Abstract
Background
A wide spectrum of disease severity has been described for Human African Trypanosomiasis (HAT) due to Trypanosoma brucei rhodesiense (T.b. rhodesiense), ranging from chronic disease patterns in southern countries of East Africa to an increase in virulence towards the north. However, only limited data on the clinical presentation of T.b. rhodesiense HAT is available. From 2006-2009 we conducted the first clinical trial program (Impamel III) in T.b. rhodesiense endemic areas of Tanzania and Uganda in accordance with international standards (ICH-GCP). The primary and secondary outcome measures were safety and efficacy of an abridged melarsoprol schedule for treatment of second stage disease. Based on diagnostic findings and clinical examinations at baseline we describe the clinical presentation of T.b. rhodesiense HAT in second stage patients from two distinct geographical settings in East Africa.
Methodology/Principal Findings:
138 second stage patients from Tanzania and Uganda were enrolled. Blood samples were collected for diagnosis and molecular identification of the infective trypanosomes, and T.b. rhodesiense infection was confirmed in all trial subjects. Significant differences in diagnostic parameters and clinical signs and symptoms were observed: the median white blood cell (WBC) count in the cerebrospinal fluid (CSF) was significantly higher in Tanzania (134cells/mm3) than in Uganda (20cells/mm3; p<0.0001). Unspecific signs of infection were more commonly seen in Uganda, whereas neurological signs and symptoms specific for HAT dominated the clinical presentation of the disease in Tanzania. Co-infections with malaria and HIV did not influence the clinical presentation nor treatment outcomes in the Tanzanian study population.
Conclusions/Significance
We describe a different clinical presentation of second stage T.b. rhodesiense HAT in two distinct geographical settings in East Africa. In the ongoing absence of sensitive diagnostic tools and safe drugs to diagnose and treat second stage T.b. rhodesiense HAT an early identification of the disease is essential. A detailed understanding of the clinical presentation of T.b. rhodesiense HAT among health personnel and affected communities is vital, and awareness of regional characteristics, as well as implications of co-infections, can support decision making and differential diagnosis.
Author Summary
Sleeping sickness, or Human African Trypanosomiasis (HAT), caused by Trypanosoma brucei rhodesiense is one of the most neglected tropical diseases. It affects mainly rural, poor East African populations and has very high socio-economic impacts. T.b. rhodesiense HAT is an acute disease; patients quickly progress from the first stage, where trypanosomes are detectable in blood and lymph, to the second stage, where parasites penetrate the central nervous system. If left untreated, T.b. rhodesiense HAT is fatal. Disease control is hampered by the absence of sensitive diagnostic tools and safe drugs. Second stage patients can only be treated with melarsoprol, a highly toxic, arsenical drug. It is more difficult to treat patients successfully at advanced stages of the disease, and late onset of treatment should be avoided. Yet, most patients are treated for other conditions prior to HAT diagnosis. Therefore, it is important that health personnel in T.b. rhodesiense endemic regions have a detailed understanding of the clinical presentation of the disease and consider regional characteristics of T.b. rhodesiense HAT for decision making and differential diagnosis.
Introduction
Human African Trypanosomiasis (HAT), also known as sleeping sickness, is caused by the protozoan parasites T.b. gambiense (West and Central Africa) and T.b. rhodesiense (East and South Africa). The disease is transmitted by tsetse flies (Glossina ssp.) predominantly in the rural areas of most of sub Saharan Africa. 60 Million people live at risk of infection, but less than 10% are under adequate surveillance [1], reflecting its neglected status. Sleeping sickness caused by either subspecies presents in two disease stages defined as the first, or haemo-lymphatic stage and the second, meningo-encephalitic stage. Diagnosis of HAT is made in blood, lymph and the cerebrospinal fluid (CSF). The second stage of the disease is indicated by the presence of trypanosomes and/or an elevated white blood cell (WBC) count (≥5WBC/mm3) in the CSF. The disease stage and the causative species of infection direct the choice of treatment. T.b. gambiense infections are treated with pentamidine in the first stage and eflornithine, a combination of eflornithine and nifurtimox, or melarsoprol in the second stage [2]–[4]. T.b. rhodesiense first and second stage infections are treated with suramin and melarsoprol respectively [2]. In the field, the trypanosome subspecies is entirely determined by the geographical location of the patient as the distinction of T.b. gambiense and T.b. rhodesiense is only possible in well equipped laboratories through PCR analysis. The detection of the human serum resistance-associated (SRA) gene unequivocally identifies T.b. rhodesiense trypanosomes [5], [6]. In Uganda, the only country where both forms of the disease are present, a potential geographical overlap of the two endemic areas has become likely [7]. This would hamper determination of infective trypanosomes under field conditions and therefore also the identification of the correct treatment.
For first stage infections there are no specific clinical signs and symptoms in both forms of the disease; fever, headache and loss of appetite are common. In T.b. rhodesiense the presence of a chancre at the site of the infective bite may be indicative for a trypanosome infection [8]. Second stage infections show disease-characteristic neuro-psychiatric signs and symptoms: severe endocrinological and mental disturbances and severe motor problems are the main signs [9]. While often considered together, Gambiense and Rhodesiense HAT are clinically and epidemiologically different diseases [10]. T.b. gambiense HAT is a chronic disease, whereas T.b. rhodesiense is characterized by an acute disease progression. If left untreated, both forms of HAT are fatal. The mean time to reach the second stage has been estimated at over one year for T.b. gambiense [11] but only 3 weeks for T.b. rhodesiense HAT [12]. Correspondingly, average times from infection to death are almost 3 years and 6 to 12 months, respectively [11], [12].
A diversity of forms of clinical progression from asymptomatic to acute have been reported for T.b. gambiense infections [13]–[15]. This seems to be even more pronounced for T.b. rhodesiense infections; a wide spectrum of disease severity ranging from a chronic disease pattern in southern countries of East Africa with existing reports of asymptomatic carriers [16] to an increase in virulence towards the north had been described [17]. Even though those differences were already described more than 60 years ago [18] the first comparative study was carried out in 2004: on the basis of the SRA gene polymorphism, trypanosomes isolates from Uganda (acute profile) and Malawi (chronic profile) confirmed to be of different genotypes. However, clinical characteristics of the study groups were limited the presence of a chancre and the self-reported duration of illness [19]. Another hypothesis postulates that the differences in disease severity could be attributed to differences in genetic resistance to trypanosomiasis among host populations [18].
From the estimated 50′000 to 70′00 cases per year [20], over 97% are T.b. gambiense cases and only a few thousand are due to T.b. rhodesiense [1]. Therefore, most literature concentrates on T.b. gambiense HAT. Its clinical picture and related cardiac and endocrinological disorders have been extensively described [21]–[28]. On the other hand, literature on the clinical aspects of T.b. rhodesiense HAT is scarce. We identified four studies (see table 1) describing its clinical presentation. Only one study in 60 patients infected with T.b. rhodesiense was designed prospectively and used a standardized questionnaire [29].
Table 1. Published literature on clinical signs & symptoms of T.b. rhodesiense HAT.
| Buyst/1977 [18] | Boatin/1986 [29] | Wellde/1989 [35] | Mbulamberi/1987 [36] | |
| Number of patients | 385 | 60 | 96 | 3152 |
| Country | Zambia | Zambia | Zambia | Uganda |
| Disease stage of patients | 2nd stage | 2nd stage | 2nd stage | 1st stagea |
| Male/female ratio | N.A. | 1.73 | 1.53 | 1.1 |
| Chancre | N.A. | 5 | 15.6 | 19 |
| Headache | 66.2 | 73.3 | 95.8 | 95.8 |
| Fever | 31.2 | 71.7 | 36.4 | 96.8 |
| Lymphadenopathy | 80.5 | N.A. | 86.4 | 17.6 |
| Itching or pruritus | N.A. | 35 | 53.1 | N.A. |
| Oedema of face | 30.1b | 21.7 | 3.1 | 27.5 |
| Swelling of legs | N.A. | 43.3 | 25.3 | N.A. |
| Joint pains | N.A. | 65 | 88.5 | 95 |
| Day time sleep | N.A. | 63.3 | 70.8 | 26.8c |
| Night time sleep | N.A. | 28.3 | N.A. | N.A. |
| Abnormal coordination | N.A. | N.A. | 51 | N.A. |
| Abnormal speech | N.A. | N.A. | 38.5 | N.A. |
| Mental confusion | 17.4 | N.A. | N.A. | N.A. |
N.A: not applicable;
98.7% of the patients were in the first stage and 1.3% of the patients in the second stage of the disease;
reported as oedema,
reported as somnolence
In this paper we describe the clinical presentation of second stage T.b. rhodesiense HAT in 138 patients from two distinct geographical settings in East Africa. We compare our findings to the existing literature and discuss factors that could explain the differences observed.
Materials and Methods
Study sites
The Kaliua Health Centre (KHC), a 50-bed missionary hospital in Tanzania (Urambo District) and the Lwala Hospital, a designated 100- bed district hospital in Uganda (Kaberamaido District) participated in the Impamel III program (improved application of melarsoprol).
Study design and data collection
A proof-of-concept trial (n = 60) followed by a utilization study (n = 78) to assess the safety and efficacy of the abridged, 10-day melarsoprol schedule for the treatment of second stage HAT [30], [31] in T.b. rhodesiense patients.
Eligible for enrolment were second stage patients with a minimum age of 6 years and confirmed second stage HAT. Patients with first stage infections, pregnant women and moribund or unconscious patients were excluded. Patients were passively enrolled at the study sites.
Diagnosis of HAT was made in blood and in CSF. Blood was examined using microscopy and/or the haematocrit centrifugation technique [32]. If trypanosomes were present, a lumbar puncture was performed for disease staging. Analysis of the CSF was done by direct microscopy and/or single modified centrifugation technique and white blood cell (WBC) count using counting chambers. Second stage infections were confirmed by the presence of trypanosomes and/or ≥5 WBC/mm3 in the CSF. The standard assessment of co-infections included malaria, filariasis and voluntary testing for HIV/AIDS.
The local Principal Investigators filled individual case report forms (CRFs). Data used for describing the clinical presentation of the disease were patient demographics, diagnostic findings, self reported duration of illness and clinical signs and symptoms on admission graded by scale of severity (grade 0, 1, 2).
Ethics statement
Each participant gave written informed consent. For the participation of children and adolescents (below 18 years) the parents, the legal representative or the guardian gave written informed consent. Ethical clearances were obtained from the Ethics Committees in Tanzania (National Institute for Medical Research), Uganda (Ministry of Health) and Switzerland (Ethics Committee of both cantons of Basel). Before first patient enrolment, the Impamel III program was registered in the database of Current Controlled Trials (ISRCTN40537886).
Data management and statistical analysis
All data were double entered and verified using Epi Data 3.1 software (www.epidata.dk) and analysis was accomplished with the statistical software package STATA Version IC10.0 (STATA, StataCorp, USA). The statistical analysis was performed comparing proportions with the Pearson Chi Square and means with the Student's t test. Logistic regression was used to test differences between groups of patients with different co-infections.
Results
The use of the abridged 10-day melarsoprol schedule for the treatment of second stage T.b. rhodesiense HAT was highly satisfactory (detailed safety and efficacy data to be published separately). In this paper we describe the clinical presentation of the disease in 138 second stage patients from Tanzania and Uganda. The majority of patients were passively detected. Nine (9) patients from Uganda (13%) were actively identified during a survey of the National Agricultural Research Organisation (NARO) in the HAT endemic region of the country. There was no significant difference between actively and passively recruited patients for the median WBC count in the CSF (actively detected: median WBC = 27, IQR = 24; passively detected: median WBC = 19, IQR = 43, p = 0.067) and the median self-reported duration of illness (actively detected: median = 3 months, IQR = 2, passively detected: median = 2 months, IQR = 4, p = 0.141). 14 patients (11 in Uganda and 3 in Tanzania) could not be examined per protocol as they died or were in a comatose state upon arrival at the study sites which led to an exclusion of those patients from the Impamel III trials.
By molecular analysis of blood samples, the presence of the SRA gene [33] was demonstrated and confirmed T.b. rhodesiense infection in all trial subjects [34].
Data on the demographic and diagnostic baseline characteristics of the study population are shown in table 2. The proportion of male (57.2%) and female (42.8%) patients was comparable. 18.8% (26/138) trial participants were younger than 16 years whereof 88.5% (23/26) were enrolled in Uganda. There were no county-specific differences for the presence of trypanosomes in blood and CSF: 99% (68/69) of patients from Tanzania and 91% (63/69) from Uganda had trypanosomes in blood (p = 0.0524) and 70% (55/69) and 86% (59/69) respectively had trypanosomes in the CSF (p = 0.3690). However, there was a significant difference for the median WBC count in the CSF in Tanzania and Uganda (134 vs. 20 WBC/mm3, p<0.0001). Also, a body mass index (BMI) below 16.5 was more frequent in patients from Uganda (p<0.0001).
Table 2. Demographic and diagnostic baseline characteristics.
| Total (n = 138) | Tanzania (n = 69) | Uganda (n = 69) | ||||
| n | % | n | % | n | % | |
| Age (years), mean ± SD | 35±19 | 38±15 | 32±22 | |||
| Age (years), range (min.-max.) | 6–85 | 9–70 | 6–85 | |||
| Male female ratio | 1.34 | 1.38 | 1.3 | |||
| Age below 16 years | 26 | 14 | 3 | 4 | 23 | 33 |
| BMIa (kg/m2) - mean ± SD | 18.5±3.4 | 19.6±2.5 | 17.3±3.8 | |||
| BMI<16.5 | 38 | 28 | 5 | 7 | 33 | 48 |
| Malaria positive on admission | 57 | 41 | 55 | 80 | 2 | 3 |
| Trypanosomes in blood | 131 | 95 | 68 | 99 | 63 | 91 |
| Trypanosomes in CSFb | 114 | 83 | 55 | 80 | 59 | 86 |
| WBCc count in CSF | ||||||
| Median | 70 | 134 | 20 | |||
| Mean ± SD | 86±82 | 135±85 | 37±40 | |||
| 0–20 cells/ul - no. (%) | 35 | 25 | 0 | 35 | 51 | |
| 21–100 cells/ul - no. (%) | 52 | 38 | 23 | 33 | 29 | 42 |
| >100 cells/ul - no. (%) | 51 | 37 | 46 | 67 | 5 | 7 |
| Patients excludedd | 14 | N.A | 3 | N.A | 11 | N.A |
| Death upon arrival | 6 | N.A | 3 | N.A | 3 | N.A |
| Comatose upon arrival | 8 | N.A | 0 | N.A | 8 | N.A |
N.A: not applicable;
Body Mass Index,
CSF: cerebrospinal fluid,
WBC: white blood cell,
exclusions due to other reasons (first stage infection, pregnancy) not shown
Clinical signs and symptoms reported at baseline and the level of significance (95%) are summarized in table 3. Headache, fever, general body pain and joint pains were common in both study populations. Clinical suspicion for cardiac insufficiency was found in both countries: 5.1% (7/138) of the patients had indication for left heart insufficiency (combination of cough and dyspnoe) and 5.8% (8/138) for right heart insufficiency (combination of oedema and hepatomegaly). Patients in Uganda had a more unspecific presentation of the disease whereas specific signs and symptoms for second stage HAT, namely sleeping disorders and aggressiveness were more common in patients from Tanzania.
Table 3. Clinical signs and symptoms at baseline and treatment outcomes.
| Total (n = 138) | Tanzania (n = 69) | Uganda (n = 69) | p-value | ||||
| n | % | n | % | n | % | ||
| Clinical signs & symptoms | |||||||
| Lymphadenopathy | 27 | 19.6 | 7 | 10.1 | 20 | 29.0 | 0.0053 |
| General body pain | 132 | 95.7 | 69 | 100.0 | 63 | 91.3 | 0.0123 |
| Headache | 128 | 92.8 | 65 | 94.2 | 63 | 91.3 | 0.5114 |
| Fever (≥37.5°C) | 37 | 26.8 | 20 | 29.0 | 17 | 24.6 | 0.5643 |
| Fever (>38.5°C) | 4 | 2.9 | 0 | 0.0 | 4 | 5.8 | 0.0424 |
| Joint pains | 129 | 93.5 | 67 | 97.1 | 62 | 89.9 | 0.0847 |
| Diarrhea | 9 | 6.5 | 1 | 1.4 | 8 | 11.6 | 0.0158 |
| Pruritus | 21 | 15.2 | 4 | 5.8 | 17 | 24.6 | 0.0003 |
| Oedema | 40 | 29.0 | 26 | 37.7 | 14 | 20.3 | 0.0244 |
| Dyspnoe | 10 | 7.2 | 1 | 1.4 | 9 | 13.0 | 0.0086 |
| Cough | 27 | 19.6 | 8 | 11.6 | 19 | 27.5 | 0.0183 |
| Tremor | 54 | 39.1 | 43 | 62.3 | 11 | 15.9 | 0.0001 |
| Hepatomegaly | 25 | 18.1 | 4 | 5.8 | 21 | 30.4 | 0.0002 |
| Splenomegaly | 51 | 37.0 | 11 | 15.9 | 40 | 58.0 | 0.0001 |
| Walking difficulties | 75 | 54.3 | 35 | 50.7 | 40 | 58.0 | 0.3928 |
| Abnormal movements | 36 | 26.1 | 31 | 44.9 | 5 | 7.2 | <0.0001 |
| Sleeping disorder daytime | 105 | 76.1 | 66 | 95.7 | 39 | 56.5 | <0.0001 |
| Sleeping disorder night time | 88 | 63.8 | 64 | 92.8 | 24 | 34.8 | <0.0001 |
| Strange behaviour | 25 | 18.1 | 15 | 21.7 | 10 | 14.5 | 0.2691 |
| Disturbed appetite | 120 | 87.0 | 60 | 87.0 | 60 | 87.0 | 1 |
| Inactivity | 100 | 72.5 | 57 | 82.6 | 43 | 62.3 | 0.0076 |
| Speech impairment | 16 | 11.6 | 6 | 8.7 | 10 | 14.5 | 0.2875 |
| Aggressiveness | 45 | 32.6 | 43 | 62.3 | 2 | 2.9 | <0.0001 |
| Treatment outcomes | |||||||
| Death | 15 | 10.1 | 7 | 10.1 | 8 | 11.6 | 0.7845 |
| Cure (parasitological & clinical) | 123 | 89.1 | 62 | 89.9 | 61 | 88.4 | 0.7845 |
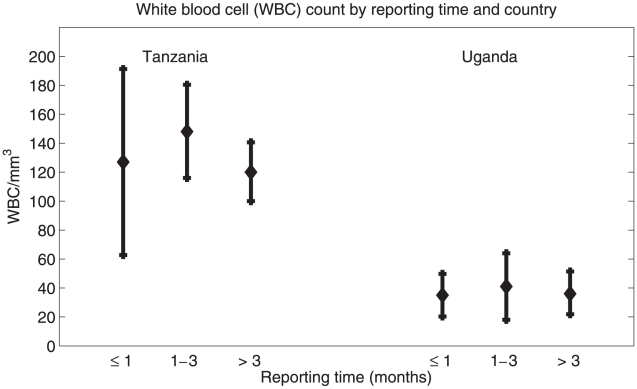
To look at changes of diagnostic markers and clinical signs and symptoms over time we compared them in patients grouped by self-reported duration of illness (see figure 1). In Tanzania and Uganda 21.7% (15/69) and 36.2% (25/69) respectively were diagnosed with HAT having signs and symptoms for one month or less. 47.8% (33/69) of patients from Tanzania and 31.9% (22/69) of patients from Uganda were diagnosed having signs and symptoms of the disease between 1 and 3 months. Respective percentages for diagnosis of HAT after feeling ill for more than 3 months were 30.4% (21/69) in Tanzania and 31.9% (22/69) in Uganda. In both countries, the presence of trypanosomes in blood and/or CSF and the WBC count in the CSF did not significantly change over time. Also, there was no change over time for most of the clinical signs and symptoms. However, we observed that tremor (p = 0.01), walking difficulties (p = 0.040), sleeping disorders at night (p = 0.029), disturbed appetite (p = 0.044) and aggressiveness (p<0.001) aggravated over time in all patients.
Figure 1. Mean and 95% confidence interval for white blood cell (WBC) count in the central nervous system (CNS) by country and reporting time.
Per protocol, standard assessment of co-infections at baseline included malaria and filariasis. 79.7% (55/69) of the patients from Tanzania and 2.9% (2/69) from Uganda were malaria positive on admission. None were found positive for filariasis. The HIV status was determined on voluntary basis. In Tanzania, 94.2% (65/69) of the patients tested their status and 24.6% (16/65) were found positive. In Uganda, 31.9% (22/69) tested their status and 9.1% (2/22) were found positive. We used the data from Tanzania to study implications of malaria and HIV co-infections on the clinical presentation and treatment outcomes of T.b. rhodesiense HAT. No significant difference either in the clinical appearance or in treatment outcomes for those patients was found. Details are shown in table 4 and 5.
Table 4. Clinical signs and symptoms and treatment outcomes in malaria co-infected patients from Tanzania.
| Total (n = 69) | Malaria negative (n = 14) | Malaria positive (n = 55) | p-value | ||||
| n | % | n | % | n | % | ||
| Clinical signs & symptoms | |||||||
| Lymphadenopathy | 7 | 10.1 | 2 | 14.3 | 5 | 9.1 | 0.569 |
| General Body Pain | 69 | 100.0 | 14 | 100.0 | 55 | 100.0 | N.A. |
| Headache | 65 | 94.2 | 14 | 100.0 | 51 | 92.7 | N.A. |
| Fever (≥37.5°C) | 20 | 29.0 | 3 | 21.4 | 17 | 30.9 | 0.488 |
| Joint pains | 67 | 97.1 | 14 | 100.0 | 53 | 96.4 | N.A. |
| Diarrhea | 1 | 1.5 | 0 | 0.0 | 1 | 1.8 | N.A. |
| Pruritus | 4 | 5.8 | 3 | 21.4 | 1 | 1.8 | 0.025 |
| Oedema | 26 | 37.7 | 7 | 50.0 | 19 | 34.6 | 0.291 |
| Dyspnoe | 1 | 1.5 | 0 | 0.0 | 1 | 1.8 | N.A. |
| Cough | 8 | 11.6 | 2 | 14.3 | 6 | 10.91 | 0.725 |
| Tremor | 43 | 62.3 | 12 | 85.7 | 31 | 56.4 | 0.058 |
| Hepatomegaly | 4 | 5.8 | 0 | 0.0 | 4 | 7.3 | N.A. |
| Splenomegaly | 11 | 15.9 | 3 | 21.4 | 8 | 14.6 | 0.533 |
| Walking difficulties | 35 | 50.7 | 8 | 57.1 | 27 | 49.1 | 0.591 |
| Abnormal movements | 31 | 44.9 | 6 | 42.9 | 25 | 45.5 | 0.862 |
| Sleeping disorder daytime | 66 | 95.7 | 11 | 78.6 | 55 | 100.0 | 0.046 |
| Sleeping disorder night time | 64 | 94.1 | 11 | 78.6 | 53 | 96.7 | 0.026 |
| Strange behaviour | 15 | 21.7 | 0 | 0.0 | 15 | 27.3 | 0.001 |
| Disturbed appetite | 60 | 87.0 | 9 | 64.3 | 51 | 92.7 | 0.010 |
| Inactivity | 57 | 82.6 | 12 | 85.7 | 45 | 81.8 | 0.732 |
| Speech impairment | 6 | 8.7 | 1 | 7.1 | 5 | 9.1 | 0.818 |
| Aggressiveness | 43 | 62.3 | 7 | 50.0 | 36 | 65.5 | 0.291 |
| Treatment outcomes | |||||||
| Death | 7 | 10.1 | 2 | 14.3 | 5 | 9.1 | 0.569 |
| Cure (parasitological & clinical) | 62 | 90.0 | 12 | 85.7 | 50 | 90.9 | 0.569 |
N.A: Malaria predicts presence/absence of sign and symptom perfectly.
Table 5. Clinical signs and symptoms and treatment outcomes of HIV co-infected patients from Tanzania.
| Total (n = 65)a | HIV negative (n = 49) | HIV positive (n = 16) | p-value | ||||
| n | % | n | % | n | % | ||
| Clinical signs & symptoms | |||||||
| Lymphadenopathy | 7 | 10.8 | 5 | 10.2 | 2 | 12.5 | 0.797 |
| General body pain | 65 | 100.0 | 49 | 100.0 | 16 | 100.0 | N.A. |
| Headache | 61 | 93.9 | 47 | 95.9 | 14 | 87.5 | 0.247 |
| Fever (≥37.5°C) | 19 | 29.2 | 17 | 34.7 | 2 | 12.5 | 0.106 |
| Joint pains | 64 | 98.5 | 49 | 100.0 | 15 | 93.8 | N.A. |
| Diarrhea | 1 | 1.5 | 1 | 2.0 | 0 | 0 | N.A. |
| Pruritus | 2 | 3.1 | 2 | 4.1 | 0 | 0 | N.A. |
| Oedema | 23 | 35.6 | 17 | 34.7 | 6 | 37.5 | 0.839 |
| Dyspnoe | 65 | 100.0 | 49 | 100.0 | 16 | 100.0 | N.A. |
| Cough | 7 | 10.8 | 4 | 8.2 | 3 | 18.6 | 0.248 |
| Tremor | 40 | 61.5 | 31 | 63.3 | 9 | 56.3 | 0.617 |
| Hepatomegaly | 4 | 6.2 | 3 | 6.1 | 1 | 6.3 | 0.985 |
| Splenomegaly | 10 | 15.4 | 8 | 16.3 | 2 | 12.5 | 0.713 |
| Walking difficulties | 32 | 49.2 | 24 | 49.0 | 8 | 50.0 | 0.943 |
| Abnormal movements | 29 | 44.6 | 22 | 44.9 | 7 | 43.8 | 0.936 |
| Sleeping disorder daytime | 63 | 96.9 | 47 | 95.9 | 16 | 100.0 | N.A. |
| Sleeping disorder night time | 62 | 95.4 | 48 | 98.0 | 14 | 87.5 | 0.127 |
| Strange behaviour | 14 | 21.5 | 9 | 18.4 | 5 | 31.3 | 0.282 |
| Disturbed appetite | 57 | 87.7 | 44 | 89.8 | 13 | 81.3 | 0.373 |
| Inactivity | 53 | 81.5 | 40 | 81.6 | 13 | 81.3 | 0.973 |
| Speech impairment | 6 | 9.2 | 5 | 10.2 | 1 | 6.3 | 0.639 |
| Aggressiveness | 43 | 66.1 | 34 | 69.4 | 9 | 56.3 | 0.338 |
| Treatment outcomes | |||||||
| Death | 7 | 10.8 | 6 | 12.2 | 1 | 6.3 | 0.510 |
| Cure (clinical & parasitological) | 58 | 84.1 | 43 | 87.8 | 15 | 93.8 | 0.510 |
N.A: HIV predicts presence/absence of sign and symptom perfectly,
four (4) patients from Tanzania did not test their HIV status.
Discussion
Based on data from the Impamel III trials we describe the clinical presentation of second stage T.b. rhodesiense HAT in Tanzania and Uganda and confirm a wide spectrum of clinical presentation in these two geographically distinct areas in East Africa. In both settings T.b. rhodesiense HAT followed the classical disease pattern, but interestingly the neurological signs and symptoms typical for HAT were seen in a relatively small percentage of patients from Uganda. In patients from Tanzania, however, they were the dominate clinical manifestation. This correlated with the significantly higher reported CSF WBC counts in patients from Tanzania.
Unspecific signs of the disease such as fever, headache, general body pain and joint pains were reported in similar proportions in both study populations. We observed fever (≥37.5) in 29.7% (41/138) of the trial subjects. In the literature, fever was reported in the range of 31–71% in second stage patients from Zambia [18], [29], [35]. In the two study populations we saw high fever (>38.5) on admission only in Uganda (5.8%, 4/69) whereof 50% were children. Fever seems to be more common in T.b. rhodesiense than T.b. gambiense second stage patients in which fever was only occasionally reported (16%) and high fever was mostly seen in children [25]. In the two study populations, oedema was reported in Uganda and Tanzania in 20.3% and in 37.7% of the patients, respectively (p = 0.0244). This was comparable to the reported range of oedema in the literature (21.7–43.3%) [18], [29], [35], [36].
The clinical aspects of T.b. gambiense HAT [21], [25], [26], [37] have been systematically studied and show that the hallmark of second stage disease are neurological signs and symptoms [21], [25]. Unfortunately, this has never been done for T.b. rhodesiense HAT and hampers comparisons. However, published data report sleeping disorders during daytime hours with 63.3–70.5% of patients being affected [29], [35]. We observed sleeping disorders during daytime hours in Uganda and Tanzania in 56.5% and in 95.7% of the patients, respectively (p<0.0001). Similarly, sleeping disorders at night time are reported in the literature in 28.3% of patients [29]. We observed it in 34.8% of the patients from Uganda and in 92.8% of the patients from Tanzania (p<0.0001). Also other neurological signs and symptoms were significantly more frequent in patients from Tanzania; tremor (p = 0.0001), abnormal movements (p<0.0001), inactivity (p = 0.0076) and aggressiveness (p<0.0001). Clearly, the neurological signs and symptoms are more pronounced in Tanzania than in Uganda, and when compared to the literature.
In Uganda, almost 50% of patients were in a poor nutritional status (48% had BMI<16.5) as food security is very poor in this part of the country. This most likely contributes to weakness and, therefore, walking difficulties in the absence of neurological symptoms. Malnutrition is associated with immunodeficiency and higher susceptibility for a wide range of infections such as tuberculosis [38], [39] and pneumonia [40], as well as a poorer response to treatment. Another potential consequence of malnutrition in Uganda is an increased number of patients admitted with severe coma indicating a more rapid progression of the disease. Yet, we assume that many HAT cases from T.b. rhodesiense endemic areas in Tanzania die without ever having had contact with the health system due to geographical isolation.
With regards to treatment outcomes, we did not see any differences in the two study populations. In both countries all patients were free of parasites at end of treatment. Also, there was no apparent difference in parasite clearance rates. Time- and treatment-dependant dynamics of CSF WBC counts in the two study populations will be published separately.
Cardiovascular involvement is typical, but rarely of clinical relevance in T.b. gambiense HAT [41], [42]. We have limited knowledge of the effects of cardiac involvement in T.b. rhodesiense patients, but there is evidence that perimyocarditis seems to play an important role in the clinical course and fatal outcomes [43], [44]. We observed symptoms of cardiac failure such as oedema (swelling of legs) in 29% of the patients. Hepatomegaly occurred in 18%, dyspnoea in 7% and cough in 20% of the patients. However, echocardiography or laboratory testing (i.e. brain natrium peptide) could not be performed to confirm heart failure.
Co-infections with malaria and HIV were studied in detail in the patient population from Tanzania as the majority of the patients were malaria-positive on admission (80%) and agreed to voluntary testing of their HIV status (94.2%). Patients that were malaria-positive on admission more often had pruritus (p = 0.025), sleeping disorders during day time hours (p = 0.026) and disturbed appetite (p = 0.01). Also, they exhibited strange behaviour more often (p = 0.001). However, there is insufficient evidence for profound differences in malaria-positive and malaria-negative subjects, possibly due to asymptomatic carriers.
We identified one study that looked at T.b. rhodesiense and HIV co-infections in 25 patients from Kenya. In terms of treatment outcomes no conclusive results were obtained [45]. Our results indicate that the HIV status of the patient does not change the clinical presentation and/or the treatment outcomes of T.b. rhodesiense HAT. For T.b. gambiense HAT, there seems to be no association between HIV and HAT infection rates [46], [47] but evidence exists for a negative association with treatment outcomes [47], [48]. More research efforts are needed to better understand the complex interactions of co- infections, especially for neglected tropical diseases [49].
Our findings on the different clinical presentation of T.b. rhodesiense HAT in the two study populations could be due to an observation bias, bias in patient selection, or in comparing patients at incongruous time points after infection. Bias due to co-infections or differences in host and/or parasite genetics is also possible.
An observation bias can not be ruled out but is however less likely as the Impamel III program was conducted with a structured case report form (CRF) and one monitoring person. We have seen variability in signs and symptoms with clear definitions (e.g. lymphadenopathy, abnormal movements or tremor) as well as subjective definitions (e.g. insomnia, headache or inactivity). We can not completely rule out a selection bias due to the exclusion of moribund and unconscious patients in which baseline examination per protocol was not possible. However, the number of excluded patients was relatively small (<10%) and the two study populations were similar in regards to self-reported duration of illness. Even though unsuccessful, active case searches were conducted in both countries which reduced a potential selection bias. Central nervous system involvement in T.b. rhodesiense HAT was previously reported within 3 weeks to 2 months of infection [12]. One third of the study population already had clear neurological signs and symptoms within one month of infection which reflects the acuteness of T.b. rhodesiense infections. The WBC count in the CSF as well as most of the clinical signs and symptoms also developed quickly and did not significantly change over time. Disease progression was noticeable by aggravation of tremor, walking difficulties, sleeping disorders at night time, disturbance of appetite and aggressiveness over time, in both study populations.
Based on the results shown we rule out a bias of our findings due to co-infections. Previous infections with trypanosomes and/or host genetics might be determinants for the different clinical presentation of the disease in Tanzania and Uganda. There are speculations that apathogenic forms of the disease could influence immune responses to pathogenic infections [50], [51] supported by the fact that HAT is more acute in white than in the black populations [52], [53]. But we also see a high variability in disease severity among African populations [17], [18], a fact that has been related to the descent of people: people of Nilotic descent, who migrated into the East African region from tsetse-free areas during the past 2,000 years may have less tolerance than people of Bantu descent, whose ancestors have been exposed to human trypanosomes for several thousand years [18]. Our findings do not align with this theory as in Tanzania, the majority of the population is of Bantu origin and in Uganda the majority of the population is of Nilotic origin. Different parasite genotypes could be responsible for the observed spectrum of disease severity, a hypothesis has already been raised 60 years ago [16], [17], [54]. Recent findings on the phylogenetic relationship between different T.b. rhodesiense strains showed that the high variability of the T.b. rhodesiense genome is attributed to multiple and independent evolutions from T.b. brucei [55]. Our data show a clear difference in the clinical presentation of T.b. rhodesiense HAT in Tanzania and Uganda but a detailed assessment of host and parasite genotypes was beyond the scope of this paper.
T.b. rhodesiense HAT is a highly neglected disease and tools for disease control are very limited. There are no sensitive diagnostics at hand and melarsoprol, the only available drug to treat second stage disease, is toxic. An early identification of the disease is vital to prevent late onset of treatment. However, most of the patients are first treated for other conditions such as malaria and pneumonia. A low degree of disease awareness among health personnel is common and aggravated by the low prevalence and the focal distribution of HAT. A detailed understanding of the clinical presentation and regional characteristics of T.b. rhodesiense HAT is important and can support decision making and differential diagnosis at health facility level.
Acknowledgments
We are indebted to our patients and their families as well as to all members of the Kaliua Health Centre and the Lwala hospital for their continuous support of the Impamel III trials. The National Institute for Medical Research in Tanzania and the Ministry of Health in Uganda are acknowledged for facilitating the trials in their countries.
Footnotes
The authors have declared that no competing interests exist.
The clinical trial program was funded by the Swiss Agency for Development and Cooperation, grant number SDC 7F-01977.02 (www.sdc.admin.ch) and the Swiss Tropical and Public Health Institute (www.swisstph.ch). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Simarro PP, Jannin J, Cattand P. Eliminating human African trypanosomiasis: where do we stand and what comes next? PLoS Med. 2008;5:e55. doi: 10.1371/journal.pmed.0050055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . Geneva: WHO; 1998. Control and surveillance of African trypanosomiasis. pp. 1–114. [PubMed] [Google Scholar]
- 3.Priotto G, Kasparian S, Mutombo W, Ngouama D, Ghorashian S, et al. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet. 2009;374:56–64. doi: 10.1016/S0140-6736(09)61117-X. [DOI] [PubMed] [Google Scholar]
- 4.Schmid C, Richer M, Bilenge CM, Josenando T, Chappuis F, et al. Effectiveness of a 10-Day Melarsoprol Schedule for the Treatment of Late-Stage Human African Trypanosomiasis: Confirmation from a Multinational Study (Impamel II). J Infect Dis. 2005;191:1922–1931. doi: 10.1086/429929. [DOI] [PubMed] [Google Scholar]
- 5.De Greef C, Hamers R. The serum resistance-associated (SRA) gene of Trypanosoma brucei rhodesiense encodes a variant surface glycoprotein-like protein. Mol Biochem Parasitol. 1994;68:277–284. doi: 10.1016/0166-6851(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 6.Gibson WC. The SRA gene: the key to understanding the nature of Trypanosoma brucei rhodesiense. Parasitology. 2005;131:143–150. doi: 10.1017/s0031182005007560. [DOI] [PubMed] [Google Scholar]
- 7.Picozzi K, Fevre EM, Odiit M, Carrington M, Eisler MC, et al. Sleeping sickness in Uganda: a thin line between two fatal diseases. Bmj. 2005;331:1238–1241. doi: 10.1136/bmj.331.7527.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buyst H. The diagnosis of sleeping sickness in a district hospital in Zambia. Ann Soc Belg Med Trop. 1975;55:551–557. [PubMed] [Google Scholar]
- 9.Burri C, Stich A, Brun R. The trypanosomiases: CABI Publishing. 2004.
- 10.Fevre EM, Picozzi K, Jannin J, Welburn SC, Maudlin I. Human African trypanosomiasis: Epidemiology and control. Adv Parasitol. 2006;61:167–221. doi: 10.1016/S0065-308X(05)61005-6. [DOI] [PubMed] [Google Scholar]
- 11.Checchi F, Filipe JA, Haydon DT, Chandramohan D, Chappuis F. Estimates of the duration of the early and late stage of gambiense sleeping sickness. BMC Infect Dis. 2008;8:16. doi: 10.1186/1471-2334-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odiit M, Kansiime F, Enyaru JC. Duration of symptoms and case fatality of sleeping sickness caused by Trypanosoma brucei rhodesiense in Tororo, Uganda. East Afr Med J. 1997;74:792–795. [PubMed] [Google Scholar]
- 13.Truc P, Formenty P, Diallo PB, Komoin-Oka C, Lauginie F. Confirmation of two distinct classes of zymodemes of Trypanosoma brucei infecting man and wild mammals in Cote d'Ivoire: suspected difference in pathogenicity. Ann Trop Med Parasitol. 1997;91:951–956. doi: 10.1080/00034989760356. [DOI] [PubMed] [Google Scholar]
- 14.Garcia A, Jamonneau V, Magnus E, Laveissiere C, Lejon V, et al. Follow-up of Card Agglutination Trypanosomiasis Test (CATT) positive but apparently aparasitaemic individuals in Cote d'Ivoire: evidence for a complex and heterogeneous population. Trop Med Int Health. 2000;5:786–793. doi: 10.1046/j.1365-3156.2000.00623.x. [DOI] [PubMed] [Google Scholar]
- 15.Sternberg JM. Human African trypanosomiasis: clinical presentation and immune response. Parasite Immunol. 2004;26:469–476. doi: 10.1111/j.0141-9838.2004.00731.x. [DOI] [PubMed] [Google Scholar]
- 16.Songa EB, Hamers R, Rickman R, Nantulya VM, Mulla AF, et al. Evidence for widespread asymptomatic Trypanosoma rhodesiense human infection in the Luangwa Valley (Zambia). Trop Med Parasitol. 1991;42:389–393. [PubMed] [Google Scholar]
- 17.Ormerod WE. Taxonomy of the sleeping sickness trypanosomes. J Parasitol. 1967;53:824–830. [PubMed] [Google Scholar]
- 18.Buyst H. The epidemiology of sleeping sickness in the historical Luangwa valley. Ann Soc Belg Med Trop. 1977;57:349–359. [PubMed] [Google Scholar]
- 19.MacLean L, Chisi JE, Odiit M, Gibson WC, Ferris V, et al. Severity of human African trypanosomiasis in East Africa is associated with geographic location, parasite genotype, and host inflammatory cytokine response profile. Infect Immun. 2004;72:7040–7044. doi: 10.1128/IAI.72.12.7040-7044.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. Weekly epidemiological record; 2006. Human African trypanosomiasis (sleeping sickness): epidemiological update. pp. 69–80. [PubMed] [Google Scholar]
- 21.Haller L, Adams H, Merouze F, Dago A. Clinical and pathological aspects of human African trypanosomiasis (T. b. gambiense) with particular reference to reactive arsenical encephalopathy. Am J Trop Med Hyg. 1986;35:94–99. doi: 10.4269/ajtmh.1986.35.94. [DOI] [PubMed] [Google Scholar]
- 22.Nkanga NG, Kazadi K, Kazyumba GL, Dechef G. [Clinical neurological signs of human African trypanosomiasis at the meningoencephalitis stage (apropos of 23 cases)]. Bull Soc Pathol Exot Filiales. 1988;81:449–458. [PubMed] [Google Scholar]
- 23.Noireau F, Apembet JD, Frezil JL. Clinical review of endocrine disorders observed in adults with trypanosomiasis. Bull Soc Pathol Exot Filiales. 1988;81:464–467. [PubMed] [Google Scholar]
- 24.Reincke M, Allolio B, Petzke F, Heppner C, Mbulamberi D, et al. Thyroid dysfunction in African trypanosomiasis: A possible role for inflammatory cytokines. Clinical Endocrinology. 1993;39:455–461. doi: 10.1111/j.1365-2265.1993.tb02393.x. [DOI] [PubMed] [Google Scholar]
- 25.Blum J, Schmid C, Burri C. Clinical aspects of 2541 patients with second stage human African trypanosomiasis. Acta Trop. 2006;97:55–64. doi: 10.1016/j.actatropica.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Blum JA, Burri C, Hatz C, Kazumba L, Mangoni P, et al. Sleeping hearts: the role of the heart in sleeping sickness (human African trypanosomiasis). Trop Med Int Health. 2007;12:1422–1432. doi: 10.1111/j.1365-3156.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- 27.Blum JA, Schmid C, Hatz C, Kazumba L, Mangoni P, et al. Sleeping glands? - The role of endocrine disorders in sleeping sickness (T.b. gambiense Human African Trypanosomiasis). Acta Trop. 2007;104:16–24. doi: 10.1016/j.actatropica.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Jamonneau V, Garcia A, Frezil JL, N'Guessan P, N'Dri L, et al. Clinical and biological evolution of human trypanosomiasis in Côte d'Ivoire. Annals of Tropical Medicine and Parasitology. 2000;94:831–835. doi: 10.1080/00034980020028004. [DOI] [PubMed] [Google Scholar]
- 29.Boatin BA, Wyatt GB, Wurapa FK, Bulsara MK. Use of symptoms and signs for diagnosis of Trypanosoma brucei rhodesiense trypanosomiasis by rural health personnel. Bull World Health Organ. 1986;64:389–395. [PMC free article] [PubMed] [Google Scholar]
- 30.Burri C, Nkunku S, Merolle A, Smith T, Blum J, et al. Efficacy of new, concise schedule for melarsoprol in treatment of sleeping sickness caused by Trypanosoma brucei gambiense: a randomised trial. Lancet. 2000;355:1419–1425. doi: 10.1016/S0140-6736(00)02141-3. [DOI] [PubMed] [Google Scholar]
- 31.Schmid C, Richer M, Bilenge CM, Josenando T, Chappuis F, et al. Effectiveness of a 10-day melarsoprol schedule for the treatment of late-stage human African trypanosomiasis: Confirmation from a multinational study (Impamel II). Journal of Infectious Diseases. 2005;191:1922–1931. doi: 10.1086/429929. [DOI] [PubMed] [Google Scholar]
- 32.Woo PT. The haematocrit centrifuge for the detection of trypanosomes in blood. Can J Zool. 1969;47:921–923. doi: 10.1139/z69-150. [DOI] [PubMed] [Google Scholar]
- 33.Njiru ZK, Mikosza AS, Armstrong T, Enyaru JC, Ndung'u JM, et al. Loop-Mediated Isothermal Amplification (LAMP) Method for Rapid Detection of Trypanosoma brucei rhodesiense. PLoS Negl Trop Dis. 2008;2:e147. doi: 10.1371/journal.pntd.0000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matovu E, Kuepfer I, Boobo A, Kibona S, Burri C. Comparative detection of trypanosomal DNA by loop-mediated isothermal amplification and PCR from flinders technology associates cards spotted with patient blood. J Clin Microbiol. 2010;48:2087–2090. doi: 10.1128/JCM.00101-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wellde BT, Chumo DA, Reardon MJ, Mwangi J, Asenti A, et al. Presenting features of Rhodesian sleeping sickness patients in the Lambwe Valley, Kenya. Ann Trop Med Parasitol. 1989;83(Suppl 1):73–89. doi: 10.1080/00034983.1989.11812411. [DOI] [PubMed] [Google Scholar]
- 36.Mbulamberi DB. Proceedings of the International Scientific Council for Trypanosomiasis Research and Control 19th Meeting, Lomé, Togo; 1987. A clinical analysis of 3151 cases of Rhodesian sleeping sickness treated in the South Eastern Uganda, during the year 1985. pp. 188–195. [Google Scholar]
- 37.Blum J, Burri C. Treatment of late stage sleeping sickness caused by T.b. gambiense: a new approach to the use of an old drug. Swiss Med Wkly. 2002;132:51–56. doi: 10.4414/smw.2002.09902. [DOI] [PubMed] [Google Scholar]
- 38.Gupta KB, Gupta R, Atreja A, Verma M, Vishvkarma S. Tuberculosis and nutrition. Lung India. 2009;26:9–16. doi: 10.4103/0970-2113.45198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podewils LJ, Holtz T, Riekstina V, Skripconoka V, Zarovska E, et al. Impact of malnutrition on clinical presentation, clinical course, and mortality in MDR-TB patients. Epidemiol Infect. 2011;139(1):113–120. doi: 10.1017/S0950268810000907. [DOI] [PubMed] [Google Scholar]
- 40.Chisti MJ, Tebruegge M, La Vincente S, Graham SM, Duke T. Pneumonia in severely malnourished children in developing countries - mortality risk, aetiology and validity of WHO clinical signs: a systematic review. Trop Med Int Health. 2009;14:1173–1189. doi: 10.1111/j.1365-3156.2009.02364.x. [DOI] [PubMed] [Google Scholar]
- 41.Blum JA, Zellweger MJ, Burri C, Hatz C. Cardiac involvement in African and American trypanosomiasis. Lancet Infect Dis. 2008;8:631–641. doi: 10.1016/S1473-3099(08)70230-5. [DOI] [PubMed] [Google Scholar]
- 42.Blum JA, Schmid C, Burri C, Hatz C, Olson C, et al. Cardiac alterations in human African trypanosomiasis (T.b. gambiense) with respect to the disease stage and antiparasitic treatment. PLoS Negl Trop Dis. 2009;3:e383. doi: 10.1371/journal.pntd.0000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Raadt P, Koten JW. Myocarditis in Rhodesiense trypanosomiasis. East Afr Med J. 1968;45:128–132. [PubMed] [Google Scholar]
- 44.Koten JW, De Raadt P. Myocarditis in Trypanosoma rhodesiense infections. Trans R Soc Trop Med Hyg. 1969;63:485–489. doi: 10.1016/0035-9203(69)90036-4. [DOI] [PubMed] [Google Scholar]
- 45.Matete GO, Kajejo OA. Human African trypanosomiasis and human immunodeficiency virus co-infection in Western Kenya. East Afr Med J. 2005;82:20–23. doi: 10.4314/eamj.v82i1.9289. [DOI] [PubMed] [Google Scholar]
- 46.Meda HA, Doua F, Laveissiere C, Miezan TW, Gaens E, et al. Human immunodeficiency virus infection and human African trypanosomiasis: a case-control study in Cote d'Ivoire. Trans R Soc Trop Med Hyg. 1995;89:639–643. doi: 10.1016/0035-9203(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 47.Pepin J, Ethier L, Kazadi C, Milord F, Ryder R. The impact of human immunodeficiency virus infection on the epidemiology and treatment of Trypanosoma brucei gambiense sleeping sickness in Nioki, Zaire. Am J Trop Med Hyg. 1992;47:133–140. doi: 10.4269/ajtmh.1992.47.133. [DOI] [PubMed] [Google Scholar]
- 48.Blum J, Nkunku S, Burri C. Clinical description of encephalopathic syndromes and risk factors for their occurrence and outcome during melarsoprol treatment of human African trypanosomiasis. Trop Med Int Health. 2001;6:390–400. doi: 10.1046/j.1365-3156.2001.00710.x. [DOI] [PubMed] [Google Scholar]
- 49.Boraschi D, Abebe Alemayehu M, Aseffa A, Chiodi F, Chisi J, et al. Immunity against HIV/AIDS, malaria, and tuberculosis during co-infections with neglected infectious diseases: recommendations for the European Union research priorities. PLoS Negl Trop Dis. 2008;2:e255. doi: 10.1371/journal.pntd.0000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jamonneau V, Ravel S, Garcia A, Koffi M, Truc P, et al. Characterization of Trypanosoma brucei s.l. infecting asymptomatic sleeping-sickness patients in Cote d'Ivoire: a new genetic group? Ann Trop Med Parasitol. 2004;98:329–337. doi: 10.1179/000349804225003406. [DOI] [PubMed] [Google Scholar]
- 51.Blum J, Beck BR, Brun R, Hatz C. Clinical and serologic responses to human ‘apathogenic’ trypanosomes. Trans R Soc Trop Med Hyg. 2005;99:795–797. doi: 10.1016/j.trstmh.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Duggan AJ, Hutchinson MP. Sleeping sickness in Europeans: a review of 109 cases. J Trop Med Hyg. 1966;69:124–131. [PubMed] [Google Scholar]
- 53.Jelinek T, Bisoffi Z, Bonazzi L, van Thiel P, Bronner U, et al. Cluster of African trypanosomiasis in travelers to Tanzanian national parks. Emerg Infect Dis. 2002;8:634–635. doi: 10.3201/eid0806.010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apted FI, Smyly DP, Ormerod WE, Stronach BW. A comparative study of the epidemiology of endemic Rhodesian sleeping sickness in different parts of Africa. J Trop Med Hyg. 1963;66:1–16. [PubMed] [Google Scholar]
- 55.MacLeod A, Welburn S, Maudlin I, Turner CM, Tait A. Evidence for multiple origins of human infectivity in Trypanosoma brucei revealed by minisatellite variant repeat mapping. J Mol Evol. 2001;52:290–301. doi: 10.1007/s002390010157. [DOI] [PubMed] [Google Scholar]