Abstract
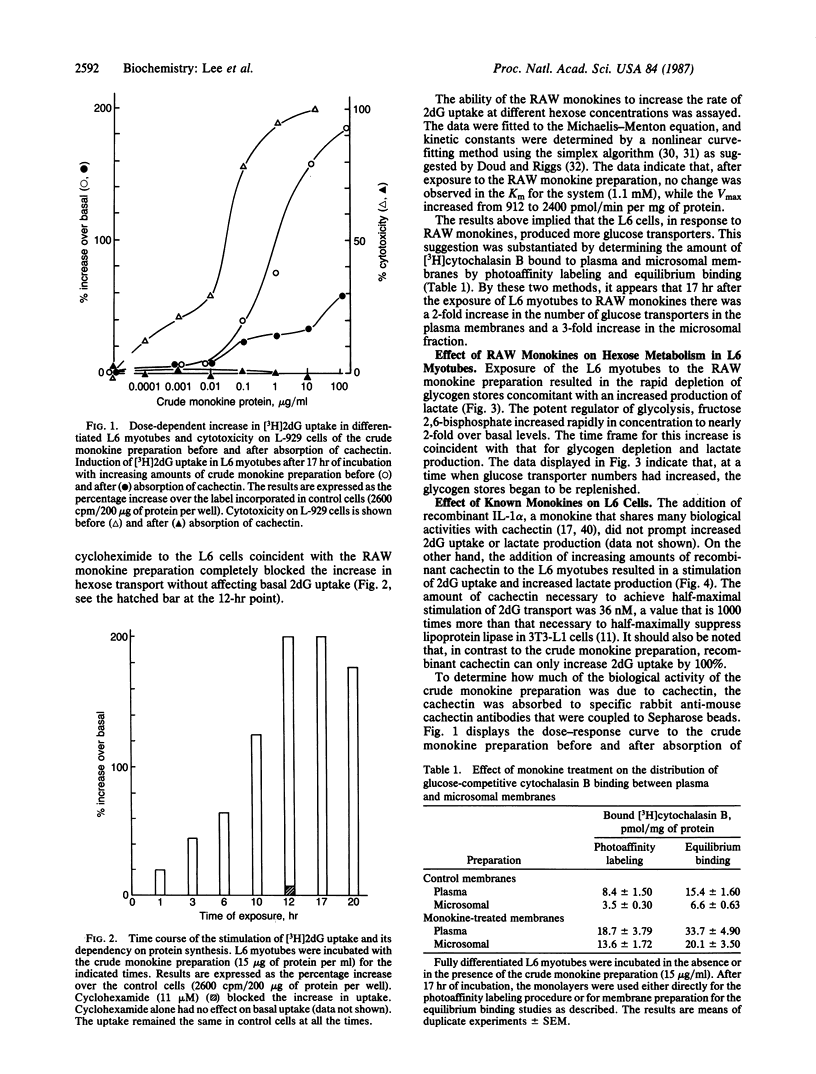
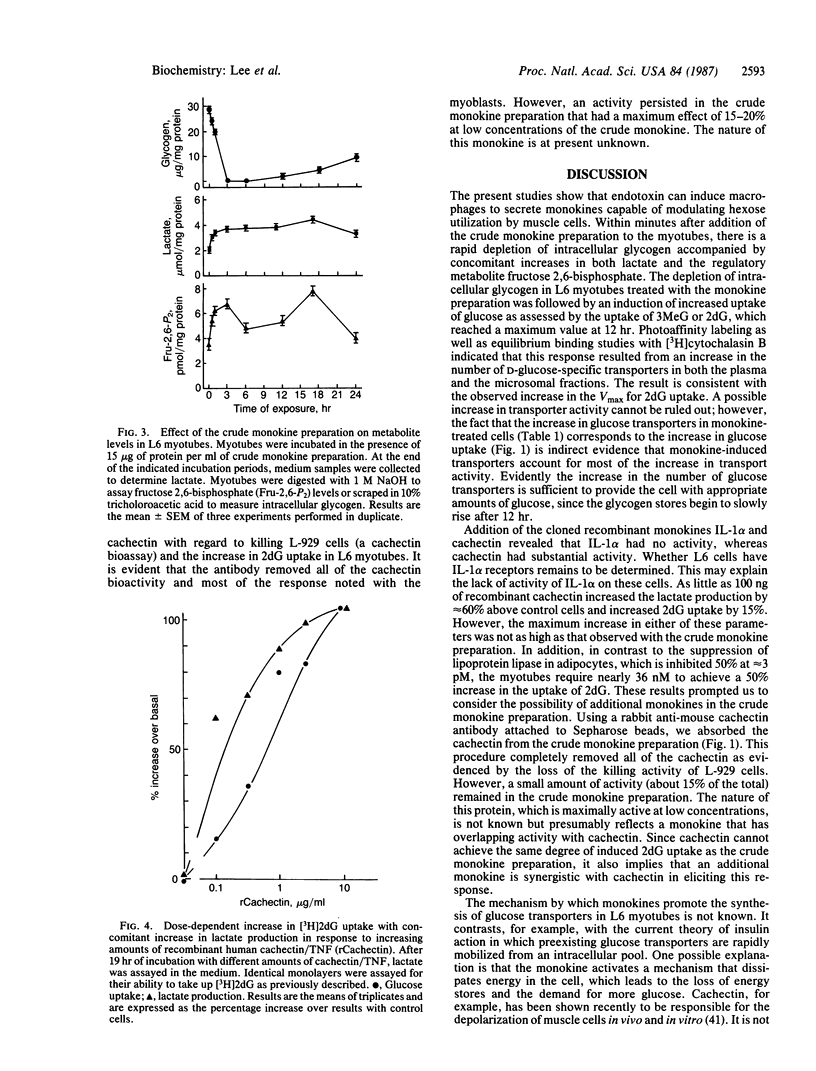
Exposure of fully differentiated L6 myotubes to a crude monokine preparation from endotoxin-stimulated RAW 264.7 cells resulted in a rapid and substantial (70%) increase in fructose 2,6-bisphosphate concentration coincident with a depletion of cellular glycogen and an increased lactate production. During the time required for glycogen depletion (3 hr), stimulation of 3-O-methyl-D-glucose and 2-deoxy-D-glucose uptake was initiated and observed to reach a maximum enhancement of 200% 12-15 hr later. The monokine had no effect on the Km value for 2-deoxy-D-glucose uptake (1.1 mM), while Vmax was increased from 912 to 2400 pmol/min per mg of protein. The increase was cytochalasin B inhibitable and was dependent on protein synthesis. Photoaffinity labeling and equilibrium binding studies with [3H]cytochalasin B support the hypothesis that this increase in hexose transport was due to an increase in hexose transporters present in the plasma membrane. Purified recombinant interleukin-1 alpha had no effect on hexose transport, whereas purified recombinant cachetin/tumor necrosis factor did stimulate hexose uptake, with half-maximal stimulation occurring at 36 nM. Although cachetin accounts for most of the biological activity associated with the crude monokine preparations, it is not the only monokine capable of inducing glucose transport in L6 cells. Specific immunoabsorption of cachectin/tumor necrosis factor from the crude monokine preparation revealed a monokine that had a similar bioactivity at extremely low concentrations on L6 cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisel W. R. Metabolic response to infection. Annu Rev Med. 1975;26:9–20. doi: 10.1146/annurev.me.26.020175.000301. [DOI] [PubMed] [Google Scholar]
- Beisel W. R., Wannemacher R. W., Jr Gluconeogenesis, ureagenesis, and ketogenesis during sepsis. JPEN J Parenter Enteral Nutr. 1980 May-Jun;4(3):277–285. doi: 10.1177/014860718000400307. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Mahoney J., Le Trang N., Pekala P., Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P. Regulation of the D-glucose transport system in isolated fat cells. Mol Cell Biochem. 1976 Mar 26;11(1):51–63. doi: 10.1007/BF01792833. [DOI] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Interleukins. Annu Rev Med. 1986;37:173–178. doi: 10.1146/annurev.me.37.020186.001133. [DOI] [PubMed] [Google Scholar]
- Filkins J. P., Figlewicz D. P. Increased insulin responsiveness in endotoxicosis. Circ Shock. 1979;6(1):1–6. [PubMed] [Google Scholar]
- Filkins J. P. Monokines and the metabolic pathophysiology of septic shock. Fed Proc. 1985 Feb;44(2):300–304. [PubMed] [Google Scholar]
- Filkins J. P. Phases of glucose dyshomeostasis in endotoxicosis. Circ Shock. 1978;5(4):347–355. [PubMed] [Google Scholar]
- Filkins J. P. Reticuloendothelial system function and glucose-insulin dyshomeostasis in sepsis. Am J Emerg Med. 1984 Jan;2(1):70–73. doi: 10.1016/0735-6757(84)90111-6. [DOI] [PubMed] [Google Scholar]
- Graff J. C., Wohlhueter R. M., Plagemann P. G. Deoxyglucose and 3-O-methylglucose transport in untreated and ATP-depleted Novikoff rat hepatoma cells. Analysis by a rapid kinetic technique, relationship to phosphorylation and effects of inhibitors. J Cell Physiol. 1978 Aug;96(2):171–188. doi: 10.1002/jcp.1040960206. [DOI] [PubMed] [Google Scholar]
- Grimditch G. K., Barnard R. J., Kaplan S. A., Sternlicht E. Insulin binding and glucose transport in rat skeletal muscle sarcolemmal vesicles. Am J Physiol. 1985 Oct;249(4 Pt 1):E398–E408. doi: 10.1152/ajpendo.1985.249.4.E398. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT W. J., BOYER P. D. Interaction of protein antigens and antibodies. I. Inhibition studies with the egg albumin-antiegg albumin system. J Immunol. 1952 Sep;69(3):247–255. [PubMed] [Google Scholar]
- Kawakami M., Pekala P. H., Lane M. D., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1982 Feb;79(3):912–916. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitcher S. A., Siddle K., Luzio J. P. A method for the determination of glucose-6-phosphatase activity in rat liver with [U-14C]glucose 6-phosphate as substrate. Anal Biochem. 1978 Jul 15;88(1):29–36. doi: 10.1016/0003-2697(78)90395-0. [DOI] [PubMed] [Google Scholar]
- Mahoney J. R., Jr, Beutler B. A., Le Trang N., Vine W., Ikeda Y., Kawakami M., Cerami A. Lipopolysaccharide-treated RAW 264.7 cells produce a mediator that inhibits lipoprotein lipase in 3T3-L1 cells. J Immunol. 1985 Mar;134(3):1673–1675. [PubMed] [Google Scholar]
- Oka Y., Czech M. P. Photoaffinity labeling of insulin-sensitive hexose transporters in intact rat adipocytes. Direct evidence that latent transporters become exposed to the extracellular space in response to insulin. J Biol Chem. 1984 Jul 10;259(13):8125–8133. [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Pekala P. H., Kawakami M., Angus C. W., Lane M. D., Cerami A. Selective inhibition of synthesis of enzymes for de novo fatty acid biosynthesis by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2743–2747. doi: 10.1073/pnas.80.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekala P., Kawakami M., Vine W., Lane M. D., Cerami A. Studies of insulin resistance in adipocytes induced by macrophage mediator. J Exp Med. 1983 Apr 1;157(4):1360–1365. doi: 10.1084/jem.157.4.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podleski T. R., Nichols S., Ravdin P., Salpeter M. M. Cloned myogenic cells during differentiation: membrane biochemistry and fine structural observations. Dev Biol. 1979 Jan;68(1):239–258. doi: 10.1016/0012-1606(79)90256-2. [DOI] [PubMed] [Google Scholar]
- Standaert M. L., Schimmel S. D., Pollet R. J. The development of insulin receptors and responses in the differentiating nonfusing muscle cell line BC3H-1. J Biol Chem. 1984 Feb 25;259(4):2337–2345. [PubMed] [Google Scholar]
- Tate S. S., Meister A. Interaction of gamma-glutamyl transpeptidase with amino acids, dipeptides, and derivatives and analogs of glutathione. J Biol Chem. 1974 Dec 10;249(23):7593–7602. [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Beutler B., Cerami A., Albert J. D., Shires G. T. Cachectin/tumor necrosis factor mediates changes of skeletal muscle plasma membrane potential. J Exp Med. 1986 Oct 1;164(4):1368–1373. doi: 10.1084/jem.164.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- Viray R. E., Filkins J. P. Nonsuppressible insulinlike activity (NSILA) and the glucose dyshomeostasis of agonal sepsis. Adv Shock Res. 1983;9:31–41. [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]



