Abstract
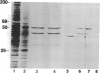
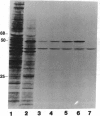
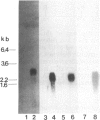
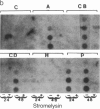
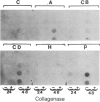
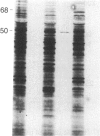
Secreted proteinases are required for tumor metastasis, angiogenesis, and tissue remodeling during wound healing and embryonic growth. Thus, the regulation of the genes of secreted proteinases may serve as an interesting model for growth-controlled genes in general. We studied the genes of the secreted proteinases stromelysin and collagenase by using molecularly cloned cDNAs from each proteinase. Stromelysin cDNA was cloned by differential screening of a total cDNA library from rabbit synovial cells treated with phorbol 12-myristate 13-acetate, which yielded a clone of 1.2 kilobase pairs; collagenase cDNA was obtained by cloning reverse transcripts of anti-collagenase-immunoadsorbed polysomal mRNA, which yielded a clone of 0.8 kilobase pairs. Stromelysin and collagenase mRNA species of 2.2 and 2.4 kilobases, respectively, were detected on hybridization blots of RNA from phorbol 12-myristate 13-acetate-treated but not untreated rabbit synovial cells. Expression of stromelysin mRNA was also induced in rabbit alveolar macrophages and rabbit brain capillary endothelial cells treated with phorbol 12-myristate 13-acetate. Stromelysin and collagenase mRNA were both induced by phorbol 12-myristate 13-acetate and cytochalasin B at a constant ratio of the two gene products; this suggests coordinate regulation. The fact that induction was blocked after inhibition of protein synthesis by cycloheximide implicates an indirect signal transduction pathway that requires new protein synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggeler J., Frisch S. M., Werb Z. Changes in cell shape correlate with collagenase gene expression in rabbit synovial fibroblasts. J Cell Biol. 1984 May;98(5):1662–1671. doi: 10.1083/jcb.98.5.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggeler J., Frisch S. M., Werb Z. Collagenase is a major gene product of induced rabbit synovial fibroblasts. J Cell Biol. 1984 May;98(5):1656–1661. doi: 10.1083/jcb.98.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda M. J., Clark E. J., Werb Z. Regulation of alpha 1 proteinase inhibitor function by rabbit alveolar macrophages. Evidence for proteolytic rather than oxidative inactivation. J Clin Invest. 1985 Jun;75(6):1758–1762. doi: 10.1172/JCI111887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda M. J., Knighton D. R., Hunt T. K., Werb Z. Isolation of a nonmitogenic angiogenesis factor from wound fluid. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7773–7777. doi: 10.1073/pnas.79.24.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Cell configuration-related control of vimentin biosynthesis and phosphorylation in cultured mammalian cells. J Cell Biol. 1983 Sep;97(3):858–865. doi: 10.1083/jcb.97.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- Farmer S. R., Wan K. M., Ben-Ze'ev A., Penman S. Regulation of actin mRNA levels and translation responds to changes in cell configuration. Mol Cell Biol. 1983 Feb;3(2):182–189. doi: 10.1128/mcb.3.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Caput D., Williams S. R., Martin D. W., Jr Cloning of human purine-nucleoside phosphorylase cDNA sequences by complementation in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4281–4285. doi: 10.1073/pnas.80.14.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Gross J. L., Moscatelli D., Rifkin D. B. Increased capillary endothelial cell protease activity in response to angiogenic stimuli in vitro. Proc Natl Acad Sci U S A. 1983 May;80(9):2623–2627. doi: 10.1073/pnas.80.9.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. H., Sheldon L. A., Fletcher C. F., Brinckerhoff C. E. Isolation of a collagenase cDNA clone and measurement of changing collagenase mRNA levels during induction in rabbit synovial fibroblasts. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1981–1985. doi: 10.1073/pnas.81.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hembry R. M., Murphy G., Cawston T. E., Dingle J. T., Reynolds J. J. Characterization of a specific antiserum for mammalian collagenase from several species: immunolocalization of collagenase in rabbit chondrocytes and uterus. J Cell Sci. 1986 Mar;81:105–123. doi: 10.1242/jcs.81.1.105. [DOI] [PubMed] [Google Scholar]
- Herron G. S., Werb Z., Dwyer K., Banda M. J. Secretion of metalloproteinases by stimulated capillary endothelial cells. I. Production of procollagenase and prostromelysin exceeds expression of proteolytic activity. J Biol Chem. 1986 Feb 25;261(6):2810–2813. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Krane S. M. Collagenases and collagen degradation. J Invest Dermatol. 1982 Jul;79 (Suppl 1):83s–86s. doi: 10.1111/1523-1747.ep12545849. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Parry G., Bissell M. J. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984 Jan;98(1):146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Leroy P., Ruhlmann C., Gesnel M. C., Breathnach R. Isolation of the oncogene and epidermal growth factor-induced transin gene: complex control in rat fibroblasts. Mol Cell Biol. 1986 May;6(5):1679–1686. doi: 10.1128/mcb.6.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. S., Paterson B. M., Ricciardi R. P., Cohen L., Roberts B. E. Methods utilizing cell-free protein-synthesizing systems for the identification of recombinant DNA molecules. Methods Enzymol. 1983;101:650–674. doi: 10.1016/0076-6879(83)01046-0. [DOI] [PubMed] [Google Scholar]
- Rabin M. S., Doherty P. J., Gottesman M. M. The tumor promoter phorbol 12-myristate 13-acetate induces a program of altered gene expression similar to that induced by platelet-derived growth factor and transforming oncogenes. Proc Natl Acad Sci U S A. 1986 Jan;83(2):357–360. doi: 10.1073/pnas.83.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Ben-Ze'ev A. Modulation of the metastatic capability in B16 melanoma by cell shape. Science. 1983 Sep 23;221(4617):1307–1310. doi: 10.1126/science.6612347. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Unemori E. N., Werb Z. Reorganization of polymerized actin: a possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J Cell Biol. 1986 Sep;103(3):1021–1031. doi: 10.1083/jcb.103.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z. Biochemical actions of glucocorticoids on macrophages in culture. Specific inhibition of elastase, collagenase, and plasminogen activator secretion and effects on other metabolic functions. J Exp Med. 1978 Jun 1;147(6):1695–1712. doi: 10.1084/jem.147.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Hembry R. M., Murphy G., Aggeler J. Commitment to expression of the metalloendopeptidases, collagenase and stromelysin: relationship of inducing events to changes in cytoskeletal architecture. J Cell Biol. 1986 Mar;102(3):697–702. doi: 10.1083/jcb.102.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Zanetti N. C., Solursh M. Induction of chondrogenesis in limb mesenchymal cultures by disruption of the actin cytoskeleton. J Cell Biol. 1984 Jul;99(1 Pt 1):115–123. doi: 10.1083/jcb.99.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]