Abstract
Purpose:
To evaluate the efficacy and safety of conductive keratoplasty (CK) for the treatment of presbyopia and analyze the differences in the effects between post- and non-laser in situ keratomileusis (LASIK) eyes. Clinical preoperative factors that could affect the predictability of CK were also analyzed.
Methods:
The visual and refractive outcomes of CK for the treatment of presbyopia in 14 eyes of 13 post-LASIK patients (post-LASIK group mean age 50.9 ± 3.4 years) and those of 25 eyes of 25 non-LASIK patients (non-LASIK group mean age 52.4 ± 4.0 years) were studied. The clinical efficacy, safety, stability, and predictability of CK were statistically evaluated.
Results:
The mean (logarithm of the minimum angle of resolution [logMAR] ± standard deviation [SD]) of preoperative uncorrected near visual acuity (UNVA) and manifest refraction spherical equivalent (MRSE) were 0.64 ± 0.25 diopter (D) and 0.35 ± 0.48 D, respectively, in the post-LASIK group, and 0.71 ± 0.20 D and 0.64 ± 0.61 D, respectively, in the non-LASIK group. At 6 months after CK, the mean UNVA and MRSE were 0.07 ± 0.13 D and −1.59 ± 0.86 D, respectively, in the post-LASIK group, and 0.07 ± 0.12 D and −1.06 ± 0.56 D, respectively, in the non-LASIK group. At 1 year after CK, the mean UNVA and MRSE were 0.30 ± 0.17 D and −0.58 ± 0.52 D, respectively, in the post-LASIK group, and 0.28 ± 0.34 D and −1.56 ± 0.62 D, respectively, in the non-LASIK group. There was no significant difference between the two groups in either factor at 6 months postoperative (Student’s t-test, P > 0.05). At 1 year after CK, all the treated eyes maintained corrected distance visual acuity better than −0.08 (logMAR). The mean cylindrical errors were within ±1.00 D in 100% of the post-LASIK and non-LASIK patients. As for the preoperative clinical factors evaluated for their potential relationship to the predictability of CK, none showed significant effect on the clinical outcomes.
Conclusion:
CK is demonstrated to be safe for the treatment of presbyopia in post-LASIK patients as well as in non-LASIK patients, though needed longer observation in terms of factors affecting predictability.
Keywords: conductive keratoplasty, post-LASIK, presbyopia, CK
Introduction
Presbyopia has been known as one of the most common aging phenomena of the eye, leading to loss of near vision, especially in emmetropic or hyperopic eyes. Conventional management is to use reading glasses to correct presbyopia. In recent years, a number of treatments have been developed to meet the needs of patients wanting to be free from reading glasses. These treatments include bifocal or multifocal contact lenses, monovision using monofocal lenses, and multifocal laser in situ keratomileusis (LASIK).1–6
US Food and Drug Administration (FDA) clinical trials in 2002 demonstrated that conductive keratoplasty (CK) was effective for the treatment of low to moderate hyperopia.7 Since then, the use of this technique has expanded to treat hyperopic astigmatism, keratoconus, and corneal ectasia (keratoectasia) after LASIK.8,9 In 2004, the results of a 1-year clinical trial demonstrated that CK was effective also for the treatment of presbyopia.10 However, there have been only a few studies on the effect of CK for presbyopia.11,12
In CK, a thin probe is inserted into the cornea, and radio waves are used to make heat coagulation of the corneal stroma to alter the corneal curvature. CK makes a myopic shift of the treated eye and improves near vision. This technique is now widely used, but there have not been many reports on the effectiveness of CK on post-LASIK eyes. In this study, we compared the visual and refractive outcomes of CK treatment on post-LASIK and non-LASIK eyes and analyzed the clinical outcomes.
Methods
Eligibility of the patients and pre- and postoperative examinations
The eligibility requirements for CK at our clinic were as follows: patients age over 40 years, only those patients who do not drive long hours and who do not require perfect distance vision, eyes with −1.0 diopter (D) to +1.5 D of sphere (manifest and cycloplegic), equivalent to −0.75 D or less than −0.75 D of cylinder, and corneal thickness of 400 μm or more at the center of the cornea and 560 μm or more at the 6 mm peripheral area. Patients with pacemakers and/or cochlear implants were excluded from CK surgery.
In this study, the preoperative examinations included uncorrected distance and near visual acuity (UDVA and UNVA, respectively), corrected distance and near visual acuity (CDVA and CNVA, respectively), binocular uncorrected distance and near visual acuity (binocular UDVA and binocular UNVA, respectively), distance and near manifest refraction and cycloplegic refraction, distance and near bilateral visual acuity, intraocular pressure, corneal topography using TMS-4® (Tomey Corp, Nagoya, Japan), ultrasonic pachymetry, endothelial cell density, slit lamp examinations, dominant eye tests, and monovision tests.
Postoperative examinations included UDVA, UNVA, CDVA, and CNVA (operated eyes only), binocular UDVA and binocular UNVA, distant manifest refraction, corneal topography, and slit lamp examinations.
The candidates also went through a monovision test using trial glasses (loose lens test). We used three different powers of lenses for the monovision trial, +1.00 D, +1.75 D, and +2.50 D, in order to evaluate the required correction and also the patients’ ability to adapt to monovison. Candidates who were not satisfied with the trial glasses were allowed to go through a similar test using contact lenses. All patients were informed that presbyopic symptoms might not be improved after CK.
In addition, the post-LASIK candidates were required to have waited at least 3 months after LASIK before undergoing CK.
Statistical analyses were conducted using Student’s t-test, Spearman’s correlation coefficient by rank, or Pearson’s correlation coefficient as appropriate. For all tests, a P value less than 0.05 was considered statistically significant.
Surgical procedure
After the preoperative slit lamp examinations were completed, one drop of 0.4% oxybuprocaine hydrochloride ophthalmic solution (Benoxil®, Santen Pharmaceutical, Osaka, Japan) was instilled, followed by one drop of 0.5% moxifloxacin hydrochloride ophthalmic solution (Vegamox®, Alcon Japan, Tokyo, Japan) and 4% lidocaine ophthalmic solution (Xylocaine®, AstraZeneca PLC, Osaka).
Under the microscopic observation, the pupil center of the cornea was marked with a marker (BD Visimark™ Gentian Violet Marking Pad, BD Ophthalmic System, Waltham, MA, USA), then the CK template (NearVison® CK or OptiPoint® Corneal Template, Refractec, Inc., CA, USA) was applanated at the center of the template and matched with the corneal center.
According to the surgical nomogram of the manufacturer, the probe was inserted into the holes of the template. For the post-LASIK eyes, the intended correction was reduced by 20%–30% from the standard nomogram, because it has been reported that the effects of CK are exaggerated in post-LASIK eyes.13
After completing the procedure, the template was removed and one drop of moxifloxacin hydrochloride, 0.3% sodium hyaluronate ophthalmic solution (Hyalein® Mini, Santen Pharmaceutical, Osaka), and 0.1% dexamethasone sodium meta-sulfobenzoate ophthalmic solution (D·E·X®, Nitto Medic, Toyama, Japan) were instilled.
The patients were instructed to rest for 20 minutes before receiving the doctor’s postoperative examination. If no problems were found, one drop of sodium hyaluronate ophthalmic solution was instilled and the patients were allowed to go home.
Postoperative care
On the day of the surgery, the patients were instructed to instil one drop of 0.1% fluorometholone ophthalmic solution (Flumetholon®, Santen Pharmaceutical, Osaka), 0.5% moxifloxacin hydrochloride ophthalmic solution, and 0.3% sodium hyaluronate ophthalmic solution once every hour.
The patients were instructed to visit our clinic for postoperative examinations at 1 day, 1 week, 1 month, 3 months, 6 months, and 1 year after surgery. From the day after the operation, one drop of fluorometholone, moxifloxacin hydrochloride, and 0.1% hyaluronate sodium ophthalmic solution (Tearbalance®, Senju Pharmaceutical, Osaka) was administrated.
Results
Patients’ demography
A total of 39 eyes of 38 patients who underwent CK between April 2007 and June 2009 were included in this study. Of those, 25 eyes of 25 patients had no history of ophthalmic surgery (9 males and 16 females) and 14 eyes of 13 patients had received LASIK before CK (8 males and 5 females). One of the post-LASIK patients underwent corrective treatment for residual hyperopia from the previous LASIK on one eye, and the fellow eye was treated for presbyopia. The mean age of the non-LASIK patients was 52.4 ± 4.0 years (n = 25) and of the post-LASIK patients was 50.9 ± 3.4 years (n = 13). For the post-LASIK patients, the mean period between their LASIK operation and CK was 408.9 ± 315.4 days (range 110–948 days). The corneal flap thickness of the previous LASIK was between 85 μm and 110 μm. The mean attempted correction was −1.60 ± 0.76 D in the non-LASIK patients, and −1.27 ± 0.74 D in the post-LASIK patients.
All of the patients received postoperative examinations at 1 day, 1 week, and 1 month after CK. Sixty-four percent (n = 16), 36.0% (n = 9), and 8.0% (n = 2) of the non-LASIK patients and 76.9% (n = 10), 53.8% (n = 7), and 23.1% (n = 3) of the post-LASIK patients received examinations at 3 months, 6 months, and 1 year after CK, respectively. As the number of the patients who attended the 1-year postoperative examination was very small, only numerical values were included without statistical analysis in this study.
Visual acuity
The mean (±standard deviation [SD]) UNVA (logarithm of the minimum angle of resolution [logMAR]) before CK and 1 week, 1 month, 3 months, 6 months, and 1 year after CK was 0.64 ± 0.25, 0.10 ± 0.20, 0.02 ± 0.13, 0.06 ± 0.17, 0.07 ± 0.13, and 0.30 ± 0.17, respectively, in the post-LASIK group, and 0.71 ± 0.20, 0.09 ± 0.20, 0.11 ± 0.17, 0.11 ± 0.14, 0.07 ± 0.12, and 0.28 ± 0.34, respectively, in the non-LASIK group. There was no significant difference in UNVA between the two groups at each postoperative examination (Student’s t-test, P > 0.05).
The mean (±SD) UDVA (logMAR) before CK and 1 week, 1 month, 3 months, 6 months, and 1 year after CK was −0.13 ± 0.11, 0.58 ± 0.38, 0.53 ± 0.38, 0.38 ± 0.39, 0.48 ± 0.40, and 0.07 ± 0.21, respectively, in the post-LASIK group, and −0.07 ± 0.13, 0.65 ± 0.39, 0.53 ± 0.37, 0.45 ± 0.34, 0.26 ± 0.24, and 0.41 ± 0.16, respectively, in the non-LASIK group. No significant difference was found in UDVA between the two groups at each postoperative examination (Student’s t-test, P > 0.05).
The preoperative, 6-month, and 1-year postoperative mean binocular UDVA (logMAR) was −0.18 ± 0.11, −0.20 ± 0.08, and −0.18 ± 0.00 in the post-LASIK group, and −0.17 ± 0.07, −0.09 ± 0.10, and −0.09 ± 0.12 in the non-LASIK group, respectively. There was a significant difference in binocular UDVA between the two groups 6 months postoperatively (Student’s t-test, P = 0.0391).
Preoperative, 6-month, and 1-year postoperative mean binocular UNVA (logMAR) was 0.50 ± 0.27, 0.06 ± 0.12, and 0.22 ± 0.25 in the post-LASIK group, and 0.53 ± 0.22, 0.03 ± 0.10, and 0.20 ± 0.17 in the non-LASIK group, respectively. There was no significant difference between the two groups in binocular UNVA at each postoperative examination (Student’s t-test, P > 0.05).
Safety of CK
The CDVA before CK and 1 week, 1 month, 3 months, 6 months, and 1 year after CK was measured in the post-LASIK eyes and the non-LASIK eyes. The mean (±SD) preoperative CDVA (logMAR) was −0.20 ± 0.06, and at 1 week, 1 month, 3 months, 6 months, and 1 year after CK was −0.14 ± 0.06, −0.15 ± 0.08, −0.17 ± 0.05, −0.18 ± 0.06, and −0.13 ± 0.07, respectively, in the non-LASIK eyes. The mean CDVA (logMAR) before CK and 1 week, 1 month, 3 months, 6 months, and 1 year after CK in the post-LASIK eyes was −0.22 ± 0.08, −0.15 ± 0.06, −0.17 ± 0.07, −0.19 ± 0.04, −0.15 ± 0.08, and −0.18 ± 0.00, respectively. All of the post-LASIK and non-LASIK patients had CDVA of −0.08 (logMAR) or better throughout the postoperative examination period until 1 year after CK, and there was no significant difference between the two groups (Student’s t-test, P > 0.05).
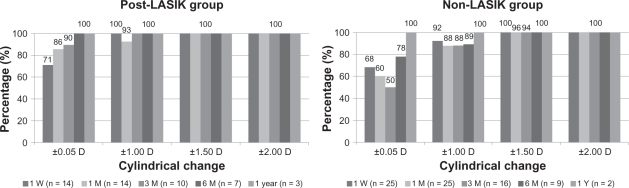
The mean postoperative cylindrical changes at 1 week, 1 month, 3 months, 6 months, and 1 year after CK in the post-LASIK group were −0.30 ± 0.47 D (±SD, range −1.00 D to +0.50 D), −0.32 ± 0.46 D (±SD, range −1.50 D to +0.25 D), −0.20 ± 0.28 D (±SD, range −0.75 D to +0.25 D), −0.11 ± 0.24 D (±SD, range −0.50 D to +0.25 D), and 0.00 ± 0.00 D (±SD), respectively. The mean postoperative cylindrical changes at 1 week, 1 month, 3 months, 6 months, and 1 year after CK in the non-LASIK group were −0.37 ± 0.54 D (±SD, range −1.50 D to +0.50 D), −0.37 ± 0.68 D (±SD, range −1.75 D to +0.75 D), 0.00 ± 0.66 D (±SD, range −1.75 D to +0.50 D), −0.42 ± 0.52 (±SD, range −1.50 D to +0.25 D), and −0.13 ± 0.18 (±SD, range −0.25 to 0.00 D), respectively. Eighty-eight percent (14 of 16 patients) of the non-LASIK patients had cylindrical changes within ±1.00 D at 6 months postoperatively, and 100% (two of two patients) had cylindrical changes within ±0.25 D at 1 year postoperatively (Figure 1). Although one patient (11%) in the non-LASIK group had more than 1.00 D of cylindrical changes, the patient maintained CDVA (logMAR) of −0.18 at 6 months postoperatively. At 6 months and 1 year postoperatively, the postoperative cylindrical changes were within 0.50 D and 0.00 D in all of the post-LASIK patients (Figure 1).
Figure 1.
Cylindrical change from preoperative for non- and post-LASIK groups. One week, 1 month, 3 months, 6 months, and 1 year postoperative absolute cylinder change from preoperative was calculated for the non- and post-LASIK groups.
Stability of CK
The refractive changes after CK were studied at 1 week, 1 month, 3 months, 6 months, and 1 year postoperatively. In the post-LASIK group, the mean MRSE before CK and 1 week, 1 month, and 3 months after CK was 0.34 ± 0.44 D, −2.54 ± 1.14 D, −2.39 ± 1.07 D, −1.75 ± 0.96 D, −1.59 ± 0.86 D, and −0.58 ± 0.52 D, respectively. In the non-LASIK group, the mean MRSE before CK and 1 week, 1 month, 3 months, 6 months, and 1 year after CK was 0.64 ± 0.59 D, −2.09 ± 1.03 D, −1.57 ± 1.04 D, −1.46 ± 0.73 D, − 1.06 ± 0.56 D, and −1.56 ± 0.62 D, respectively. There was no significant difference between the two groups before CK and 1 week, 3 months, and 6 months after CK (Student’s t-test, P > 0.05). However, there was significant difference between the groups at 1 month postoperatively (Student’s t-test, P = 0.0244). In the post-LASIK group, the mean MRSE change between 1 week and 1 month was 0.16 D, between 1 month and 3 months was 0.63 D, between 3 months and 6 months was 0.16 D, and between 6 months and 1 year was 1.01 D, respectively. The mean MRSE change between 1 week and 1 month was 0.52 D, between 1 month and 3 months was 0.11 D, between 3 months and 6 months was 0.40 D, and between 6 months and 1 year was 0.50 D, respectively, in the non-LASIK group.
Predictability of CK
The predictability of CK was evaluated based on the clinical results at 1 week, 1 month, 3 months, 6 months, and 1 year postoperatively. The nomogram used in this study ranged from +0.50 D to +3.00 D, and the appropriate value was applied depending on the patients’ need for correction according to the preoperative loose lens test (Table 1). In the post-LASIK patients, the attempted correction was lowered by 20%–30% in order to avoid overcorrection. The mean errors in MRSE from the attempted correction at 1 week, 1 month, 3 months, 6 months, and 1 year postoperatively were −1.28 ± 0.88 D, −1.12 ± 0.80 D, −0.63 ± 0.68 D, −0.38 ± 0.82 D, and 0.00 ± 0.00 D, respectively, in the post-LASIK group, and −0.50 ± 0.71 D, 0.03 ± 0.98 D, 0.29 ± 0.66 D, 0.85 ± 0.74 D, and 0.06 ± 0.80 D in the non-LASIK group. There were significant differences between the two groups at 1 week, 1 month, 3 months, and 6 months postoperatively (Student’s t-test, P = 0.0046, 0.0007, 0.0024, and 0.0073, respectively). The post-LASIK group showed greater myopic shift than the non-LASIK group.
Table 1.
Nomogram for conductive keratoplasty
| Intended correction | Number of spots | Optical zone |
|---|---|---|
| 0.5–1.0 D | 8 | 7 mm |
| 1.0–1.5 D | 16 | 7–8 mm |
| 1.5–2.0 D | 8 | 6 mm |
| 2.5–3.0 D | 16 | 6–8 mm |
| 3.0–3.5 D | 16 | 6–7 mm |
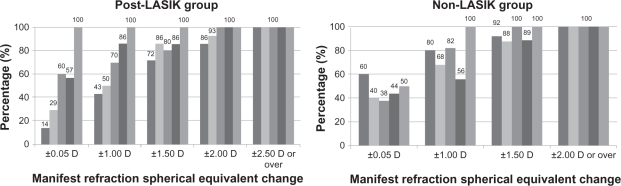
At 6 months after CK, 44% of the eyes were within ±0.50 D, 56% were within ±1.00 D, and 89% were within ±1.50 D of the intended correction, and at 1 year after CK, 50% were within ±0.50 D and 100% were within ±1.00 D of the intended correction in the non-LASIK group (Figure 2). Fifty-seven percent of the eyes were within ±0.50 D, 86% were within ±1.00 D, and 100% were within ±2.00 D of the intended correction 6 months postoperative, and 100% did not differ from the intended correction 1 year postoperative in the post-LASIK group (Figure 2).
Figure 2.
The error from the intended correction for non- and post-LASIK groups. The predictability at 1 week, 1 month, 3 months, 6 months, and 1 year postoperative for the non- and post-LASIK groups was evaluated by calculating the distance of manifest refraction spherical equivalent value from the intended correction.
In order to elucidate the predictability of CK, we studied other factors that might have affected the clinical outcomes. These included pre-CK pachymetry, the amount of correction in the previous LASIK, and residual corneal bed thickness. The mean pachymetry in the non-LASIK group was 543.4 ± 28.7 μm (±SD, range 502–609 μm) and 476.6 ± 50.3 μm (±SD, range 406–560 μm) in the post-LASIK group. The mean residual corneal bed thickness was 380 ± 45.1 μm (±SD, range 316–456 μm) and the mean attempted correction in the previous LASIK was 4.65 ± 2.52 D (±SD, range 1.25–9.50 D) in the post-LASIK group. The relationships between these factors and postoperative errors from the attempted correction were analyzed based on the clinical data at 3 months after CK (Figure 3). There was no significant relationship between these factors and the corrective errors of CK (Pearson’s correlation coefficient, P > 0.05). Furthermore, no relationships between the factors were observed by Spearman’s correlation coefficient by rank (P > 0.05).
Figure 3.
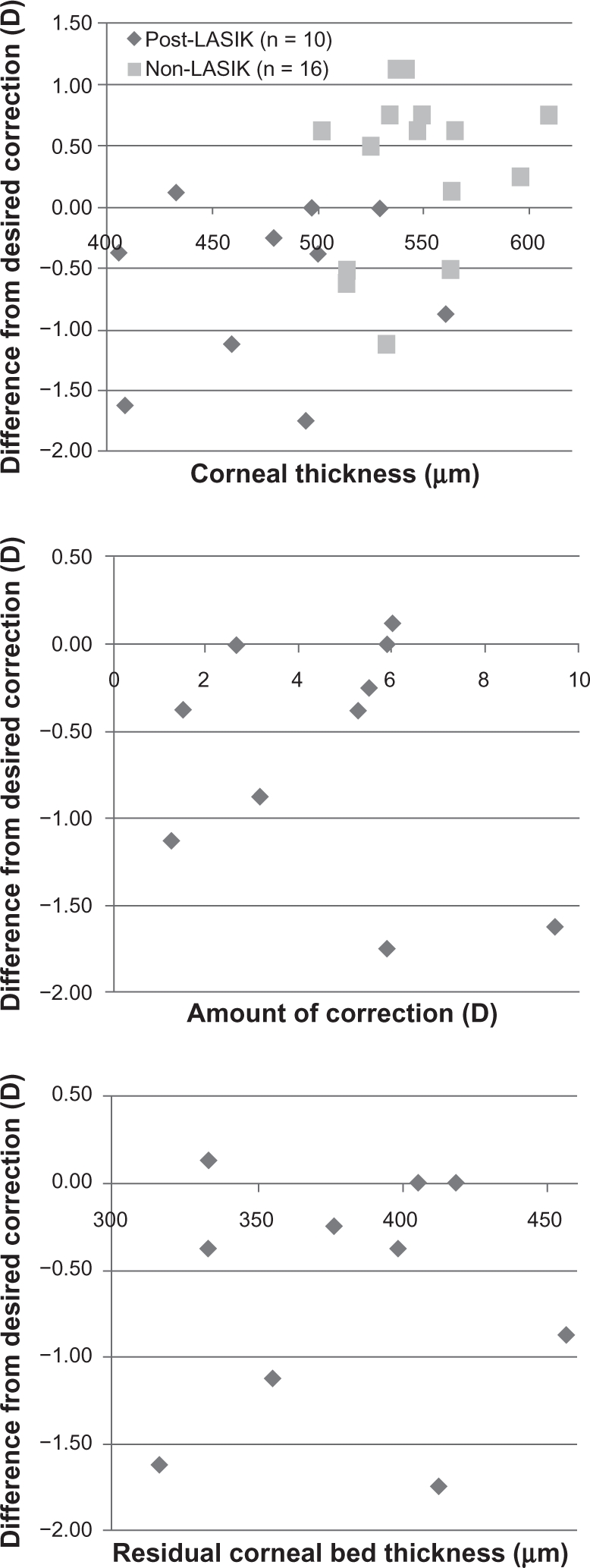
Comparison of corneal thickness, amount of correction made during LASIK, and residual corneal bed thickness after LASIK and the predictability at 3 months postoperative for both groups.
Discussion
In recent years, a number of surgical treatments of presbyopia have been reported. As LASIK has become one of the most popular choices for the correction of refractive error, we expect that the number of patients seeking treatment of presbyopia after LASIK will increase. Some of these patients might not have enough residual corneal bed thickness to receive the second refractive correction. As CK does not further reduce the residual corneal bed thickness nor does it affect the corneal center, CK is one of the most favorable treatments of presbyopia after LASIK at present. However, there have been a few reports of comparative studies on the effects of CK between post-LASIK eyes and non-LASIK eyes.
All of the post-LASIK and non-LASIK patients achieved improvement in UNVA at 6 months after CK, and there was no significant difference between the two groups. The binocular UNVA improved in both groups at 6 months after CK examinations, and there was no significant difference between the two groups. On the whole, treatment using CK has improved the patients’ near vision while maintaining their good binocular distance vision.
The CDVA at 6 months post-CK was −0.08 (logMAR) or better in both groups, and there was no significant difference between the two groups. In all patients of the post-LASIK group and 89% of the non-LASIK patients, the cylindrical change from preoperation was within 1.00 D at 6 months after CK. Although the cylindrical changes were greater than 1.00 D in one patient of the non-LASIK group, the patient’s UNVA was as good as the other patients in the group. The patients’ visual outcomes were not affected when looking at the numerical results, but large cylindrical changes may adversely affect their quality of vision. These patients need to be monitored closely in the longer term.
It has been reported that the regression rate of post-CK patients mitigates from 3 months to 1 year after surgery.11 In our study, the regression rate was varied between the examined period in both post-LASIK and non-LASIK groups. It is suggested that regression was not a stable event after CK. Further observation is needed to evaluate what may affect the stability of CK. Additionally, at 1 year postoperative, further refractive and visual (UNVA) regression was noted in some patients. However, they maintained good corrected distance and near visual acuity, suggesting that CK is a safe procedure.
The predictability of refractive outcomes was better in the non-LASIK group than in the post-LASIK group. Alió et al demonstrated that the predictability of CK on patients who have had previous corneal refractive surgery was not good.13 Although we set the target 20%–30% weaker than the standard nomogram, the effects of CK were stronger in the post-LASIK eyes than in the non-LASIK eyes, resulting in greater myopic shift in the post-LASIK eyes.
It has been reported that the amount of residual corneal thickness after LASIK and the amount of myopic correction by LASIK affect the biomechanical properties and strength of the cornea.14,15 Therefore, we evaluated the relationship between the two factors and the predictability of CK in the post-LASIK eyes. We demonstrated that there was little relationship between these two factors and the predictability of CK. In principle, we preserve a minimum of 300 μm residual corneal bed thickness after LASIK to allow follow-on enhancements and also for patients’ safety. Even with this safety margin, we assumed that the integrity of the cornea was weaker in the post-LASIK eyes than in the non-LASIK eyes, and the reduced integrity might have affected the predictability of the CK.
In this study, no significant relationship between the predictability of CK and the residual bed thickness nor the amount of correction by the LASIK procedure was found. Additionally, the relationship between corneal thickness and the predictability at 3 months after CK was also compared between the post-LASIK and the non-LASIK groups. We noted that there was significant difference in the mean corneal thickness between the two groups. The mean corneal thickness for the post-LASIK group was thinner by 68.8 μm. The post-LASIK group also had a greater tendency to have a myopic shift. Even with these differences, neither group demonstrated significant correlation between corneal thickness and predictability.
As none of the above three factors, residual corneal bed thickness, amount of LASIK correction, and corneal thickness, was related to the predictability of CK on post-LASIK eyes, we hypothesize that corneal structural changes such as flap creation might have reduced corneal integrity and induced an exaggerated reaction to the CK treatment, leading to overcorrection. Our results were compatible with the hypothesis that the structural alteration of the anterior and posterior lamellar possibly caused an unusually large response to CK treatment.16 As the central corneal thickness was more reduced but the peripheral regions were relatively unchanged in LASIK, it has been suggested that corneal elasticity has been decreased, and this induces a strong steepening effect, which could result in an approximate doubling of the effect of CK when compared with the effect on eyes without previous corneal surgery.17 Further investigation is needed to determine whether these structural changes may be the cause of the resultant overcorrection seen in post-LASIK eyes.
With the introduction of the special templates (NearVison® CK, OptiPoint® Corneal Template) for CK probe application, problems such as the increased cylindrical errors caused by the unsymmetrical application of the probe to the cornea and/or the failure to mark the pupil center properly are mitigated.18
No specific intra- or postoperative complications were demonstrated by CK treatment on the non-LASIK nor the post-LASIK patients in this study. However, rare complications have been reported, such as diffuse lamellar keratitis followed by epithelial defects and corneal perforation after CK treatment on post-LASIK eyes.19,20 We need to consider the possibility of severe complications and carefully follow the post-LASIK CK patients.
In our study, few patients attended their 1-year follow-up. We hypothesised that those patients who did not return for the follow-up retained stable refraction and visual acuity and did not feel the need for a doctor consultation.
In conclusion, CK is demonstrated to be a safe procedure for the treatment of presbyopia in post-LASIK eyes as well as in non-LASIK eyes. However, we need further investigation into its stability and the factors that may affect the predictability of CK.
Acknowledgments
Miss Naoko Inoue (Shinagawa LASIK Center, Tokyo, Japan) kindly corrected and assembled the patients’ data for the study.
Footnotes
Disclosure
None of the authors has a financial or proprietary interest in any material or method mentioned.
References
- 1.Guputa N, Naroo SA, Wolffsohn S. Visual comparison of multifocal lens to monovision. Optom Vis Sci. 2009;86:E98–E105. doi: 10.1097/OPX.0b013e318194eb18. [DOI] [PubMed] [Google Scholar]
- 2.Reilly CD, Lee WB, Alvarenga L, et al. Surgical monovision and monovision reversal in LASIK. Cornea. 2006;25:136–138. doi: 10.1097/01.ico.0000178722.19317.7b. [DOI] [PubMed] [Google Scholar]
- 3.Braun EHP, Lee J, Steinert RF. Monovision in LASIK. Ophthalmology. 2008;115:1196–1202. doi: 10.1016/j.ophtha.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Telandro A. The pseudoaccommodative cornea multifocal ablation with a center-distance pattern: a Review. J Refract Surg. 2009;25(Suppl):S156–S159. doi: 10.3928/1081597X-20090115-14. [DOI] [PubMed] [Google Scholar]
- 5.El Danasoury AM, Gamaly TO, Hantera M. Multizone. LASIK with peripheral near zone for correction of presbyopia in myopic and hyperopic eyes: 1-year results. J Refract Surg. 2009;25:296–305. doi: 10.3928/1081597X-20090301-10. [DOI] [PubMed] [Google Scholar]
- 6.Uy E, Go R. Pseudoaccommodative cornea treatment using the NIDEK EC-5000 CXIII excimer laser in myopic and hyperopic presbyopes. J Refract Surg. 2009;25(Suppl):S148–S155. doi: 10.3928/1081597X-20090115-13. [DOI] [PubMed] [Google Scholar]
- 7.McDonald MB, Hersh PS, Manche EE, et al. the Conductive Keratoplasty United States Investigators Group Conductive keratoplasty for the correction of low to moderate hyperopia: US clinical trial 1-year results on 355 eyes. Ophthalmology. 2002;109:1978–1989. doi: 10.1016/s0161-6420(02)01255-1. [DOI] [PubMed] [Google Scholar]
- 8.Alió JL, Claramonte PJ, Cáliz A, Ramzy MI. Corneal modeling of keratoconus by conductive keratoplasty. J Cataract Refract Surg. 2005;31:190–197. doi: 10.1016/j.jcrs.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 9.Naoumidi TL, Kounis GA, Astyrakakis NI, et al. Two-year follow-up of conductive keratoplasty for the treatment of hyperopic astigmatism. J Cataract Refract Surg. 2006;32:732–741. doi: 10.1016/j.jcrs.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 10.McDonald MB, Durrie D, Asbell P, et al. Treatment of presbyopia with conductive keratoplasty® six-month results of the 1-year United States FDA clinical trial. Cornea. 2004;23:661–668. doi: 10.1097/01.ico.0000126321.13143.a0. [DOI] [PubMed] [Google Scholar]
- 11.Stahl JE. Conductive keratoplasty for presbyopia: 1-year results. J Refract Surg. 2006;22:137–144. doi: 10.3928/1081-597X-20060201-10. [DOI] [PubMed] [Google Scholar]
- 12.Stahl JE. Conductive keratoplasty for presbyopia: 3-year results. J Refract Surg. 2007;23:905–910. doi: 10.3928/1081-597X-20071101-07. [DOI] [PubMed] [Google Scholar]
- 13.Alió JL, Ramzy MI, Galal A, Claramonte PJ. Conductive keratoplasty for the correction of residual hyperopia after LASIK. J Refract Surg. 2005;21:698–704. doi: 10.3928/1081-597X-20051101-07. [DOI] [PubMed] [Google Scholar]
- 14.Seiler T, Koufala K, Richter G. Iatrogenic keratectasia after laser in situ keratomileusis. J Refract Surg. 1998;14:312–317. doi: 10.3928/1081-597X-19980501-15. [DOI] [PubMed] [Google Scholar]
- 15.Kamiya K, Shimizu K, Ohmoto F. Comparison of the changes in corneal biomechanical properties after photorefractive keratectomy and laser in situ keratomileusis. Cornea. 2009;28:765–769. doi: 10.1097/ICO.0b013e3181967082. [DOI] [PubMed] [Google Scholar]
- 16.Comaish IF, Lawless MA. Conductive keratoplasty to correct residual hyperopia after corneal surgery. J Cataract Refract Surg. 2003;29:202–206. doi: 10.1016/s0886-3350(02)01498-0. [DOI] [PubMed] [Google Scholar]
- 17.Hersh PS, Fry KL, Chandrashekhar R, Fikaris DS. Conductive keratoplasty to treat complications of LASIK and photorefractive keratectomy. Ophthalmology. 2005;112:1941–1947. doi: 10.1016/j.ophtha.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Daivis EA, Fahmy AM. Stage III diffuse lamellar keratitis following conductive keratoplasty over a LASIK flap. J Cataract Refract Surg. 2009;35:1141–1143. doi: 10.1016/j.jcrs.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Kymionis GD, Titze P, Markomanolakis MM, et al. Corneal perforation after conductive keratoplasty with previous refractive surgery. J Cataract Refract Surg. 2003;29:2452–2454. doi: 10.1016/s0886-3350(03)00347-x. [DOI] [PubMed] [Google Scholar]
- 20.Pallikaris IG, Naoumidi TL, Astyrakakis NI. Long-term results of conductive keratoplasty for low to moderate hyperopia. J Cataract Refract Surg. 2005;31:1520–1529. doi: 10.1016/j.jcrs.2005.01.032. [DOI] [PubMed] [Google Scholar]