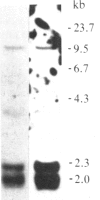
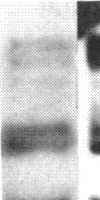
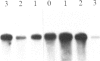
Abstract
beta-Actin mutations in chemically transformed human cell lines have been associated with tumorigenicity, an association consistent with other evidence suggesting that altered cytoskeletal proteins may have an important role in cancer initiation or progression. From a human promyelocytic leukemia cell line, we have isolated a gamma-actin cDNA clone with amino acid substitutions in a region highly conserved in the many actins analyzed. To our knowledge, this is the first example of a variant gamma-actin in a human neoplasm. A separate finding from the analysis of this clone is that the gamma-actin 3'-untranslated region is among the most highly conserved of all 3'-untranslated sequences so far reported, but is entirely different from the beta-actin 3'-untranslated region. The high degree of evolutionary conservation suggests that the 3'-untranslated regions of these two mRNAs have important and distinct functional roles that were already fully differentiated more than 100 million years ago. Mutations affecting four major cytoskeletal components have now been identified in human neoplastic cells. These findings suggest that mutated cytoskeletal genes may be members of a class of oncogenes, fundamentally different from both the nuclear-acting (e.g., myc and simian virus 40 large tumor antigen) and growth factor/receptor/protein kinase-related (e.g., sis, erbB, and ras) types of oncogenes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bains W., Ponte P., Blau H., Kedes L. Cardiac actin is the major actin gene product in skeletal muscle cell differentiation in vitro. Mol Cell Biol. 1984 Aug;4(8):1449–1453. doi: 10.1128/mcb.4.8.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict W. F., Murphree A. L., Banerjee A., Spina C. A., Sparkes M. C., Sparkes R. S. Patient with 13 chromosome deletion: evidence that the retinoblastoma gene is a recessive cancer gene. Science. 1983 Feb 25;219(4587):973–975. doi: 10.1126/science.6336308. [DOI] [PubMed] [Google Scholar]
- Bergsma D. J., Chang K. S., Schwartz R. J. Novel chicken actin gene: third cytoplasmic isoform. Mol Cell Biol. 1985 May;5(5):1151–1162. doi: 10.1128/mcb.5.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikel I., Mamon H., Brown E. L., Boltax J., Agha M., Livingston D. M. The t-unique coding domain is important to the transformation maintenance function of the simian virus 40 small t antigen. Mol Cell Biol. 1986 Apr;6(4):1172–1178. doi: 10.1128/mcb.6.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. F., Farmer S. R. Regulation of tubulin and actin mRNA production in rat brain: expression of a new beta-tubulin mRNA with development. Mol Cell Biol. 1983 Aug;3(8):1333–1342. doi: 10.1128/mcb.3.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J., Dorey F., Cragoe E., Jr, Koeffler H. P. Amiloride potentiation of differentiation of human promyelocytic cell line HL-60. J Natl Cancer Inst. 1984 Jan;72(1):13–17. doi: 10.1093/jnci/72.1.13. [DOI] [PubMed] [Google Scholar]
- Chou C. C., Gatti R. A., Fuller M. L., Concannon P., Wong A., Chada S., Davis R. C., Salser W. A. Structure and expression of ferritin genes in a human promyelocytic cell line that differentiates in vitro. Mol Cell Biol. 1986 Feb;6(2):566–573. doi: 10.1128/mcb.6.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- Davis R. C., Thomason A. R., Fuller M. L., Slovin J. P., Chou C. C., Chada S., Gatti R. A., Salser W. A. mRNA species regulated during the differentiation of HL-60 cells to macrophages and neutrophils. Dev Biol. 1987 Jan;119(1):164–174. doi: 10.1016/0012-1606(87)90218-1. [DOI] [PubMed] [Google Scholar]
- Degen J. L., Neubauer M. G., Degen S. J., Seyfried C. E., Morris D. R. Regulation of protein synthesis in mitogen-activated bovine lymphocytes. Analysis of actin-specific and total mRNA accumulation and utilization. J Biol Chem. 1983 Oct 25;258(20):12153–12162. [PubMed] [Google Scholar]
- Erba H. P., Gunning P., Kedes L. Nucleotide sequence of the human gamma cytoskeletal actin mRNA: anomalous evolution of vertebrate non-muscle actin genes. Nucleic Acids Res. 1986 Jul 11;14(13):5275–5294. doi: 10.1093/nar/14.13.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. R., Wan K. M., Ben-Ze'ev A., Penman S. Regulation of actin mRNA levels and translation responds to changes in cell configuration. Mol Cell Biol. 1983 Feb;3(2):182–189. doi: 10.1128/mcb.3.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstenfeld L. C., Finer M. H., Boedtker H. Altered beta-actin gene expression in phorbol myristate acetate-treated chondrocytes and fibroblasts. Mol Cell Biol. 1985 Jun;5(6):1425–1433. doi: 10.1128/mcb.5.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Leavitt J., Kakunaga T. Mutated beta-actin gene: coexpression with an unmutated allele in a chemically transformed human fibroblast cell line. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3634–3638. doi: 10.1073/pnas.78.6.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I., Tanese N., Fuchs E. Complementary DNA sequence of a human cytoplasmic actin. Interspecies divergence of 3' non-coding regions. J Mol Biol. 1983 Feb 5;163(4):673–678. doi: 10.1016/0022-2836(83)90117-1. [DOI] [PubMed] [Google Scholar]
- Hegyi G., Szilagyi L., Elzinga M. Photoaffinity labeling of the nucleotide binding site of actin. Biochemistry. 1986 Sep 23;25(19):5793–5798. doi: 10.1021/bi00367a067. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell. 1986 May 9;45(3):407–415. doi: 10.1016/0092-8674(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Bushar G., Kakunaga T., Hamada H., Hirakawa T., Goldman D., Merril C. Variations in expression of mutant beta actin accompanying incremental increases in human fibroblast tumorigenicity. Cell. 1982 Feb;28(2):259–268. doi: 10.1016/0092-8674(82)90344-0. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Porreca P., Ng S. Y., Lin C. S., Kedes L. Molecular cloning and characterization of mutant and wild-type human beta-actin genes. Mol Cell Biol. 1984 Oct;4(10):1961–1969. doi: 10.1128/mcb.4.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt J., Latter G., Lutomski L., Goldstein D., Burbeck S. Tropomyosin isoform switching in tumorigenic human fibroblasts. Mol Cell Biol. 1986 Jul;6(7):2721–2726. doi: 10.1128/mcb.6.7.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberman R., Egner U. Homologies in the primary structure of GTP-binding proteins: the nucleotide-binding site of EF-Tu and p21. EMBO J. 1984 Feb;3(2):339–341. doi: 10.1002/j.1460-2075.1984.tb01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. S., Ng S. Y., Gunning P., Kedes L., Leavitt J. Identification and order of sequential mutations in beta-actin genes isolated from increasingly tumorigenic human fibroblast strains. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6995–6999. doi: 10.1073/pnas.82.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R. C., Elzinga M. Partial amino acid sequence of brain actin and its homology with muscle actin. Biochemistry. 1977 Dec 27;16(26):5801–5806. doi: 10.1021/bi00645a025. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S. H., Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. 1986 Feb 27-Mar 5Nature. 319(6056):743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Shilo B. Z., Shih C., Cowing D., Hsu H. W., Weinberg R. A. Three different human tumor cell lines contain different oncogenes. Cell. 1981 Aug;25(2):355–361. doi: 10.1016/0092-8674(81)90054-4. [DOI] [PubMed] [Google Scholar]
- Naharro G., Robbins K. C., Reddy E. P. Gene product of v-fgr onc: hybrid protein containing a portion of actin and a tyrosine-specific protein kinase. Science. 1984 Jan 6;223(4631):63–66. doi: 10.1126/science.6318314. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga K., Yoda K., Sakiyama S. Structure of a processed gene of mouse cytoplasmic gamma-actin transposed into a BAM5 sequence: insertion has created 13 base-pair direct repeats. Nucleic Acids Res. 1985 May 10;13(9):3031–3042. doi: 10.1093/nar/13.9.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. W., Sanford K. K., Frankel R. Tubulin and actin in paired nonneoplastic and spontaneously transformed neoplastic cell lines in vitro: fluorescent antibody studies. Cell. 1978 Apr;13(4):629–642. doi: 10.1016/0092-8674(78)90213-1. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Leavitt J., Kakunaga T., Weber K. Coexpression of a mutant beta-actin and the two normal beta- and gamma-cytoplasmic actins in a stably transformed human cell line. Cell. 1980 Dec;22(3):893–899. doi: 10.1016/0092-8674(80)90566-8. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Actin amino-acid sequences. Comparison of actins from calf thymus, bovine brain, and SV40-transformed mouse 3T3 cells with rabbit skeletal muscle actin. Eur J Biochem. 1978 Oct 16;90(3):451–462. doi: 10.1111/j.1432-1033.1978.tb12624.x. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. A protein-chemical analysis of muscle actin differentiation. Differentiation. 1979;14(3):123–133. doi: 10.1111/j.1432-0436.1979.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D., Rotter V. Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):790–794. doi: 10.1073/pnas.82.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]