Abstract
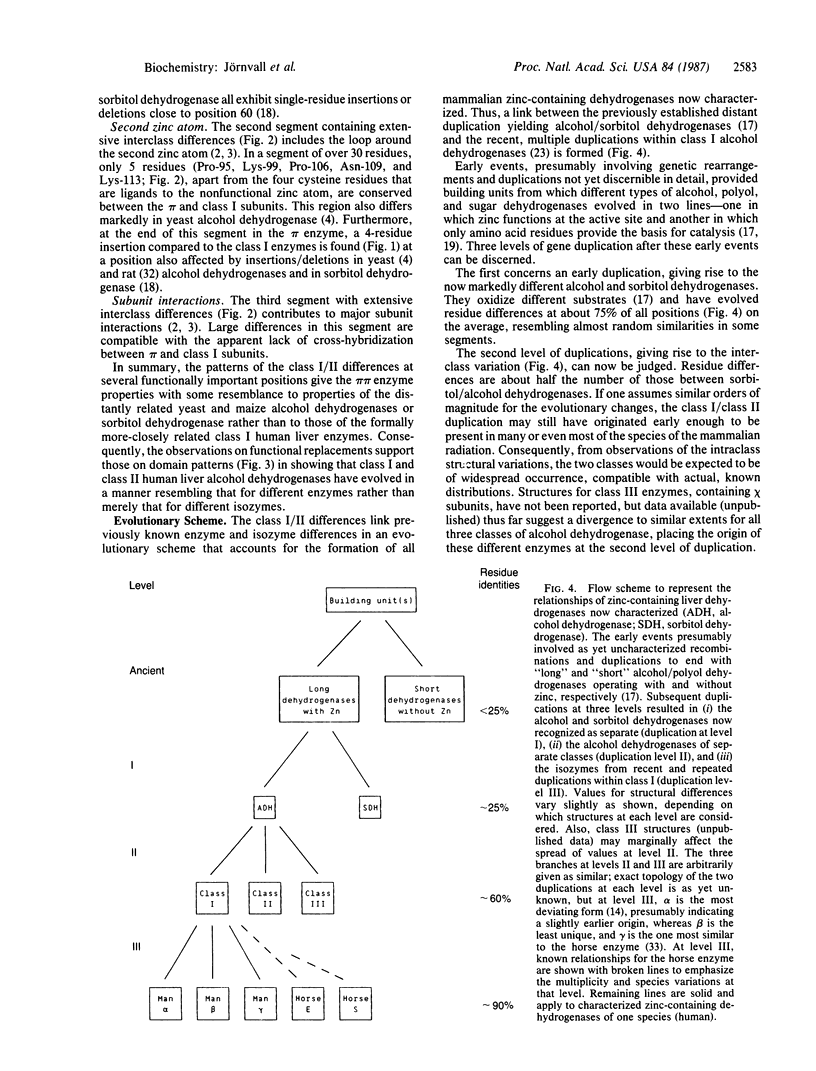
A comparison of the structure of class II human liver alcohol dehydrogenase (alcohol:NAD+ oxidoreductase, EC 1.1.1.1) (containing pi subunits) with those of the human class I isozymes (containing alpha, beta, and gamma subunits) reveals differences at about 40% of all positions. Variations are large for active-site regions, the segment around the second zinc atom, and for segments involved in subunit interactions. The two classes of alcohol dehydrogenase have diverged to exhibit structural differences to about half the extent of those between alcohol and polyol dehydrogenases. Hence, the two classes of alcohol dehydrogenase represent steps in enzyme rather than isozyme divergence. An evolutionary scheme that relates different types of zinc-containing mammalian dehydrogenases to one another encompasses at least three levels of gene duplication subsequent to the early step(s) of assembly of building unit(s). The first level of duplication results in the formation of now clearly different enzymes. The second level concerns the various classes of alcohol dehydrogenase, forming steps between typical enzymes and isozymes. The third level encompasses recent and multiple duplications in isozyme evolution of alcohol dehydrogenases. This scheme, linking zinc-containing dehydrogenases at different levels, resembles that in other protein families and reflects general patterns in protein relationships.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brändén C. I., Eklund H., Cambillau C., Pryor A. J. Correlation of exons with structural domains in alcohol dehydrogenase. EMBO J. 1984 Jun;3(6):1307–1310. doi: 10.1002/j.1460-2075.1984.tb01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler R., Hempel J., Kaiser R., von Wartburg J. P., Vallee B. L., Jörnvall H. Human alcohol dehydrogenase: structural differences between the beta and gamma subunits suggest parallel duplications in isoenzyme evolution and predominant expression of separate gene descendants in livers of different mammals. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6320–6324. doi: 10.1073/pnas.81.20.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Meyerowitz E. M. Molecular cloning and DNA sequence of the Arabidopsis thaliana alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1408–1412. doi: 10.1073/pnas.83.5.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. S., Sachs M. M., Gerlach W. L., Finnegan E. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase 2 (Adh2) gene of maize. Nucleic Acids Res. 1985 Feb 11;13(3):727–743. doi: 10.1093/nar/13.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G., Hatfield G. W., Smith M. Molecular genetic analysis of human alcohol dehydrogenase. Alcohol. 1985 Jan-Feb;2(1):53–56. doi: 10.1016/0741-8329(85)90015-1. [DOI] [PubMed] [Google Scholar]
- Duester G., Jörnvall H., Hatfield G. W. Intron-dependent evolution of the nucleotide-binding domains within alcohol dehydrogenase and related enzymes. Nucleic Acids Res. 1986 Mar 11;14(5):1931–1941. doi: 10.1093/nar/14.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G., Smith M., Bilanchone V., Hatfield G. W. Molecular analysis of the human class I alcohol dehydrogenase gene family and nucleotide sequence of the gene encoding the beta subunit. J Biol Chem. 1986 Feb 15;261(5):2027–2033. [PubMed] [Google Scholar]
- Edenberg H. J., Zhang K., Fong K., Bosron W. F., Li T. K. Cloning and sequencing of cDNA encoding the complete mouse liver alcohol dehydrogenase. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2262–2266. doi: 10.1073/pnas.82.8.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund H., Horjales E., Jörnvall H., Brändén C. I., Jeffery J. Molecular aspects of functional differences between alcohol and sorbitol dehydrogenases. Biochemistry. 1985 Dec 31;24(27):8005–8012. doi: 10.1021/bi00348a025. [DOI] [PubMed] [Google Scholar]
- Eklund H., Nordström B., Zeppezauer E., Söderlund G., Ohlsson I., Boiwe T., Söderberg B. O., Tapia O., Brändén C. I., Akeson A. Three-dimensional structure of horse liver alcohol dehydrogenase at 2-4 A resolution. J Mol Biol. 1976 Mar 25;102(1):27–59. doi: 10.1016/0022-2836(76)90072-3. [DOI] [PubMed] [Google Scholar]
- Hög J. O., Hedén L. O., Larsson K., Jörnvall H., von Bahr-Lindström H. The gamma 1 and gamma 2 subunits of human liver alcohol dehydrogenase. cDNA structures, two amino acid replacements, and compatibility with changes in the enzymatic properties. Eur J Biochem. 1986 Sep 1;159(2):215–218. doi: 10.1111/j.1432-1033.1986.tb09855.x. [DOI] [PubMed] [Google Scholar]
- Ikuta T., Szeto S., Yoshida A. Three human alcohol dehydrogenase subunits: cDNA structure and molecular and evolutionary divergence. Proc Natl Acad Sci U S A. 1986 Feb;83(3):634–638. doi: 10.1073/pnas.83.3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H. Differences in E and S chains from isoenzymes of horse liver alcohol dehydrogenase. Nature. 1970 Mar 21;225(5238):1133–1134. doi: 10.1038/2251133a0. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Eklund H., Brändén C. I. Subunit conformation of yeast alcohol dehydrogenase. J Biol Chem. 1978 Dec 10;253(23):8414–8419. [PubMed] [Google Scholar]
- Jörnvall H., Hempel J., Vallee B. L., Bosron W. F., Li T. K. Human liver alcohol dehydrogenase: amino acid substitution in the beta 2 beta 2 Oriental isozyme explains functional properties, establishes an active site structure, and parallels mutational exchanges in the yeast enzyme. Proc Natl Acad Sci U S A. 1984 May;81(10):3024–3028. doi: 10.1073/pnas.81.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H., Hempel J., Vallee B. Structures of human alcohol and aldehyde dehydrogenases. Enzyme. 1987;37(1-2):5–18. doi: 10.1159/000469237. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Persson M., Jeffery J. Alcohol and polyol dehydrogenases are both divided into two protein types, and structural properties cross-relate the different enzyme activities within each type. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4226–4230. doi: 10.1073/pnas.78.7.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H., von Bahr-Lindström H., Jany K. D., Ulmer W., Fröschle M. Extended superfamily of short alcohol-polyol-sugar dehydrogenases: structural similarities between glucose and ribitol dehydrogenases. FEBS Lett. 1984 Jan 9;165(2):190–196. doi: 10.1016/0014-5793(84)80167-2. [DOI] [PubMed] [Google Scholar]
- Li T. K., Bosron W. F., Dafeldecker W. P., Lange L. G., Vallee B. L. Isolation of pi-alcohol dehydrogenase of human liver: is it a determinant of alcoholism? Proc Natl Acad Sci U S A. 1977 Oct;74(10):4378–4381. doi: 10.1073/pnas.74.10.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Fritsch E. F., Lauer J., Lawn R. M. The molecular genetics of human hemoglobins. Annu Rev Genet. 1980;14:145–178. doi: 10.1146/annurev.ge.14.120180.001045. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. L., Kato H., Upshall A., Parker M. D., Saari G., O'Hara P. J. Identification and molecular analysis of a third Aspergillus nidulans alcohol dehydrogenase gene. EMBO J. 1985 Aug;4(8):2093–2099. doi: 10.1002/j.1460-2075.1985.tb03897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parés X., Vallee B. L. New human liver alcohol dehydrogenase forms with unique kinetic characteristics. Biochem Biophys Res Commun. 1981 Jan 15;98(1):122–130. doi: 10.1016/0006-291x(81)91878-7. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Smith M., Williamson V. M., Young E. T. Nucleotide sequence of the yeast alcohol dehydrogenase II gene. J Biol Chem. 1983 Feb 25;258(4):2674–2682. [PubMed] [Google Scholar]
- Russell P. R., Hall B. D. The primary structure of the alcohol dehydrogenase gene from the fission yeast Schizosaccharomyces pombe. J Biol Chem. 1983 Jan 10;258(1):143–149. [PubMed] [Google Scholar]
- Smith M., Hopkinson D. A., Harris H. Developmental changes and polymorphism in human alcohol dehydrogenase. Ann Hum Genet. 1971 Feb;34(3):251–271. doi: 10.1111/j.1469-1809.1971.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Young E. T., Pilgrim D. Isolation and DNA sequence of ADH3, a nuclear gene encoding the mitochondrial isozyme of alcohol dehydrogenase in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Nov;5(11):3024–3034. doi: 10.1128/mcb.5.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bahr-Lindström H., Hög J. O., Hedén L. O., Kaiser R., Fleetwood L., Larsson K., Lake M., Holmquist B., Holmgren A., Hempel J. cDNA and protein structure for the alpha subunit of human liver alcohol dehydrogenase. Biochemistry. 1986 May 6;25(9):2465–2470. doi: 10.1021/bi00357a026. [DOI] [PubMed] [Google Scholar]



