequence of a more general cytotoxic effect? Rather not: when we overexpressed SpvB alone in HeLa cells from a strong eukaryotic promoter, transfected cells did show considerable morphological changes and reduction of F-actin staining yet PB numbers remained unaffected (data not shown).
Whilst the precise mechanism of SpvB action is yet to be unraveled, its impact on PBs is likely part of a multi-factorial process. First, although the ΔspvB strain was consistently impaired in PB disruption, the lack of SpvB alone does not match the stronger impairment of strains deficient in SPI-2 activity, suggesting redundant function of another factor(s) in the same pathway. Second, expression of SpvB in HeLa cells does result in actin depolymerization yet is not sufficient to disperse PB, suggesting that SpvB either needs the activity of other bacterial or cellular proteins either translocated by Salmonella or induced in the host by the infection, respectively. Third, the spvB gene is induced rather early during infection [50], [51], whereas PB disruption is a late process. Moreover, although Shigella also induces PB dispersion, none of the known secreted Shigella effectors shows obvious homology with SpvB, and none of the characterized effectors in Shigella has been shown to have ADP-ribosyl-transferase activity. Thus, future work to shed light on the mechanisms of PB dispersion will require a comprehensive analysis of protein abundance changes and putative SpvB interaction partners during Salmonella infection.
Recent work in viral infection systems, specifically infection with Dengue and West Nile viruses, has shown that the PB number per cell decreases at late times post-infection, but no insights regarding the proteins, pathways or relevance of this phenotype were obtained [17]. Interestingly, in the case of SGs and poliovirus infection, which early on induces the formation of these granules, but later loses this ability, a viral protease was shown to cleave G3BP, a critical SG component, and by this mechanism block SG formation and increase viral replication [52]. Undoubtedly more research is necessary to determine what is the mechanism of PB disassembly induced by bacterial and viral infection, and if this phenotype is a requirement and/or a consequence of infection. Nevertheless, our study identifying PB integrity as a novel target of bacterial infection, following similar observations for some viruses, highlights the physiological importance of PB disruption by pathogens.
Materials and Methods
Bacterial strains
Salmonella enterica serovar Typhimurium strain SL1344 constitutively expressing GFP from a chromosomal locus (strain JVS-3858) is referred to as wild-type (WT) throughout this work. The mutant strains were constructed from a non-tagged strain (JVS1574) using the lambda red recombinase method [53]. A complete list of the primers used to construct and verify the mutant strains is provided in Table S1. The collection of mutant strains was tagged with GFP by phage P22 transduction of the respective strain with a lysate of strain JVS-3858. In the complemented strains, the proteins SpiC and SpvB are expressed from the pXG0 low copy plasmid, under the control of the respective promoter. The primers used for cloning are indicated in Table S2. Shigella flexneri serotype 5 strain M90T was kindly provided by Anna Zumsteg (Max Planck Institute for Infection Biology, Berlin Germany).
Cell culture, heat-shock, LPS and puromycin treatment
HeLa (ATCC, CCL-2), RAW 264.7 (ATCC, TIB-71) and A431 (ATCC, CRL-1555) cells were grown in RPMI 1640 (GIBCO) supplemented with 10% fetal calf serum (Biochrom), 2 mM L-glutamine (GIBCO), 1 mM sodium-pyrovate (GIBCO) and 0.5% β-mercapto-ethanol (GIBCO) in a 5% CO2, humidified atmosphere, at 37°C.
SG formation was induced by heat-shock, by incubating the cells 1 hour at 46°C in a 5% CO2, humidified atmosphere. For LPS treatment, cells were incubated with 1 µg/ml purified LPS from Salmonella enteric serovar Typhimurium (Sigma) for 20 hours. Puromycin treatment was performed by incubating the cells for 1 hour with 100 µg/ml puromycin (Calbiochem).
Bacterial infection and quantification of Salmonella intracellular replication
Twenty-four hours prior to infection, 2.0×105 of cells were seeded into Lab-Tek II chamber slides (Nalge Nunc; for immunofluorescence) or 12-well plates (for protein samples and intracellular replication assays).
Overnight cultures of Salmonella or Shigella were diluted 1∶100 in fresh L-broth medium and grown aerobically until OD 2. Bacteria were harvested by centrifugation and resuspended in complete RPMI medium. For immunofluorescence and intracellular replication assays cells were infected at MOI of 5, and for preparation of protein extracts at an MOI of 50. After addition of bacteria, cells were centrifuged at 250×g for 10 min at room temperature followed by 20 min incubation in 5% CO2, humidified atmosphere, at 37°C. Medium was then replaced with complete RPMI containing gentamicin (50 µg/ml) to kill extracellular bacteria. Following 30 min incubation, the medium was replaced with new complete RPMI containing 10 µg/ml gentamicin for the rest of the experiment. Unless otherwise indicated, cells were collected 20 hours after infection.
To quantify Salmonella intracellular replication, infected HeLa cells were thoroughly washed with phosphate-buffered saline (PBS) and lysed with PBS containing 0.1% Triton-X-100. Samples were then serially diluted in PBS and plated on LB plates. The number of colonies formed from the recovered bacteria was compared to that obtained from the input bacteria used for infection.
Immunofluorescence and total RNA staining
Cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.5% Triton X-100 in PBS during 5 min, followed by 30 min blocking in 1% bovine serum albumin (BSA). Cells were then stained for 1 hour at room temperature with the following primary antibodies diluted in blocking solution: rabbit anti-DDX6 (Bethyl; 1∶500), human anti-GW182 (IC-6 serum, kindly provided by Marvin J. Fritzler, University of Calgary, Calgary, Canada; 1∶5000), mouse anti-TIAR (BD Transduction Laboratories; 1∶200), or rabbit anti-Shigella flexneri (Axell; 1∶200). Cells were washed with PBS and incubated for 1 hour with the respective secondary antibodies conjugated to Alexa Fluor 488 or 594 (Invitrogen; 1∶500).
For staining of total RNA the Click-iT RNA imaging kit (Invitrogen) was used according to the manufacturer's instructions. Briefly, mock-treated or Salmonella infected cells were labelled with 0.5 mM 5-ethynyl uridine (EU) for the 20 hours of infection, cells were then fixed, permeabilized as described above, and the Click-iT reaction cocktail containing the Alexa Fluor 594 azide was added for 30 min. Cells were then washed and processed for immunofluorescence using mouse anti-Salmonella LPS (Abcam: 1∶1000) and Alexa 488 labelled goat anti-mouse (Invitrogen; 1∶500), as described above.
In all cases, cells were stained with Hoechst 33342 (Invitrogen; 1∶5000) diluted in PBS and mounted in Vectashield mounting medium (Vector Laboratories). Images were acquired using a Leica DMR microscope equipped with a Nikon Dxm1200F digital camera.
P-body quantification
PB quantification was performed at the ICGEB High-Throughput Screening Facility (http://www.icgeb.org/high-throughput-screening.html). Image acquisition was performed using an ImageXpress Micro automated high-content screening microscope (Molecular Devices) equipped with a 40x objective; a total of 48 fields were acquired from each coverslip, which corresponds to an average of ca. 1000 cells imaged and analysed per experimental condition and replicate. Automated image analysis was then performed in two sequential steps using the MetaXpress software (Molecular Devices). Firstly, nuclei were segmented and cells were classified as positive or negative for Salmonella, depending on the total area of Salmonella staining (GFP, green channel) above the background, and subsequently the number of PB was quantified for each cell (red channel); analysis were performed using the “Multi Wavelength Cell Scoring” and “Transfluor” application modules built in MetaXpress. Salmonella and PBs are assigned to a specific cell based on their proximity to neighboring nuclei. Results were then combined, on a cell by cell basis, using AcuityXpress software (Molecular Devices) and the average number of PB per cell was calculated for the population of cells positive or negative for intracellular Salmonella.
Whole-cell protein extracts and Western-blot
Cell pellets were resuspended in 1x sample loading buffer (Fermentas), and protein samples were separated in a 10% SDS-PAGE, followed by Western-blotting. The following antibodies were used: mouse anti-GFP (Roche; 1∶1000), rabbit anti-DDX6 (Bethyl; 1∶1000), rat anti-AGO2 (kindly provided by Gunter Meister, Max Planck Institute of Biochemistry, Martinsried, Germany; 1∶2000), mouse anti-GW182 (Santa-Cruz; 1∶50) and mouse anti-β-actin (Applied Biosystems; 1∶2000). Bound primary antibodies were detected with horseradish peroxidase-coupled secondary antibodies (Amersham; 1∶5000) followed by detection using Western Lightning reagent (PerkinElmer). Signals were detected with a Fuji LAS-3000 CCD camera.
Transfection and Luciferase assays
microRNA reporter plasmids containing binding sites for miR-21, miR-23a, miR-16, miR-1 or miR-122 downstream of the renilla luciferase coding sequence of the psiCHECK2 luciferase reporter vector (Promega) were kindly designed, cloned and provided as a gift for research purposes by Thermo Fisher Scientific Research and Development scientists.
For the transfection experiments 5.0×104 HeLa cells were seeded onto 12-well plates 24 hours prior to transfection. Reporter vectors (1 µg) were transfected using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. Twenty-four hours after transfection cells were mock-treated or infected with Salmonella as described previously, and cells were collected 20 hours later. Firefly and renilla luciferase activities were measured on a Wallac 1420 Victor3 multilabel counter (PerkinElmer) using beetle-Juice and renilla-juice (PJK GmbH).
Statistics
The mean ± standard deviations for at least three independent experiments is shown in figures, and p values were calculated using One-way ANOVA and Tukey's Multiple Comparison Test (Graph Pad, Prism Software). A p value of less than 0.05 was considered to be statistically significant.
Supporting Information
Overview of the procedure to quantify the number of PBs in Salmonella infected cells. (A) Low-resolution montage showing 48 image fields acquired at a 40x magnification. (B) High-resolution image of Salmonella infected cells. Cell nucleus was stained with Hoechst 33342 (blue), Salmonella was detected in the green channel and PBs were detected using anti-DDX6 antibody (red channel). A total of 48 fields were imaged per coverslip, which corresponds to approx. 1000 cells per experimental condition. (C) Original images and results from the image segmentation showing Salmonella positive cells (green cells, bottom Salmonella pannel) and PBs (rightmost bottom panels).
(TIF)
PB disruption induced by Salmonella infection is cell-type dependent. (A) HeLa cells were mock-treated or infected with Salmonella for 20 hours. PBs were detected using anti-GW182 (red channel). Scale bar, 10 µm. The region highlighted by a white square is enlarged on the right side of the panel. (B) RAW264.7 and A431 cells were mock-treated or infected with Salmonella for 20 hours. PBs were stained with anti-DDX6 antibody (red channel). (C) SG formation was tested in HeLa cells infected for 2 and 4 hours with Salmonella. Cells were stained with anti-TIAR antibody (red channel).
(TIF)
SpiC/SsaM/SsaL complex is essential for Salmonella interference with PB integrity. HeLa cells were infected with ΔSsaM and ΔSsaL Salmonella strains for 20 hours. PBs were stained with anti-DDX6 antibody (red channel). Scale bar, 10 µm. The region indicated by a white square is enlarged on the rightmost panel.
(TIF)
PB integrity is affected by infection with Salmonella mutant strains of the tested SPI-2 T3SS dependent effector proteins. HeLa cells were infected with the indicated Salmonella mutant strains for 20 hours. PBs were stained with anti-DDX6 antibody (red channel). Scale bar, 10 µm. The region indicated by a white square is enlarged in the panel below.
(TIF)
List of the primers used to construct and verify the Salmonella mutant strains.
(PDF)
List of the primers used for cloning in pXG0-amp.
(PDF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Work in the Vogel lab is supported by NGFN and by the Sixth Framework Programme of the European Commission through the SIROCCO Integrated Project. M.M. was supported by Project “SeND - Search for New Drugs,” funded by Region Friuli Venezia Giulia and III supplementary contract of Framework Programme on Scientific Research in Region Friuli Venezia Giulia, Italy. This publication was funded by the German Research Foundation (DFG) in the funding programme Open Access Publishing. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Balagopal V, Parker R. Polysomes, P bodies and stress granules: states and fates of eukaryotic mRNAs. Curr Opin Cell Biol. 2009;21:403–408. doi: 10.1016/j.ceb.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol. 2007;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- 3.Eulalio A, Behm-Ansmant I, Schweizer D, Izaurralde E. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol Cell Biol. 2007;27:3970–3981. doi: 10.1128/MCB.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauley KM, Eystathioy T, Jakymiw A, Hamel JC, Fritzler MJ, et al. Formation of GW bodies is a consequence of microRNA genesis. EMBO Rep. 2006;7:904–910. doi: 10.1038/sj.embor.7400783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu CY, Rana TM. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 2006;4:e210. doi: 10.1371/journal.pbio.0040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lian S, Fritzler MJ, Katz J, Hamazaki T, Terada N, et al. Small interfering RNA-mediated silencing induces target-dependent assembly of GW/P bodies. Mol Biol Cell. 2007;18:3375–3387. doi: 10.1091/mbc.E07-01-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson P, Kedersha N. RNA granules. J Cell Biol. 2006;172:803–808. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, et al. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckham CJ, Parker R. P bodies, stress granules, and viral life cycles. Cell Host Microbe. 2008;3:206–212. doi: 10.1016/j.chom.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mas A, Alves-Rodrigues I, Noueiry A, Ahlquist P, Diez J. Host deadenylation-dependent mRNA decapping factors are required for a key step in brome mosaic virus RNA replication. J Virol. 2006;80:246–251. doi: 10.1128/JVI.80.1.246-251.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheller N, Mina LB, Galao RP, Chari A, Gimenez-Barcons M, et al. Translation and replication of hepatitis C virus genomic RNA depends on ancient cellular proteins that control mRNA fates. Proc Natl Acad Sci U S A. 2009;106:13517–13522. doi: 10.1073/pnas.0906413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diez J, Ishikawa M, Kaido M, Ahlquist P. Identification and characterization of a host protein required for efficient template selection in viral RNA replication. Proc Natl Acad Sci U S A. 2000;97:3913–3918. doi: 10.1073/pnas.080072997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noueiry AO, Diez J, Falk SP, Chen J, Ahlquist P. Yeast Lsm1p-7p/Pat1p deadenylation-dependent mRNA-decapping factors are required for brome mosaic virus genomic RNA translation. Mol Cell Biol. 2003;23:4094–4106. doi: 10.1128/MCB.23.12.4094-4106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathans R, Chu CY, Serquina AK, Lu CC, Cao H, et al. Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol Cell. 2009;34:696–709. doi: 10.1016/j.molcel.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougherty JD, White JP, Lloyd RE. Poliovirus Mediated Disruption of Cytoplasmic Processing Bodies. J Virol. doi: 10.1128/JVI.01657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emara MM, Brinton MA. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc Natl Acad Sci U S A. 2007;104:9041–9046. doi: 10.1073/pnas.0703348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JA, Schmechel SC, Raghavan A, Abelson M, Reilly C, et al. Reovirus induces and benefits from an integrated cellular stress response. J Virol. 2006;80:2019–2033. doi: 10.1128/JVI.80.4.2019-2033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piotrowska J, Hansen SJ, Park N, Jamka K, Sarnow P, et al. Stable formation of compositionally unique stress granules in virus-infected cells. J Virol. 2010;84:3654–3665. doi: 10.1128/JVI.01320-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McInerney GM, Kedersha NL, Kaufman RJ, Anderson P, Liljestrom P. Importance of eIF2alpha phosphorylation and stress granule assembly in alphavirus translation regulation. Mol Biol Cell. 2005;16:3753–3763. doi: 10.1091/mbc.E05-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 22.McGhie EJ, Brawn LC, Hume PJ, Humphreys D, Koronakis V. Salmonella takes control: effector-driven manipulation of the host. Curr Opin Microbiol. 2009;12:117–124. doi: 10.1016/j.mib.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galan JE. Common themes in the design and function of bacterial effectors. Cell Host Microbe. 2009;5:571–579. doi: 10.1016/j.chom.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 25.Mastroeni P, Grant A, Restif O, Maskell D. A dynamic view of the spread and intracellular distribution of Salmonella enterica. Nat Rev Microbiol. 2009;7:73–80. doi: 10.1038/nrmicro2034. [DOI] [PubMed] [Google Scholar]
- 26.Bhanji RA, Eystathioy T, Chan EK, Bloch DB, Fritzler MJ. Clinical and serological features of patients with autoantibodies to GW/P bodies. Clin Immunol. 2007;125:247–256. doi: 10.1016/j.clim.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blobel G, Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971;68:390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maroney PA, Yu Y, Fisher J, Nilsen TW. Evidence that microRNAs are associated with translating messenger RNAs in human cells. Nat Struct Mol Biol. 2006;13:1102–1107. doi: 10.1038/nsmb1174. [DOI] [PubMed] [Google Scholar]
- 29.Zheng D, Ezzeddine N, Chen CY, Zhu W, He X, et al. Deadenylation is prerequisite for P-body formation and mRNA decay in mammalian cells. J Cell Biol. 2008;182:89–101. doi: 10.1083/jcb.200801196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999;147:1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nonhoff U, Ralser M, Welzel F, Piccini I, Balzereit D, et al. Ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6 and interferes with P-bodies and stress granules. Mol Biol Cell. 2007;18:1385–1396. doi: 10.1091/mbc.E06-12-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jao CY, Salic A. Exploring RNA transcription and turnover in vivo by using click chemistry. Proc Natl Acad Sci U S A. 2008;105:15779–15784. doi: 10.1073/pnas.0808480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu XJ, McGourty K, Liu M, Unsworth KE, Holden DW. pH sensing by intracellular Salmonella induces effector translocation. Science. 2010;328:1040–1043. doi: 10.1126/science.1189000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu XJ, Liu M, Holden DW. SsaM and SpiC interact and regulate secretion of Salmonella pathogenicity island 2 type III secretion system effectors and translocators. Mol Microbiol. 2004;54:604–619. doi: 10.1111/j.1365-2958.2004.04297.x. [DOI] [PubMed] [Google Scholar]
- 35.Lesnick ML, Reiner NE, Fierer J, Guiney DG. The Salmonella spvB virulence gene encodes an enzyme that ADP-ribosylates actin and destabilizes the cytoskeleton of eukaryotic cells. Mol Microbiol. 2001;39:1464–1470. doi: 10.1046/j.1365-2958.2001.02360.x. [DOI] [PubMed] [Google Scholar]
- 36.Browne SH, Lesnick ML, Guiney DG. Genetic requirements for salmonella-induced cytopathology in human monocyte-derived macrophages. Infect Immun. 2002;70:7126–7135. doi: 10.1128/IAI.70.12.7126-7135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tezcan-Merdol D, Nyman T, Lindberg U, Haag F, Koch-Nolte F, et al. Actin is ADP-ribosylated by the Salmonella enterica virulence-associated protein SpvB. Mol Microbiol. 2001;39:606–619. doi: 10.1046/j.1365-2958.2001.02258.x. [DOI] [PubMed] [Google Scholar]
- 38.Kurita A, Gotoh H, Eguchi M, Okada N, Matsuura S, et al. Intracellular expression of the Salmonella plasmid virulence protein, SpvB, causes apoptotic cell death in eukaryotic cells. Microb Pathog. 2003;35:43–48. doi: 10.1016/s0882-4010(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 39.Otto H, Tezcan-Merdol D, Girisch R, Haag F, Rhen M, et al. The spvB gene-product of the Salmonella enterica virulence plasmid is a mono(ADP-ribosyl)transferase. Mol Microbiol. 2000;37:1106–1115. doi: 10.1046/j.1365-2958.2000.02064.x. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa M, Handa Y, Ashida H, Suzuki M, Sasakawa C. The versatility of Shigella effectors. Nat Rev Microbiol. 2008;6:11–16. doi: 10.1038/nrmicro1814. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz-Ferrer V, Voinnet O. Roles of plant small RNAs in biotic stress responses. Annu Rev Plant Biol. 2009;60:485–510. doi: 10.1146/annurev.arplant.043008.092111. [DOI] [PubMed] [Google Scholar]
- 42.Yang Z, Jakymiw A, Wood MR, Eystathioy T, Rubin RL, et al. GW182 is critical for the stability of GW bodies expressed during the cell cycle and cell proliferation. J Cell Sci. 2004;117:5567–5578. doi: 10.1242/jcs.01477. [DOI] [PubMed] [Google Scholar]
- 43.Pauley KM, Satoh M, Pauley BA, Dominguez-Gutierrez PR, Wallet SM, et al. Formation of GW/P bodies as marker for microRNA-mediated regulation of innate immune signaling in THP-1 cells. Immunol Cell Biol. 2010;88:205–212. doi: 10.1038/icb.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wyllie DH, Kiss-Toth E, Visintin A, Smith SC, Boussouf S, et al. Evidence for an accessory protein function for Toll-like receptor 1 in anti-bacterial responses. J Immunol. 2000;165:7125–7132. doi: 10.4049/jimmunol.165.12.7125. [DOI] [PubMed] [Google Scholar]
- 45.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uchiya K, Barbieri MA, Funato K, Shah AH, Stahl PD, et al. A Salmonella virulence protein that inhibits cellular trafficking. Embo J. 1999;18:3924–3933. doi: 10.1093/emboj/18.14.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sweet TJ, Boyer B, Hu W, Baker KE, Coller J. Microtubule disruption stimulates P-body formation. Rna. 2007;13:493–502. doi: 10.1261/rna.355807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aizer A, Brody Y, Ler LW, Sonenberg N, Singer RH, et al. The dynamics of mammalian P body transport, assembly, and disassembly in vivo. Mol Biol Cell. 2008;19:4154–4166. doi: 10.1091/mbc.E08-05-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowen D, Rocheleau TA, Blackburn M, Andreev O, Golubeva E, et al. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science. 1998;280:2129–2132. doi: 10.1126/science.280.5372.2129. [DOI] [PubMed] [Google Scholar]
- 50.Hautefort I, Thompson A, Eriksson-Ygberg S, Parker ML, Lucchini S, et al. During infection of epithelial cells Salmonella enterica serovar Typhimurium undergoes a time-dependent transcriptional adaptation that results in simultaneous expression of three type 3 secretion systems. Cell Microbiol. 2008;10:958–984. doi: 10.1111/j.1462-5822.2007.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fierer J, Eckmann L, Fang F, Pfeifer C, Finlay BB, et al. Expression of the Salmonella virulence plasmid gene spvB in cultured macrophages and nonphagocytic cells. Infect Immun. 1993;61:5231–5236. doi: 10.1128/iai.61.12.5231-5236.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White JP, Cardenas AM, Marissen WE, Lloyd RE. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe. 2007;2:295–305. doi: 10.1016/j.chom.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of the procedure to quantify the number of PBs in Salmonella infected cells. (A) Low-resolution montage showing 48 image fields acquired at a 40x magnification. (B) High-resolution image of Salmonella infected cells. Cell nucleus was stained with Hoechst 33342 (blue), Salmonella was detected in the green channel and PBs were detected using anti-DDX6 antibody (red channel). A total of 48 fields were imaged per coverslip, which corresponds to approx. 1000 cells per experimental condition. (C) Original images and results from the image segmentation showing Salmonella positive cells (green cells, bottom Salmonella pannel) and PBs (rightmost bottom panels).
(TIF)
PB disruption induced by Salmonella infection is cell-type dependent. (A) HeLa cells were mock-treated or infected with Salmonella for 20 hours. PBs were detected using anti-GW182 (red channel). Scale bar, 10 µm. The region highlighted by a white square is enlarged on the right side of the panel. (B) RAW264.7 and A431 cells were mock-treated or infected with Salmonella for 20 hours. PBs were stained with anti-DDX6 antibody (red channel). (C) SG formation was tested in HeLa cells infected for 2 and 4 hours with Salmonella. Cells were stained with anti-TIAR antibody (red channel).
(TIF)
SpiC/SsaM/SsaL complex is essential for Salmonella interference with PB integrity. HeLa cells were infected with ΔSsaM and ΔSsaL Salmonella strains for 20 hours. PBs were stained with anti-DDX6 antibody (red channel). Scale bar, 10 µm. The region indicated by a white square is enlarged on the rightmost panel.
(TIF)
PB integrity is affected by infection with Salmonella mutant strains of the tested SPI-2 T3SS dependent effector proteins. HeLa cells were infected with the indicated Salmonella mutant strains for 20 hours. PBs were stained with anti-DDX6 antibody (red channel). Scale bar, 10 µm. The region indicated by a white square is enlarged in the panel below.
(TIF)
List of the primers used to construct and verify the Salmonella mutant strains.
(PDF)
List of the primers used for cloning in pXG0-amp.
(PDF)