Abstract
Increased understanding of the molecular heterogeneity that is intrinsic to the various subtypes of breast cancer will likely shape the future of breast cancer diagnosis, prognosis and treatment. Advances in the field over the last several decades have been remarkable and have clearly translated into better patient care as evidenced by the earlier detection, better prognosis and new targeted therapies. There have been two recent advances in the breast cancer research field that have lead to paradigm shifts: first, the identification of intrinsic breast tumor subtypes, which has changed the way we think about breast cancer and second, the recent characterization of cancer stem cells (CSCs), which are suspected to be responsible for tumor initiation, recurrence and resistance to therapy. These findings have opened new exciting avenues to think about breast cancer therapeutic strategies. While these advances constitute major paradigm shifts within the research realm, the clinical arena has yet to adopt and apply our understanding of the molecular basis of the disease to early diagnosis, prognosis and therapy of breast cancers. Here, we will review the current clinical approach to classification of breast cancers, newer molecular-based classification schemes and potential future of biomarkers representing a functional classification of breast cancer.
Key words: breast cancer, tumor heterogeneity, cancer subtypes, stem cells
Introduction
Breast cancer is a genetically and clinically heterogeneous disease.1 In order to organize this heterogeneity and standardize the language, breast cancer classification systems have been developed. These classification schemes have evolved over many decades into a tool that is used to aid in treatment and prognosis. However, with recent advances in cancer research and an increased molecular understanding of breast cancer heterogeneity, the current clinical model for breast cancer classification may benefit from the addition of several factors. The identification of tumor initiating cancer stem cells and the five molecular subtypes of breast cancer will help provide an even more comprehensive and clinically relevant classification of breast cancer heterogeneity.
Histological Classifications of Breast Cancer Subtypes
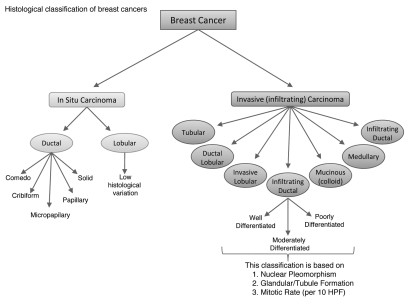
Unlike colon cancers, defining the progression of breast cancer has not been possible due to lack of markers that define hyperplasia (typical and atypical), carcinoma in situ and invasive cancer.1 However, breast cancer can be broadly categorized into in situ carcinoma and invasive (infiltrating) carcinoma. Breast carcinoma in situ is further sub-classified as either ductal or lobular; growth patterns and cytological features form the basis to distinguish between the two types. Ductal carcinoma in situ (DCIS) is considerably more common than its lobular carcinoma in situ (LCIS) counterpart and encompasses a heterogeneous group of tumors. DCIS has traditionally been further sub-classified based on the architectural features of the tumor which has given rise to five well recognized subtypes: Comedo, Cribiform, Micropapillary, Papillary and Solid (Fig. 1).2 While this classification scheme has been a valuable tool for several decades, it relies solely on histology without utilizing newer molecular markers that have a proven prognostic significance.
Figure 1.
Histological classification of breast cancer subtypes. This scheme, currently used by clinicians, categorizes the heterogeneity found in breast cancer based on architectural features and growth patterns. HPF: high power field.
In light of surgical advances leading to breast-conserving therapy, it has become necessary to more accurately stratify patients based on relative risk of recurrence or progression. These demands have led to the generation of several newer classification systems that incorporate molecular markers such as ER, PR, ErbB2 (Her2/neu) and p53.3–6 While the routine use of these markers for DCIS has not been accepted by the larger medical community, it is notable that the National Comprehensive Cancer Network has included determination of ER status as part of their DCIS workup.7 This paradigm shift foreshadows the future of molecular medicine that we have only recently begun to appreciate.
Similar to in situ carcinomas, invasive carcinomas are a heterogeneous group of tumors differentiated into histological subtypes. The major invasive tumor types include infiltrating ductal, invasive lobular, ductal/lobular, mucinous (colloid), tubular, medullary and papillary carcinomas (Fig. 1). Of these, infiltrating ductal carcinoma (IDC) is, by far, the most common subtype accounting for 70–80% of all invasive lesions.8 IDC is further sub-classified as either well-differentiated (grade 1), moderately differentiated (grade 2) or poorly differentiated (grade 3) based on the levels of nuclear pleomorphism, glandular/tubule formation and mitotic index.9
In contrast to DCIS, where the use of molecular markers is still debated, the utility of ER, PR and HER2/neu is well accepted for IDC and it is recommended that their status be determined on all invasive carcinomas.7,10 Furthermore, the College of American Pathologists acknowledges, but does not require or recommend, the use of other ancillary tests (e.g., gene array profiling or immunohistochemical staining for markers other than ER, PR and HER2/neu) as long as sufficient tissue is available.9 The use of ER, PR and HER2/neu determination in IDC exemplifies the potential of molecular biomarkers in guiding clinical decisions.11 Already, the status of these markers helps determine which patients are likely to respond to targeted therapies (i.e., tamoxifen or aromatase inhibitors for ER+/PR+ patients and trastuzumab or lapatinib for HER2/neu patients).12,13
Molecular Classifications of Breast Cancer Subtypes
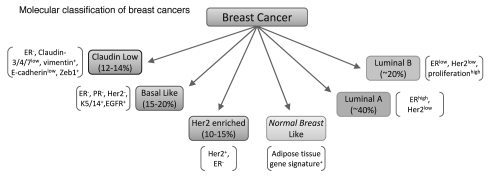
While the current model for breast cancer classification has prognostic value, lack of a molecular component to the classification scheme limits the ability to predict a response to newer targeted therapies. The recent identification of the molecular subtypes of breast cancer has begun to address this issue. Recent studies identified several intrinsic molecular subtypes of breast cancer that were later confirmed and classified as: basal-like, ErbB2+, normal breast like, luminal subtype A and luminal subtype B (Fig. 2).14–16 More recently, a new subtype classified as “claudin-low” has also been identified.17,18 These molecular subtypes of cancer were identified by microarray-based gene expression analysis and unbiased hierarchical clustering. Notably, the molecular subtypes display highly significant differences in prediction of overall survival, as well as disease-free survival with the basal-like/triple-negative (ER−/PR−/ErbB2−) subtype having the shortest survival.15,16 Furthermore, this molecular classification was able to stratify the ER+ population into several subtypes that, again, demonstrated a difference in patient survival. This is significant because even though clinical assessment of IDC utilizes ER, PR and ErbB2 status, these markers did not allow separation of the two distinct ER+ subtypes (i.e., Luminal A and Luminal B) that have very different clinical outcomes.15,16
Figure 2.
Molecular classification of breast cancer. This classification is based on the intrinsic molecular subtypes of breast cancer identified by microarray analysis of patient tumor specimens.14–16
The utility of this new molecular classification to predict outcomes has raised hopes of its adaptation in clinical practice; however, routine use of microarray analysis or genome sequencing is still cost prohibitive. To overcome this obstacle, investigators narrowed down a 50-gene signature that can effectively differentiate the molecular subtypes using quantitative real time PCR (qRT-PCR). This 50-gene signature, termed PAM50, has been shown to be an effective replacement for full microarray analysis with an ability to classify tumors into one of the intrinsic subtypes.19 Furthermore, it was demonstrated that a model using the PAM50 gene set for molecular classification had a significantly improved ability to predict risk of relapse as compared to a model utilizing only clinical variables (tumor size, node status and histologic grade) when tested on ER+/node-negative patients.19 However, it is important to mention that utilizing both clinical variables and molecular subtyping resulted in significantly better predictive value than either one alone. In light of the tremendous variability in response to therapy, it is perhaps most notable that using the molecular subtypes generated a model with 94% sensitivity and 97% negative predictive value for predicting pathological complete response.19
Thus, it is reasonable to expect that the application of molecular subtyping in clinical practice will provide useful information regarding patient-specific prognosis, risk of relapse and probability for pathological complete response. A major benefit of improved risk stratification will be the identification of patients for whom the benefits of neoadjuvant therapy outweigh the risks. Alternatively, patients with increased risk of relapse may benefit from a more aggressive treatment strategy or increased surveillance. It is important to note that the PAM50 is not the only multi-gene predictor for breast cancer; there are many others that are useful for cancer classification, grading, prognosis and response to therapy (reviewed in ref. 20).
Functional Classification of Breast Cancer Subtypes—Mammary Stem Cells and Cancer Stem Cells
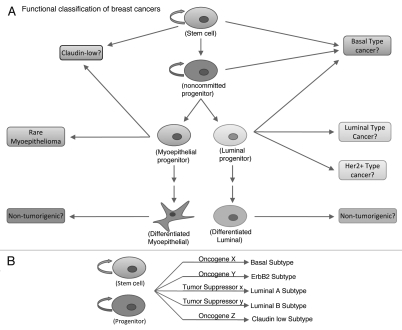
While identification of the molecular subtypes of breast cancers represents an area of significant advance in our understanding of breast cancer, yet another emerging area of intense research has been that of breast cancer stem cells (CSCs).21 Although the concept of CSCs is not recent, it has gained significant attention of late due to the isolation and characterization of CSCs as tumor initiating cells in several common malignancies. The cancer stem cell theory simply states that, within a tumor, there exists a small subset of cells that is responsible for tumor initiation and progression, while the remaining bulk of the tumor cells is of low tumorigenic potential.1 The cell of origin for CSCs is still undetermined but two prevailing hypotheses propose that CSCs either originate from normal cells within the stem cell hierarchy (Fig. 3A) or arise from a common normal stem cell (Fig. 3B).
Figure 3.
Functional classification of breast cancer. This scheme will classify breast cancer based on the tumor initiating cells. Currently there are two proposed hypothesis: (A) either the heterogeneity seen in breast cancer arises from distinct mammary stem/progenitor cells at various levels within the mammary stem cell hierarchy or, (B) breast cancer heterogeneity is the result of a single mammary stem/progenitor cell being transformed by various oncogenes which give rise to various types of cancer.
Due to the rapid growth of the field, there is considerable confusion regarding the appropriate methods of isolation and identification of CSCs. Particularly, there are no conclusive markers to differentiate between stem and non-committed progenitor cells. The cellular hierarchy of the mammary gland is not as clearly delineated as that of the hematopoietic system. Several laboratories have independently identified markers to isolate CSCs that appear to work for their respective systems; unfortunately, there has not been a careful comparison of these various identification strategies. Notably, markers used to identify normal mammary stem cells (MaSCs) are the same as those used to identify CSCs.22–26 It is likely that there will be significant overlap between the markers but ultimately some will be more useful than others. In this section, we will review various methods that have been reported to effectively identify and isolate both normal and cancer stem cells.
CD44/Lin.
While the existence of MaSCs was first shown in the late 1990s by Kordon and Smith,27 and later conclusively proven by mammary gland development from single cells,23,24 breast CSCs were not clearly characterized until 2003.26 Clarke and colleagues isolated a subset of cancer cells identified as CD44high/CD24low/lin− that were able to generate tumors when as few as 200 cells were implanted in immune-compromised mouse recipients. In contrast, cancer cells that did not display this marker set were unable to generate tumors with even 20,000 cells.26 Notably, tumors generated with CD44high/CD24low/lin− cells were able to recapitulate the histopathology of the initial tumor demonstrating the ability of these cells to regenerate the full range of tumor heterogeneity. In order to demonstrate the self-renewal ability of these cells, the authors showed that the cells retained the ability to generate tumors after serial passages.26
These markers have now been used by several groups to isolate CSCs from both primary tumors and established breast cancer cell lines. While this has been tremendously useful as a tool for laboratory research, its potential for clinical use relies on its ability to predict patient prognosis or response to therapy. To investigate this, an invasiveness gene signature was generated by comparing gene expression profiles from CD44high/CD24low cancer and normal breast epithelium. This gene signature was shown to correlate with both overall and metastasis free survival of breast cancer patients.28 This implies that patient prognosis may be correlated with the level of CSCs (and hence the invasiveness gene signature). In support of this, it was shown that the gene signature correlated with prognosis in several other cancers including medulloblastoma, lung cancer and prostate cancer.
ALDEFLUOR (ALDH1).
The appeal of using cell surface markers for the identification of CSCs is the ability to isolate viable CSCs via flow cytometry. Recently, investigators have identified an intracellular enzyme aldehyde dehydrogenase 1 (ALDH1) as a marker of both normal and cancer stem cells.22 A cell permeant fluorescent substrate allows quantification of ALDH activity in live cells, facilitating the use of this marker to identify and isolate CSCs. A fluorescent substrate-based “ALDEFLUOR” assay (Stem Cell Technologies) has been shown to effectively isolate adult tissue stem cells from a variety of tissue types including the hematopoietic system,29,30 central nervous system31 and mammary gland.22 The initial usefulness of ALDH1 in identifying stem cells across tissue types is consistent with its suspected role in the early differentiation of stem cells.32 Notably, ALDH1 activity has also been used to identify CSCs,22,33 implying some retention of normal stem cell signaling in transformed CSCs.
The ALDEFLUOR assay was originally designed to isolate adult hematopoietic stem cells, but Ginstier et al. have recently demonstrated its ability to isolate both normal mammary stem cells and breast CSCs.22 Although there hasn't been a comprehensive comparison between the full spectrum of stem cell markers, Ginster and colleagues did compare the ALDH1 marker with CD44high/CD24low/lin− marker set; they report approximately 20% overlap between the two markers and demonstrate that cells displaying both markers carry enhanced tumorigenic potential.22 Moreover, they show that cells within the CD44high/CD24low/lin− population that are able to generate tumors are also ALDEFLUOR-positive. This suggests that CD44high/CD24low/lin− markers are able to enrich for breast CSCs but there is likely considerable heterogeneity within that population.
Again, the ability of a marker to predict clinical outcome is a critical factor in determining its clinical utility. ALDH1 has been shown to correlate with the overall survival of patients by several research groups. One study using two independent sets of breast tumors with 481 collective samples found that ALDH1-positive tumors showed strong association with poor clinical outcome and high histological grade. Notably, high tumor grade has recently been associated with a higher content of CSCs.34 Furthermore, the basal subtype of breast tumors, which are typically triple-negative and carry a poorer prognosis, contain high numbers of CSCs.35 Another study utilizing four series of tumor samples found a significant correlation between ALDH1 expression and basal-like breast tumors that were mostly triple-negative (ER−/PR−/ErbB2−), which is consistent with other published data for association with CD44+/CD24− marker set.36 More recently, Neumeister et al. using a multiplexed flow-based assay, showed that the coexpression of ALDH1, CD44 and cytokeratins was significantly correlated with poor outcome.37
In addition to an association with patient outcome, ALDH1-positive tumors have also been associated with low pathologic complete response to sequential paclitaxel and epirubicin-based chemotherapy. Interestingly, the CD44/CD24 analysis was done in conjunction but did not show any significant differences in pathologic complete response rates.38 Furthermore, it was shown that the proportion of ALDH1-positive cells increased significantly after neoadjuvant therapy, indicating inherent chemotherapy resistance in the ALDH1-positive CSCs. These studies are useful in establishing the clinical significance of ALDH1 as a biomarker for prognosis and therapy response/resistance.
CD49f/α6-integrin and CD29/β1-integrin.
In addition to the use of CD44/CD24, several other cell surface markers have been used to isolate prospective mammary stem cells. In 2006, two groups isolated mouse mammary stem cells by first excluding cells of endothelial and hematopoietic origin and then selecting for cells based on their expression of CD24, CD29 or CD49f.23,24 These studies showed that a single cell was able to reconstitute a full mammary gland in vivo.23,24 Although the initial experiments involving these markers were done in a mouse model system, they have successfully been utilized for isolation of MaSCs from human tissue.39 More importantly, elevated levels of CD49f in breast cancer tissues correlate with poor prognosis and reduced survival. When α6-integrin levels were used in conjunction with clinical risk factors (e.g., histologic grade, steroid receptor expression) the prognostic value was significantly better than either one alone.40
Issues and Considerations
Failures with the one size fits all approaches to cancer therapy will no doubt be replaced by more personalized cancer therapy in the future. Understanding histological and molecular characteristics of a tumor will therefore be necessary as tools to individualize such therapy. Above, we reviewed the various approaches that can be taken to classify breast cancer, namely histological, molecular and functional. The current clinical classification system relies heavily on the histological aspects of the primary lesion; while this system has some prognostic value, it lacks a molecular basis. Through advanced technologies and tools, we are now beginning to achieve a more comprehensive understanding of breast cancer, which has led to the identification of five intrinsic subtypes of breast cancer. This represents a molecular classification/approach to breast cancer that has tremendous potential for therapeutic response and prognostic predictions. Finally, the functional classification of breast cancer is likely to use one or more of the CSC markers to quantify the percentage of CSCs in a patient's tumor. Initial studies have clearly associated CSCs with poor clinical outcome and CSCs will likely be the focus of the next generation of targeted therapies.
Importantly, we emphasize that at present there is not a clear basis to adopt one molecular marker or parameter over another; rather parts of several marker sets will likely need to be retained and incorporated into a more comprehensive system that will be most useful to clinicians in providing the most predictive classification of disease for the benefit of the patients. It has consistently been shown that combination of clinical variables with either molecular or functional biomarkers yields a system that is more robust and capable than any one system alone. This type of approach, coupled with the rapid progress that is being made in the field of breast cancer research is likely to produce better diagnostic and prognostic biomarkers to improve patient outcomes.
Acknowledgements
Work in our laboratories is supported by the NIH grant CA96844 and Department of Defense grant W81XWH-07-1-0351 to V.B.; the NIH grants CA87986, CA105489, CA99163 and CA116552 to H.B.; and the NCI Core Support Grant to UNMC-Eppley Cancer Center.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/13879
References
- 1.Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7:791–799. doi: 10.1038/nrc2212. [DOI] [PubMed] [Google Scholar]
- 2.Connolly J, Kempson R, LiVolsi V, Page D, Patchefsky A, Silverberg S. Recommendations for the reporting of breast carcinoma. Association of Directors of Anatomic and Surgical Pathology. 2004 [Google Scholar]
- 3.Lagios MD, Margolin FR, Westdahl PR, Rose MR. Mammographically detected duct carcinoma in situ. Frequency of local recurrence following tylectomy and prognostic effect of nuclear grade on local recurrence. Cancer. 1989;63:618–624. doi: 10.1002/1097-0142(19890215)63:4<618::aid-cncr2820630403>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Poller DN, Silverstein MJ, Galea M, Locker AP, Elston CW, Blamey RW, et al. Ideas in pathology. Ductal carcinoma in situ of the breast: a proposal for a new simplified histological classification association between cellular proliferation and c-erbB-2 protein expression. Mod Pathol. 1994;7:257–262. [PubMed] [Google Scholar]
- 5.Holland R, Peterse JL, Millis RR, Eusebi V, Faverly D, van de Vijver MJ, et al. Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol. 1994;11:167–180. [PubMed] [Google Scholar]
- 6.Silverstein MJ, Poller DN, Waisman JR, Colburn WJ, Barth A, Gierson ED, et al. Prognostic classification of breast ductal carcinoma-in-situ. Lancet. 1995;345:1154–1157. doi: 10.1016/s0140-6736(95)90982-6. [DOI] [PubMed] [Google Scholar]
- 7.NCCN clinical practice guidelines in oncology. Breast Cancer. 2010:2. [Google Scholar]
- 8.Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93:1046–1052. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lester SC, Bose S, Chen YY, Connolly JL, de Baca ME, Fitzgibbons PL, et al. Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Arch Pathol Lab Med. 2009;133:1515–1538. doi: 10.5858/133.10.1515. [DOI] [PubMed] [Google Scholar]
- 10.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 11.Maughan KL, Lutterbie MA, Ham PS. Treatment of breast cancer. Am Fam Physician. 2010;81:1339–1346. [PubMed] [Google Scholar]
- 12.Rakha EA, Reis-Filho JS, Ellis IO. Combinatorial biomarker expression in breast cancer. Breast Cancer Res Treat. 2010;120:293–308. doi: 10.1007/s10549-010-0746-x. [DOI] [PubMed] [Google Scholar]
- 13.Payne SJ, Bowen RL, Jones JL, Wells CA. Predictive markers in breast cancer—the present. Histopathology. 2008;52:82–90. doi: 10.1111/j.1365-2559.2007.02897.x. [DOI] [PubMed] [Google Scholar]
- 14.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 15.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross JS, Hatzis C, Symmans WF, Pusztai L, Hortobagyi GN. Commercialized multigene predictors of clinical outcome for breast cancer. Oncologist. 2008;13:477–493. doi: 10.1634/theoncologist.2007-0248. [DOI] [PubMed] [Google Scholar]
- 21.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 24.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X, Malhotra GK, Lele SM, Lele MS, West WW, Eudy JD, et al. Telomerase-immortalized human mammary stem/progenitor cells with ability to self-renew and differentiate. Proc Natl Acad Sci USA. 2010;107:14146–14151. doi: 10.1073/pnas.1009030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 28.Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 29.Storms RW, Trujillo AP, Springer JB, Shah L, Colvin OM, Ludeman SM, et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci USA. 1999;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hess DA, Meyerrose TE, Wirthlin L, Craft TP, Herrbrich PE, Creer MH, et al. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 31.Corti S, Locatelli F, Papadimitriou D, Donadoni C, Salani S, Del Bo R, et al. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24:975–985. doi: 10.1634/stemcells.2005-0217. [DOI] [PubMed] [Google Scholar]
- 32.Chute JP, Muramoto GG, Whitesides J, Colvin M, Safi R, Chao NJ, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci USA. 2006;103:11707–11712. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Park SY, Lee HE, Li H, Shipitsin M, Gelman R, Polyak K. Heterogeneity for stem cell-related markers according to tumor subtype and histologic stage in breast cancer. Clin Cancer Res. 2010;16:876–887. doi: 10.1158/1078-0432.CCR-09-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Resetkova E, Reis-Filho JS, Jain RK, Mehta R, Thorat MA, Nakshatri H, et al. Prognostic impact of ALDH1 in breast cancer: a story of stem cells and tumor microenvironment. Breast Cancer Res Treat. 2010;123:97–108. doi: 10.1007/s10549-009-0619-3. [DOI] [PubMed] [Google Scholar]
- 37.Neumeister V, Agarwal S, Bordeaux J, Camp RL, Rimm DL. In situ identification of putative cancer stem cells by multiplexing ALDH1, CD44 and cytokeratin identifies breast cancer patients with poor prognosis. Am J Pathol. 2010;176:2131–2138. doi: 10.2353/ajpath.2010.090712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 39.Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- 40.Friedrichs K, Ruiz P, Franke F, Gille I, Terpe HJ, Imhof BA. High expression level of alpha 6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res. 1995;55:901–906. [PubMed] [Google Scholar]