Abstract
Background
Single-dose infusion of the agonistic anti-CD40 monoclonal antibody (mAb) CP-870,893 accomplishes immune activation and clinical responses in patients with advanced cancers, but repeat dosing of this agent has not been reported.
Results
Twenty-seven patients were enrolled. The most common adverse event was transient, infusion-related cytokine release syndrome (CRS). Dose-limiting toxicities included grade 3 CRS and grade 3 urticaria; the maximum tolerated dose (MTD) was estimated to be 0.2 mg/kg. Seven patients (26%) had stable disease as the best clinical response; no partial or complete responses were observed. At the MTD, patient B lymphocytes exhibited persistently increased expression of costimulatory and adhesion molecules without resetting to baseline between doses. In four of eight patients (50%) evaluated at the MTD, there were marked declines in total CD3+ T lymphocytes, as well as CD4+ and CD8+ subsets.
Patients and Methods
Patients with advanced solid tumor malignancies received weekly intravenous infusions of CP-870,893 in four dose level cohorts. Safety and immune pharmacodynamics were assessed.
Conclusions
Weekly infusions of the agonist CD40 antibody CP-870,893 were well-tolerated, but there was little clinical activity in advanced cancer patients. Correlative studies demonstrate chronic B-cell activation and in some patients, T-cell depletion. Longer dosing intervals may be desirable for optimal immune pharmacodynamics.
Key words: CD40, immunotherapy, antibody, T cell, B cell
Introduction
The cell-surface molecule CD40 is a member of the tumor necrosis factor (TNF) receptor superfamily and regulates immune activation by virtue of its expression on antigen-presenting cells (APC) including B cells, monocytes and dendritic cells.1 Considerable in vivo and in vitro data demonstrate that signaling via CD40 activates APCs by binding CD154, the natural ligand for CD40 on activated T cells.1 Data from mouse models suggest that agonistic CD40 antibodies substitute for the function of CD4+ T cells in models of T-cell-mediated immunity2–4 and trigger effective immune responses against tumor antigens.5–8 Additionally, CD40 is often expressed by solid tumor cells and upon ligation, mediates tumor cell apoptosis and growth impairment.1 Consequently, CD40 agonists are being explored as potential novel therapy for cancer.
CP-870,893 is a fully human agonistic CD40 monoclonal antibody (mAb) that has been shown to activate human APC in vitro, including dendritic cells and B cells.9,10 In addition, CP-870,893-activated APC induce T-cell proliferation and secretion of effector cytokines including IFNγ and IL-2.10 CP-870,893 has been shown to inhibit growth of human tumors in both immune-deficient and immune-reconstituted SCID-beige mice.11,12 In the first-in-human clinical study of CP-870,893,13 a single infusion was given to patients with advanced cancer at doses ranging from 0.01 mg/kg to 0.3 mg/kg with the most common adverse event being transient grade 1 to 2 cytokine release syndrome (CRS). Four patients with advanced melanoma experienced a partial response to a single infusion. All patients eventually relapsed except for one patient whose remission was maintained while receiving repeat doses of CP-870,893 every 6–8 weeks.13 Pharmacodynamic studies demonstrated a marked, rapid and dose-dependent decrease in the percentage of CD19+ B cells among peripheral blood lymphocytes and concomitant upregulation of CD86 on the remaining B cells. This effect was evident within 1 hour of infusion and peaked between 2–3 days after infusion.13
Given the promising clinical results of this single dose phase I study, we performed a second phase I study investigating the effects of administering CP-870,893 on a weekly basis. The rationale for this schedule was based on the observation that the majority of both the pharmacodynamic effect of CP-870,893 and changes in laboratory parameters (such as liver function tests) peaked then resolved within one week of a single infusion. It was appreciated from previously published mouse tumor models of anti-CD40 mAb therapy that certain schedules of mAb administration, particularly daily dosing, could result in deleterious effects on T cells secondary to hyperstimulation.14,15 The goal of this study was to determine the safety and maximum tolerated dose (MTD) of weekly CP-870,893 infusion, and to compare safety data with clinical response and immune pharmacodynamics.
Results
Patient characteristics, toxicity and determination of MTD.
Twenty-seven patients with advanced solid tumors were treated in this study (Table 1). Patients had a wide range of 13 distinct tumor histologies, but 11 patients (41%) had melanoma. Four weekly dose levels were explored, with the majority of patients being treated with 0.2 mg/kg (n = 13) or 0.25 mg/kg (n = 6) of CP-870,893. Infusion of the drug was well-tolerated, and adverse events are summarized in Table 2. Two dose limiting toxicities (DLT) were observed in the initial cohorts, both occurring in the 0.25 mg/kg dose level (grade 3 CRS in one patient and grade 3 urticaria in another). No DLTs were observed among the first six patients treated with 0.2 mg/kg of CP-870,893 and the MTD of weekly infusion of CP-870,893 was estimated as 0.2 mg/kg in accordance with the clinical protocol. This is the same MTD as was estimated for single-dose CP-870,893 administration in the first-in-human study.13 In an MTD expansion cohort of seven additional patients treated with 0.2 mg/kg weekly CP-870,893, two additional events of grade 3 infusion-related CRS were observed, with the overall rate of grade 3 CRS at this dose level being 15%.
Table 1.
Patient characteristics
| Characteristic | Number | % | |
| Sex | |||
| Male | 18 | 67 | |
| Female | 9 | 33 | |
| Age, years | |||
| Mean | 61.6 | ||
| Range | (45–80) | ||
| Race/ethnicity | |||
| White | 24 | 89 | |
| Asian | 2 | 7 | |
| Black | 1 | 4 | |
| ECOG PS | |||
| 0 | 19 | 70 | |
| 1 | 8 | 30 | |
| Tumor types | |||
| Anal cancer | 1 | 4 | |
| Bladder cancer | 1 | 4 | |
| Breast cancer | 3 | 11 | |
| Colon cancer | 1 | 4 | |
| Hepatocellular carcinoma | 1 | 4 | |
| Melanoma | 11 | 41 | |
| Mesothelioma | 2 | 7 | |
| Nasopharyngeal cancer | 1 | 4 | |
| Neuroendocrine tumor | 1 | 4 | |
| Non-small cell lung cancer | 1 | 4 | |
| Ovarian cancer | 1 | 4 | |
| Renal cell carcinoma | 2 | 7 | |
| Uterine cancer | 1 | 4 | |
| Prior treatment | |||
| Chemotherapy | 26 | 96 | |
| Radiation | 13 | 48 | |
| Surgery | 27 | 100 | |
| Immunotherapy* | 4 | 15 | |
| Treatment Group | |||
| 0.05 mg/kg | 5 | 19 | |
| 0.1 mg/kg | 3 | 11 | |
| 0.2 mg/kg | 13 | 48 | |
| 0.25 mg/kg | 6 | 22 | |
includes interferon-alpha, IL-2, peptide vaccines, interferon-beta adenovirus gene therapy.
Table 2.
Treatment emergent adverse events (all causalities)
| Adverse event | Any grade, n (%) | Grade 3, n (%) | Grade 4, n (%) |
| CP-870,893 0.05 mg/kg (n = 5) | |||
| Abdominal pain | 4 (80) | 1 (20) | 0 |
| Back pain | 2 (40) | 0 | 0 |
| Chills | 4 (80) | 0 | 0 |
| Constipation | 1 (20) | 1 (20) | 0 |
| Dehydration | 1 (20) | 1 (20) | 0 |
| CP-870,893 0.1 mg/kg (n = 3) | |||
| Arthralgias | 2 (67) | 0 | 0 |
| Autoimmune disorder | 1 (33) | 1 (33) | 0 |
| Dyspnea | 2 (67) | 0 | 0 |
| Eye lid ptosis | 1 (33) | 1 (33) | 0 |
| Musculoskeletal pain | 2 (67) | 0 | 0 |
| Nausea | 2 (67) | 0 | 0 |
| Non-cardiac chest pain | 1 (33) | 1 (33) | 0 |
| CP-870,893 0.2 mg/kg (n = 13) | |||
| Abdominal distention | 2 (15) | 0 | 0 |
| Abdominal pain | 2 (15) | 1 (8) | 0 |
| Arthralgias | 3 (23) | 0 | 0 |
| AST increase | 2 (15) | 1 (8) | 0 |
| Bronchitis | 2 (15) | 0 | 0 |
| Chills | 3 (23) | 0 | 0 |
| Confusion | 1 (8) | 1 (8) | 0 |
| Constipation | 3 (23) | 0 | 0 |
| Cough | 4 (31) | 0 | 0 |
| Cytokine release syndrome | 11 (85) | 2 (16)* | 0 |
| Dehydration | 2 (15) | 0 | 0 |
| Diarrhea | 3 (23) | 0 | 0 |
| Dizziness | 3 (23) | 0 | 0 |
| Dyspnea | 2 (15) | 1 (8) | 0 |
| Fatigue | 7 (54) | 1 (8) | 0 |
| Flushing | 2 (15) | 0 | 0 |
| Headache | 5 (39) | 0 | 0 |
| Hemoptysis | 2 (15) | 0 | 0 |
| Hypophosphatemia | 1 (8) | 1 (8) | 0 |
| Lethargy | 1 (8) | 1 (8) | 0 |
| Lymphedema | 1 (8) | 1 (8) | 0 |
| Musculoskeletal chest pain | 2 (15) | 1 (8) | 0 |
| Nausea | 5 (38) | 0 | 0 |
| Pleuritic pain | 2 (15) | 0 | 0 |
| Pneumonia | 1 (8) | 1 (8) | 0 |
| Pulmonary embolism | 1 (8) | 0 | 1 (8) |
| Vomiting | 3 (23) | 0 | 0 |
| CP-870,893 0.25 mg/kg (n = 6) | |||
| Alk Phos increase | 3 (50) | 1 (17) | 0 |
| ALT increase | 3 (50) | 0 | 0 |
| AST increase | 4 (67) | 0 | 0 |
| Anemia | 2 (33) | 0 | 0 |
| Arthralgias | 2 (33) | 0 | 0 |
| Back pain | 3 (50) | 1 (17) | 0 |
| Constipation | 3 (50) | 0 | 0 |
| Cytokine release syndrome | 4 (67) | 1 (17)* | 0 |
| Dizziness | 2 (33) | 0 | 0 |
| Dyspnea | 2 (33) | 0 | 0 |
| Fatigue | 2 (33) | 0 | 0 |
| Headache | 2 (33) | 0 | 0 |
| LDH increase | 1 (17) | 1 (17) | 0 |
| Muscle weakness | 2 (33) | 1 (17) | 0 |
| Pyrexia | 2 (33) | 0 | 0 |
| Upper respiratory tract infection | 2 (33) | 0 | 0 |
| Urticaria | 1 (17) | 1 (17)* | 0 |
Events listed if present in ≥ 2 for toxicities grade 1 and 2, or in ≥ 1 if grades 3 or 4 toxicity, *dose-limiting toxicity (DLT).
CRS of any grade was the most common adverse event observed in this study diagnosed in 15 patients, including 11 out of 13 patients treated with 0.2 mg/kg of CP-870,893 and four out of six patients treated with 0.25 mg/kg. CRS typically manifest with transient rigors, chills, fever and other symptoms within one hour after the end of the infusion, responsive to meperidine and resolving within 2–12 hrs after infusion. In those patients developing CRS, it typically but not always occurred with the first infusion and typically but not always featured the same or less intensity with each subsequent dose. CRS was judged to be grade 3 when symptoms were prolonged or not rapidly responsive to medical management or recurred following initial improvement. No patients were hospitalized for CRS. No patients in the two lower dose cohorts had CRS.
Treatment delay and dose alterations due to toxicities.
A total of 224 doses of CP-870,893 were given in this study (Table 3). Of these, only six doses (2.7%) had to be delayed by a week or more due to toxicities. Two of these dose delays were related to DLTs (grade 3 CRS and grade 3 urticaria). Other toxicities leading to dose delays included recurrent grade 2 gastroparesis associated with grade 2 elevation of alkaline phosphatase during cycle 2 of therapy in one patient and grade 2 elevation of D-dimer during week 6 of cycle 1, neither considered to be treatment related.
Table 3.
Dose, diagnoses, time to progression and number of doses for patients with stable and progressive disease as best clinical response
| Response | Patient | Dose (mg/kg) | Diagnosis | TTP (weeks) | # of doses |
| SD | |||||
| 4001 | 0.05 | Uterine cancer | 20 | 21 | |
| 3004 | 0.1 | Melanoma | 19 | 16 | |
| 4004 | 0.1 | Melanoma | 15 | 16 | |
| 3005 | 0.2 | Mesothelioma | 18 | 16 | |
| 3009 | 0.2 | Mesothelioma | 17 | 15 | |
| 3006 | 0.25 | RCC | 18 | 13 | |
| 3008 | 0.25 | RCC | 16 | 13 | |
| Median | 18 | 16 | |||
| PD | |||||
| 3001 | 0.05 | Melanoma | 4 | ||
| 3002 | 0.05 | Melanoma | 7 | 8 | |
| 3003 | 0.05 | Breast cancer | 2 | 3 | |
| 4002 | 0.05 | HCC | 6 | 7 | |
| 4003 | 0.1 | Melanoma | 4 | 5 | |
| 3010 | 0.2 | Bladder cancer | 7 | 8 | |
| 3011 | 0.2 | Melanoma | 5 | 4 | |
| 3012 | 0.2 | Nasopharyngeal cancer | 4 | 5 | |
| 3013 | 0.2 | Breast cancer | 5 | ||
| 3015 | 0.2 | Neuroendocrine tumor | 7 | 8 | |
| 4005 | 0.2 | Melanoma | 2 | 3 | |
| 4006 | 0.2 | Breast cancer | 3 | 4 | |
| 4009 | 0.2 | Sarcoma | 3 | 4 | |
| 4010 | 0.2 | Melanoma | 7 | 6 | |
| 5001 | 0.2 | Ovarian cancer | 7 | 8 | |
| 5004 | 0.2 | Melanoma | 3 | 4 | |
| 3007 | 0.25 | Melanoma | 4 | 5 | |
| 4008 | 0.25 | Anal cancer | 7 | 8 | |
| 5002 | 0.25 | Colon cancer | 7 | 7 | |
| 5003 | 0.25 | Melanoma | 7 | 8 | |
| Median | 4.5 | 5 |
RCC, renal cell carcinoma; HCC, hepatocellular carcinoma.
Hematologic toxicity.
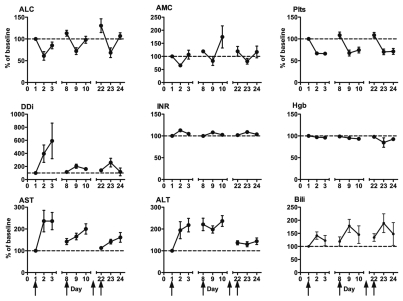
Infusion of CP-870,893 was associated with transient decreases in lymphocytes, monocytes and platelets (shown for patients treated at the MTD or higher in Fig. 1). Nadir counts were observed 24 hrs after infusion. Lymphocyte and monocyte counts recovered within 48 hrs and returned to baseline at the time of the following infusion on day 8. The decrease in platelet count after infusion was more sustained than the decrease in lymphocyte counts. For most patients, platelet counts remained at around 60% of the baseline value for 2 days following the infusion, but returned to baseline on the day of the next weekly infusion of CP-870,893 and no thrombocytopenia of any grade was observed. D-dimer levels increased significantly after infusion of CP-870,893, but again, these increases were transient, clinically insignificant and returned to baseline values within one week. PT/INR and hemoglobin levels did not change significantly with treatment, and no case of disseminated intravascular coagulation was diagnosed on study.
Figure 1.
Change relative to baseline in hematologic and serum chemistry parameters after first, second and fourth infusion of CP-870,893 in patients at MTD or higher. ALC, absolute lymphocyte count; AMC, absolute monocyte count; Plts, platelets; Ddi, D-dimer; INR, international ratio; Hgb, hemoglobin; AST, asparate aminotransferase; ALT, alanine aminotransferase; Bili, total bilirubin. Arrows indicate treatment days (day 1, 8, 15, 22). Values expressed as mean ± SEM, n = 8–18.
Liver toxicity.
Transient increases in asparate aminotransferase (AST), alanine aminotransferase (ALT) and total bilirubin were observed after CP-870,893 infusion (again shown for patients at the MTD or higher in Fig. 1). Increased AST and ALT was most prominent with the first dose of CP-870,893 with the effect extinguished with subsequent dosing. In contrast, mild transient elevations in total bilirubin were observed with each dose. The observed increases in AST and ALT were registered as grade 1 or higher in 6 and 3 occurrences, respectively (Table 2). One patient (0.2 mg/kg cohort) had a grade 3 increase in AST which was not dose limiting as it was not considered to be treatment-related. In all but one patient (in the 0.25 mg/kg cohort), liver enzyme elevations returned to baseline at the time of the next infusion.
Other toxicities.
Several other grade 3 toxicities were observed on study (Table 2) but were not considered DLTs because they were not deemed treatment-related (nearly always related to tumor progression). In the one case of grade 4 toxicity (pulmonary embolism), the event occurred on day 44 of study in a patient with highly advanced melanoma who had originally been treated at 0.2 mg/kg cohort but dose reduced for grade 3 CRS after the second infusion.
In one case of grade 3 autoimmune disorder, a melanoma patient in the 0.1 mg/kg cohort was diagnosed with autoimmune diabetes on day 153 of the study after 16 doses of CP-870,893. Prior to enrollment on the study, this patient, a thin Caucasian female, had not been diagnosed with diabetes and she had no family history of type 1 or type 2 diabetes. On screening laboratory analysis prior to treatment, glucose was 158 mg/dl and the islet cell antibody (ICA) titer was elevated at 1:32. While on study, she had fluctuating glucose levels between 105 and 207 mg/dl with ICA titers varying between 1:4 to 1:256. When her glucose level was >200 mg/dl on two successive treatment days, she was formally diagnosed with diabetes mellitus and taken off of the study with the suspicion for the development of autoimmune diabetes. At that time, fasting and stimulated c-peptide concentrations were <0.5 and 1.0 ng/mL, respectively. Epitope specific antibodies for insulin and GAD65 were undetectable. Initial treatment with a sulfonylurea was sub-optimal and the patient was subsequently treated with glargine insulin and repaglinide and remained on treatment for type 1 diabetes mellitus for a year until her death from progressive melanoma.
Antitumor activity.
Seven patients (26%), each with progressive disease prior to enrollment, had stable disease (SD) as the best clinical response (Table 3). The median time to progression in these patients with SD was 18 weeks (range 16–20 weeks) and the median number of CP-870,893 infusions was 16 (range 13–21 infusions). Of the patients with SD, two patients had melanoma, two had renal cell carcinoma, two had mesothelioma and one had a uterine cancer. Stable disease was observed at each dose level. Twenty patients with progressive disease as best response had a median TTP of 4.5 weeks (range 2–7 weeks), receiving a median of five infusions of CP-87,893 (range 3–8 infusions). There were no partial or complete responses. We did not observe any correlation between the observation of stable disease and clinical parameters such as autoimmunity or severity of CRS; however, the small number of patients limits firm conclusions on this issue.
Pharmcokinetics and human anti-human antibody.
At the 0.2 mg/kg dose level, the serum half life of CP-870,893 was graphically estimated to be less than 6 hrs on both the first and fourth doses (with AUC of 7.45 ± 3.93 and 5.12 ± 3.35 hrmg/mL, respectively; and Cmax of 1.49 ± 0.63 and 0.87 ± 0.31 ug/ml, respectively), similar to the kinetics observed in the first-in-human study. No patient developed human anti-human antibody after treatment with CP-870,893.
Pharmacodynamics.
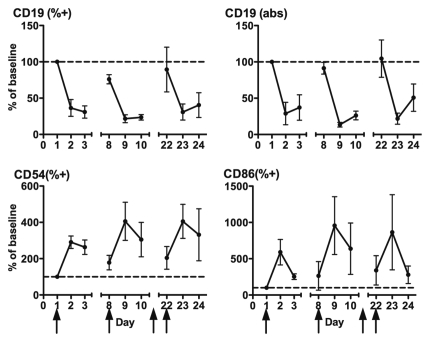
The first-in-human study with CP-870,893 identified depletion and activation of peripheral blood B cells as a major pharmacodynamic effect.13 Here, we used flow cytometry to measure B cell parameters around the first, second and fourth infusion of CP-870,893 in patients treated in the MTD expansion cohort (Fig. 2). We observed a marked decrease in the fraction of CD19+ B cells among lymphocytes as well as the absolute numbers of CD19+ B cells (Fig. 2). However, this effect was relatively short-lived and CD19+ cell counts returned or nearly returned to day 1 baseline by the time of the next infusion (i.e., day 8 or day 22). We then evaluated the expression of CD86 and CD54 on patients' B cells around the first, second and fourth infusion of CP-870,893, in light of observations that in vitro, CP-870,893 upregulates these markers on human B cells.10 With each infusion, both the percentage of CD86+ and CD54+ B cells among total B cells markedly increased, up to a four-fold increase for CD54 and 10-fold increase for CD86. Importantly, on average, the percentage of CD54+ or CD86+ B cells did not reset to baseline by the time of subsequent infusions. For example, at the time of the fourth infusion of CP-870,893 (day 22), the percentage of B cells expressing CD86 was 3 fold higher than on day 1, despite that the absolute number of CD19+ B cells was the same between day 1 and day 22.
Figure 2.
Pharmacodynamics of CP-870,893 after first, second and fourth infusion of CP-870,893. Parameters are shown relative to baseline. CD19 (%+) = percentage of CD19+ cells among lymphocytes, CD19 (abs) = absolute number of CD19+ cells, CD54 (%+) = CD54+ cells among CD19+ cells, CD86 (%+) CD86+ cells among CD19+ cells. Arrows indicate treatment days (day 1, 8, 15, 22). Values expressed as mean ± SEM, n = 8–18.
Peripheral blood mononuclear cells (PBMC) from five patients treated at the MTD or higher were available for flow cytometric analysis of myeloid dendritic cells (mDC) and plasmacytoid dendritic cells (pDC) on days 1, 2 and 8. For mDC (defined phenotypically as CD11c+ CD123-dim CD14-negative cells), there was depletion of cells from the blood of most patients, and there was modest (25–40%) upregulation of HLA-DR MFI in some patients but no change in CD86 expression (data not shown). For pDC (CD123+ CD11c-dim CD14-negative), changes in the circulating percentage and absolute count were highly variably in response to treatment and only modest increases in HLA-DR was observed in two patients after therapy (data not shown).
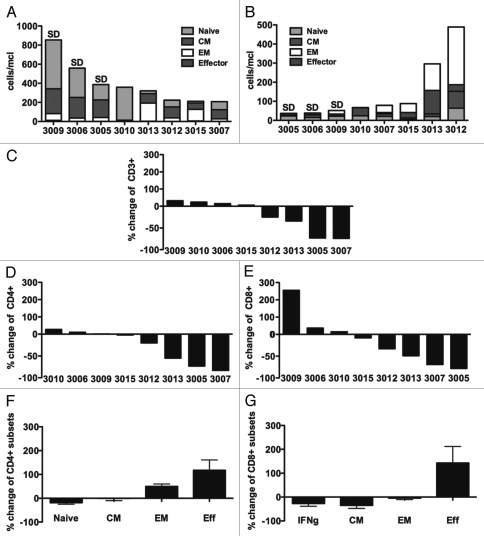
Modulation in T-cell subsets.
To assess the impact of weekly CP-870,893 infusion on T-cell physiology, subsets of peripheral blood T cells were measured at baseline and at the end of study (Fig. 3) in eight patients treated at the MTD. At baseline, there was great patient-to-patient variability in the absolute numbers of peripheral blood CD4 T cells, CD8 T cells (Fig. 3) and FoxP3+ CD4+ regulatory T cells (Fig. 4). Interestingly, the three patients who had SD in response to weekly CP-870,893 were the three patients with both the highest absolute CD4 count (>375/mcl, median 559/mcl) and the lowest absolute CD8 count (<60/mcl, median 38/mcl); however, in the single dose study, there was no suggestion that patients with tumor response after therapy had at baseline the highest CD4 counts or the lowest CD8 counts.
Figure 3.
Modulation in T-cell subsets following treatment with CP-870,893. (A and B) Baseline parameters of eight patients treated at the MTD or higher study expressed in absolute numbers for each patient (unique patient number noted on x-axis) for (A) CD4+ and (B) CD8+ T cell subsets. Naïve = CD45RA+CCR7+CD27+CD28+, CM = Central Memory = CD45RA-CCR7+CD27+CD28+, EM = Effector Memory = CD45RA−CCR7−CD27+/−CD28+/−, Effector = CD45RA+CCR7−CD27−CD28−. SD denotes patients who had stable disease as best clinical response. Change in (C) absolute CD3 counts, (D) absolute CD4 counts and (E) absolute CD8 counts, comparing end of study to baseline. Change relative to baseline in the percentages of four T-cell subsets, defined above, for (F) CD4 and (G) CD8 subsets in response to treatment, shown as mean ± SEM.
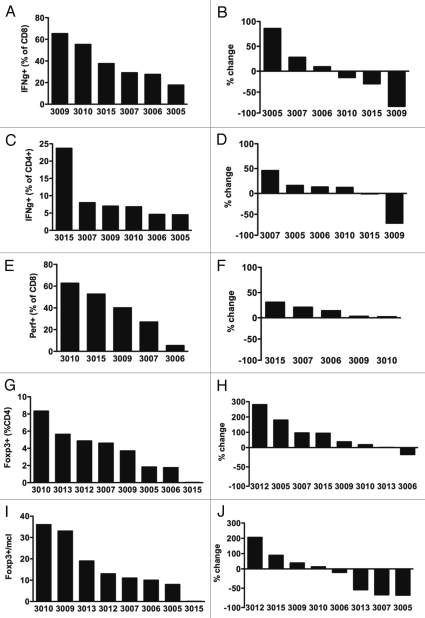
Figure 4.
Baseline parameters and treatment-related changes of IFNγ and perforin expression and regulatory T cells. Fraction of IFNγ among CD8+ (A) and CD4+ (C), fraction of perforin among CD8+ (E), fraction of FoxP3+ among CD4+ (G) and absolute Foxp3+ CD4+ cells per ul blood (I) at baseline. Relative changes comparing end of study to baseline in IFNγ among CD8+ (B) and CD4+ (D), fraction of perforin among CD8+ (F), fraction of FoxP3+ among CD4+ (H) and absolute count of Foxp3+ CD4+ cells (J).
Post-treatment samples were obtained after a median of eight CP-870,893 infusions (range 4–16) and median of 1.5 weeks following the last infusion (range, 1–3 weeks). For four of eight patients (50%) studied in this analysis, there were marked reductions of total CD3+ T cells, involving depletion in both the CD4+ and CD8+ T-cell compartments (median CD4 decrease, 55%, range −2 to −83%; median CD8 decrease, −49%, range −8 to −78%). In three of four patients, CD4 counts dropped below <200 cells/µl. For the four other patients, there was minimal change in absolute CD3, CD4 or CD8 counts, except in one patient for which a 2.5-fold increase in CD8 T cells was observed. Within the CD4 and CD8 compartments, many patients exhibited relative shifts in the percentages of naive, effector, effector memory (EM) and central memory (CM) T cells before and after treatment to a more differentiated phenotype (effector) (Fig. 3).
To analyze the functional capacity, we assessed the capacity of patient T cells to produce IFNγ in response to stimulation with PMA and ionomycin. Additionally, we assessed perforin expression of CD8+ T cells as a marker of potential cytolytic T-cell function. At baseline, IFNγ production and perforin expression varied widely (Fig. 4). Change in these parameters after CP-870,893 treatment was inconsistent; for example, changes from baseline in IFNγ production ranged from an increase of 86% to a decrease of 84%. Only mild increases (2–31%) in perforin-expressing cells were observed in a fraction of patients.
With regard to regulatory FoxP3+ T cells, the percentage of FoxP3+ CD4+ T cells among CD4+ T cells increased in several but not all patients, but these increases did not parallel changes in the absolute count of FoxP3+ CD4+ T cells. Four patients exhibited decreases in the absolute count of FoxP3+ CD4+ T cells but three of these patients had marked decreases in total CD4+ T cells to explain this effect (Fig. 4).
Discussion
The goal of this clinical investigation was to evaluate the safety and the biologic activity of treating cancer patients with multiple, intravenous doses of CP-870,893, a fully human agonist mAb specific for the cell-surface molecule CD40. We show here that weekly dosing schedule of CP-870,893 is feasible, associated with cytokine release syndrome which defines the MTD, but was not associated with objective clinical responses. Correlative immune assessment studies suggest that administration of CP-870,893 on a weekly schedule led to persistently increased expression of costimulatory and adhesion molecules of B cells without resetting to baseline. Additionally, in up to 50% of patients, there were potentially deleterious consequences on T-cell populations as evidenced by marked declines in the absolute count of total CD3+ T cells and both the CD4 and CD8 T-cell subsets. In the previous trial of single-dose CP-870,893 infusion conducted in a similar group of patients, chronic B-cell stimulation and T cell depletion were not observed. These findings have important implications for future trials exploring CP-870,893 as an immunomodulatory agent in cancer patients and suggest that an interval of longer than one week may be desirable for optimal immune pharmacodynamics.
There was a high degree of similarity in the toxicology and pharmacology of weekly dose CP-870,893 evaluated in this study and that of single-infusion dosing previously reported.13 As in the single-dose trial, the MTD for weekly infusion of CP-870,893 was estimated at 0.2 mg/kg, suggesting that the administration of multiple doses at weekly intervals did not significantly increase toxicity of this drug. Good tolerability of the weekly dosing schedule was further validated by the small fraction of scheduled infusions (2.7%) that were delayed due to toxicities. CRS was again observed to be the most common adverse event. Grade 3 CRS constituted two out of three observed DLTs. Transient and mild-to-moderate elevations of liver function tests (AST, ALT, bilirubin) and D-dimer with each CP-870,893 infusion were observed as were transient declines in lymphocytes, monocytes and platelets. The changes in laboratory abnormalities most likely reflect CP-870,893 interaction with CD40-expressing lymphocytes, monocytes, platelets and endothelial cells. As with the clinical adverse events, these changes in laboratory test results were very similar in nature and grade to those observed following a single infusion of CP-870,893.13 Only one immune-mediated toxicity was observed and diagnosed as autoimmune diabetes, which was most likely an exacerbation of an underlying condition as the patient had measurable anti-islet cell antibodies at baseline that increased during 16 weeks of therapy, associated with a new insulin requirement. Importantly, immune mediated events such as those classically observed for blocking anti-CTLA-4 mAb,16 such as colitis, dermatitis and hypophysitis, were not observed in this or previous studies of CP-870,893. Serum half-life of CP-870,893 and other pharmacokinetic parameters were also very similar between weekly dose and single dose. No human anti-human antibodies have been identified for weekly dosing or single dosing of CP-870,893.
The best clinical response in this trial was stable disease (SD) in 26% of patients. There were no objective clinical responses (PR or CR), unlike the single-dose study in which 4 of 27 patients (26%) had a PR (all responders had melanoma).13 Although it is impossible to compare objective response rates between these two small studies of single-dose vs. weekly CP-870,893, it was disappointing not to be able to confirm some level of objective antitumor activity in this study, especially considering that the eligibility criteria and two investigational sites were otherwise the same and that melanoma patients were also represented in the weekly dose clinical trial.
A prominent pharmacodynamic effect of CP-870,893 treatment in vivo is depletion and activation of peripheral blood B cells,13 which we have also recently modeled in vitro.10 Here, we found that B cells rapidly but transiently decreased in the circulation with each weekly dose, similar to our observations in patients treated with a single infusion, but returned to baseline both with respect to percent of lymphocytes and absolute counts on the day of subsequent infusions. As expected, B cells isolated one or two days after infusion in this study exhibited increased expression of CD86 and CD54, consistent with activation, and importantly this effect was observed with each dose. However, expression of CD54 or CD86 B cells did not reset to baseline by the time of subsequent infusions. In the single dose trial, B cell activation parameters always eventually reset to baseline,13 and presumably would have also reset in this study except that CP-870,893 was re-administered once per week. At the time of the fourth infusion of CP-870,893, for example, the percentage of B cells expressing CD86 was 3-fold higher compared to baseline, despite the fact that the absolute number of B cells was the same. The pharmacodynamic effect of B cell activation may or may not represent a mechanism of action of CP-870,893. In vitro, B cells that are activated via CD40 in general,17 and via CP-870,893 in particular,10 act as potent antigen presenting cells and in certain model systems, drive the induction of the antitumor T cells.18–20 Whether or not such a mechanism occurs in vivo in response to CP-870,893 remains to be determined. More classically, the immunological power of CD40 activation has been linked to activation of dendritic cells,21 with potent T-cell responses being observed even in the absence of B cells;4 however, recent data underscore the role of B cells and their potential function as APC in regulating T cell function.22 Regardless of the role of B cells in the mechanism of action of CP-870,893, our findings that peripheral B cells appear persistently activated with weekly CP-870,893 infusion raise the possibility of chronic, even global, immune stimulation induced with the antibody used on this schedule. Such chronic B-cell activation was not observed with a single dose of CP-870,893.13
Our observations that weekly CP-870,893 may have induced chronic immune stimulation are concerning in light of published results from mouse models suggesting that overstimulation with CD40 agonists may be deleterious to adaptive immune responses.14,15,23 Similar to many of the patients treated with weekly CP-870,893 reported here, mice given frequent or high doses of anti-CD40 mAb exhibit T-cell depletion. For example, in an implantable model of melanoma, weekly injection of an agonistic anti-CD40 antibody into tumor-bearing mice accelerated the deletion of tumor-antigen specific T cells, even though an initial expansion of antigen-specific T cells was seen after the first dose of antibody.14 In another study, mice injected with repeated daily doses of an agonist anti-CD40 mAb (with IL-2 or IL-15) exhibited marked CD4+ T-cell apoptosis.15 Tumor-bearing mice treated with anti-CD40 mAb and IL-2 were initially protected but were unable to mount effective memory responses and succumbed to secondary tumor challenge.15 Similarly, anti-CD40 mAb resulted in severe depression of the CD8+ T-cell response to challenge with lymphocytic choriomeningitis virus.23 Indeed, high daily dosing of anti-CD40 mAb has been used to control the autoimmune inflammatory process associated with chronic collagen-induced arthritis,24 emphasizing the potential immunosuppressive quality of certain dose and schedule regimens of anti-CD40 mAb. Thus, although many important issues distinguish these mouse models from our clinical trial, our findings raise the hypothesis that too frequent dosing of CP-870,893 may result in counterproductive immune modulation manifesting as persistent B-cell stimulation and in some patients, peripheral blood T-cell depletion. In patients receiving a single dose of CP-870,893 at similar doses, marked T-cell depletion in the peripheral blood was not observed at the end of study, and no patients in this previous study at the MTD or higher had CD4+ T-cell counts <200 cells/µl at the end of study. Still, we cannot exclude the possibility that T-cell depletion observed at the end of study in some patients was associated with tumor progression. We speculate that the lack of objective tumor responses observed with weekly infusion of CP-870,893—in contrast to the responses seen in the single dose study—may be related to counterproductive immune hyperstimulation with short-interval dosing. On the other hand, it is important to keep in mind that CD40 agonists may also mediate antitumor effects directly (non-immunologically). Certain tumors express high levels of CD40 and direct activation of CD40 results in growth inhibition and sensitization to cytotoxic agents. CP-870,893, for example, has been reported to have antitumor effects in murine systems for which lymphocytes are absent.12 Our observations here of stable disease in patients with renal cell carcinoma and mesothelioma may be important in this regard, but we did not have the opportunity to measure CD40 expression of tumors from patients in this study.
The observed half life of CP-870,893 of less than 6 hrs is unusually short for a fully human IgG2 molecule. This finding was similar to the CP-870,893 half life observed in the single dose study and was one of the reasons that a relatively short dosing interval of one week was chosen for a repeat dose study. The reasons for this short half life remain unknown, but it has been speculated that it reflects a large sink of CD40 (receptor) molecules in vivo. When given to mice, the half life of CP-870,893 is about 3 weeks. For another agonist CD40 mAb SGN-40 (which is IgG1 not IgG2), initial pharmacokinetic data in humans demonstrated that the half-life was also unusually short and dependent on the dose given, ranging from 0.9–2.9 days after the first dose.25 The doses of SGN-40 used were higher than we have explored for CP-870,893. It was suggested that for SGN-40 there may be a rapid elimination pathway or redistribution volume that had not been saturated,25 but in a subsequent publication regarding SGN-40, pharmacokinetic parameters could not be estimated because of limited sample collections.26
Current trials of CP-870,893 are utilizing dosing intervals of 3 or 4 weeks to address the potential concern of CD40-mediated overstimulation. Other approaches to improve efficacy include strategies for combination therapy.1 Active approaches are combining CP-870,893 with chemotherapy (e.g., with carboplatin and paclitaxel for patients with advanced solid tumors or with gemcitabine for chemotherapy naive patients with pancreatic cancer) or with a cancer vaccine (e.g., poly IC:LC and NY-ESO-1/gp100/MART-1 peptides emulsified with Montanide ISA 51 for patients with melanoma). Recently a trial has opened combining CP-870,893 every three weeks with the anti-CTLA-4 blocking mAb tremelimumab every 12 weeks for patients with metastatic melanoma. Careful immunological assessments are important endpoints of these clinical trials.
In summary, this phase I study of weekly infusions of the agonistic, fully human CD40 mAb CP-870,893 showed that a weekly dosing schedule was well tolerated and feasible, but there was little antitumor clinical activity of this infusion schedule. Although the reasons for this remain speculative, correlative studies presented here suggest that less frequent administration of CP-870,893 may be advantageous to avoid undesirable immune modulation. It will be important to consider these findings in the design of future trials of this and other agonistic CD40 antibodies.
Patients and Methods
Patients.
Twenty-seven patients with advanced solid malignancies were enrolled between September 2005 and June 2007 at the Abramson Cancer Center, the Moffitt Cancer Center and Research Institute and the Sarah Cannon Research Institute. The protocol and consent form were approved by the local institution review boards. Patients had to be at least 18 years old with an eastern Cooperative Oncology Group performance status of 0 to 1 and adequate end organ function. Signed informed consent was required. Patients with autoimmune disorders, coagulopathies or major illness and those who were pregnant or lactating were excluded. Concurrent treatment with anticancer drugs was not allowed.
Study design.
Weekly intravenous infusions of CP-870,893 were administered to eligible patients in cohorts receiving 0.05, 0.1, 0.2 or 0.25 mg/kg per infusion. CP-870,893 was supplied by Pfizer Inc. (New London, CT) as a solution in vials containing 10 mg/mL of IgG2 protein. One cycle consisted of eight weekly injections, and the study drug was administered on days 1, 8, 15, 22, 29, 36, 43 and 50 of each cycle. The primary objective was to characterize the safety and tolerability and to define the MTD of CP-870,893 when administered on a continuous weekly schedule. Secondary objectives were to characterize pharmacokinetics, pharmacodynamics, immune modulation and antitumor activity.
At least three patients per dose level were enrolled, with additional patients enrolled in case of toxicity, which was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. DLT was defined as treatment-related nonhematologic grade 3 to 4 adverse events despite optimal supportive care; grade 3 hematologic adverse events, other than lymphopenia, that do not recover to grade 0–1 or baseline; grade 4 lymphopenia if complicated by infection or other grade 4 hematologic adverse event; or grade 2 or worse infusion reaction that affects vital organs. The MTD was estimated as the highest dose at which fewer than two of six patients experienced DLT. An additional seven patients were treated at the MTD in an expansion cohort. Patients developing study drug-related DLT were re-treated at a reduced dose, following a 1-week dose delay.
Study procedures.
Patients were assessed for adverse events continuously while on study and at least one more time 4 weeks after the last infusion of the study drug. Disease-appropriate imaging was performed at baseline and at the end of each treatment cycle and tumor response was assessed according to Response Evaluation Criteria in Solid Tumors version 1.0. A panel of nine autoantibodies was measured at baseline and day 29 of each cycle. Serum for pharmacokinetics and human anti-human antibody analyses was obtained at baseline and various times after infusion. Blood specimens for flow cytometric analysis were collected on day 1–3, 8–10, 22–24 in cycle 1 and at the end of study.
Pharmacokinetic, anti-human antibodies and pharmacodynamics.
Serum samples were used to quantify levels of CP-870,893 and half life, AUC and Cmax were determined, as previously described.13 Pharmacodynamics of CP-870,893 was assessed by flow cytometry of peripheral blood performed according to good laboratory practices, as previously described.13
Human peripheral blood and lymphocyte isolation.
PBMC was obtained from patients treated at the University of Pennsylvania Abramson Cancer Center after signed, informed consent for an additional protocol allowing phlebotomy of patients enrolled on the weekly CD40 treatment study. The protocol was approved by the Institutional Review Board at the University of Pennsylvania. Absolute lymphocyte count was obtained from a complete blood count and differential as measured by an accredited clinical lab. PBMC were obtained by Ficoll centrifugation (Amersham Pharmacia Biotech) and viably frozen at −150°C until use.
Flow cytometry.
Flow cytometry was performed on PBMC in PBS with 5% heat-inactivated fetal calf serum using a FACSCanto cytometer and FACSDiva (BD Biosciences) and FlowJo (TreeStar, Inc.) software. Intracellular staining was performed using a fixation/permeabilization kit (eBioscience), according to the manufacturer's instructions. PBMC were washed twice in staining buffer and analyzed immediately by flow cytometry. Fluorochromeconjugated mAb used were: PE-Cy7- or APC-H7-CD3 clone SK7, PerCP-CD4 clone SK3, APC-CD4 clone RPA-T4, PerCP-CD8 clone SK1, APC-CD11c clone B-ly6, PerCP-CD14 clone MP9, APC-H7-CD27 clone M-T271, PE-CD28 clone L293, APC-CD45RA clone HI100, FITC-CD86 clone FUN-1, PE-CD123 clone 9F5 (all BD Biosciences); PE-CCR7 clone 150503 (R&D Systems, Minneapolis, MN); APC-Alexa Fluor 750-anti-HLA-DR clone LN3 (eBioscience, San Diego, CA); Alexa fluor 488-anti-FoxP3 clone 259D and APC-perforin clone dG9 (Biolegend, San Diego, CA).
Intracellular cytokine staining.
Peripheral blood mononuclear cells in complete media comprising RPMI 1640 (Invitrogen) supplemented with 10% heat-inactivated human AB serum, 20 mM HEPES (Sigma-Aldrich) and 2 mM l-glutamine (Invitrogen) were stimulated with or without phorbol 12-myristate 13-acetate (PMA) (5 ng/ml) and ionomycin (1 µM) (Sigma, St. Louis, MO) for 4 hrs in the presence of 1 µM monensin (BD Biosciences). PBMC were then washed twice, re-suspended in staining buffer and stained for the surface markers CD3, CD4 and CD8 followed by intracellular staining for IFNγ using a fixation/permeabilization kit (eBioscience), according to the manufacturer's instructions. PBMC were washed twice in staining buffer and analyzed immediately by flow cytometry.
Acknowledgements
This study was supported by funding from Pfizer, Inc. This work was also supported in part by NIH grant P50 CA093372 (to R.H.V.) to perform immunologic analyses of patient samples. We thank Maryann Gallagher, Amy Kramer, Jackie McGilligan, Theresa Colligon and Jenna Trosko for their expert assistance with the clinical trial and Drs. Peter O'Dwyer and Keith Flaherty for helpful discussions.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/13251
Disclosure
R.D.H. owns stock in Pfizer Inc. and is employed by Pfizer Inc.
References
- 1.Vonderheide RH. Prospect of targeting the CD40 pathway for cancer therapy. Clin Cancer Res. 2007;13:1083–1088. doi: 10.1158/1078-0432.CCR-06-1893. [DOI] [PubMed] [Google Scholar]
- 2.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 3.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T- helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 4.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 5.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 6.Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 7.Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5:780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 8.van Mierlo GJ, den Boer AT, Medema JP, van der Voort EI, Fransen MF, Offringa R, et al. CD40 stimulation leads to effective therapy of CD40(−) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci USA. 2002;99:5561–5566. doi: 10.1073/pnas.082107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter TB, Alsarraj M, Gladue RP, Bedian V, Antonia SJ. An agonist antibody specific for CD40 induces dendritic cell maturation and promotes autologous anti-tumour T-cell responses in an in vitro mixed autologous tumour cell/lymph node cell model. Scand J Immunol. 2007;65:479–486. doi: 10.1111/j.1365-3083.2007.01927.x. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter EL, Mick R, Ruter J, Vonderheide RH. Activation of human B cells by the agonist CD40 antibody CP-870,893 and augmentation with simultaneous toll-like receptor 9 stimulation. J Transl Med. 2009;7:93. doi: 10.1186/1479-5876-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedian V, Donovan C, Garder J, Natoli E, Paradis T, Alpert R, et al. In vitro characterization and pre-clinical pharmacokinetics of CP-870,893, a human anti-CD40 agonist antibody. J Clin Oncol. 2006;24:109. [Google Scholar]
- 12.Gladue R, Cole S, Donovan C, Paradis T, Alpert R, Natoli E, et al. In vivo efficacy of the CD40 agonist antibody CP-870,893 against a broad range of tumor types: impact of tumor CD40 expression, dendritic cells and chemotherapy. J Clin Oncol. 2006;24:103. [Google Scholar]
- 13.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 14.Kedl RM, Jordan M, Potter T, Kappler J, Marrack P, Dow S. CD40 stimulation accelerates deletion of tumor-specific CD8(+) T cells in the absence of tumor-antigen vaccination. Proc Natl Acad Sci USA. 2001;98:10811–10816. doi: 10.1073/pnas.191371898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berner V, Liu H, Zhou Q, Alderson KL, Sun K, Weiss JM, et al. IFNgamma mediates CD4+ T-cell loss and impairs secondary antitumor responses after successful initial immunotherapy. Nat Med. 2007;13:354–360. doi: 10.1038/nm1554. [DOI] [PubMed] [Google Scholar]
- 16.Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2007;13:5238–5242. doi: 10.1158/1078-0432.CCR-07-0813. [DOI] [PubMed] [Google Scholar]
- 17.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, et al. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 18.Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, et al. CD40 activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–2765. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–2054. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 20.Coughlin CM, Fleming MD, Carroll RG, Pawel BR, Hogarty MD, Shan X, et al. Immunosurveillance and survivin-specific T-cell immunity in children with high-risk neuroblastoma. J Clin Oncol. 2006;24:5725–5734. doi: 10.1200/JCO.2005.05.3314. [DOI] [PubMed] [Google Scholar]
- 21.Lanzavecchia A. Immunology. Licence to kill. Nature. 1998;393:413–414. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- 22.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartholdy C, Kauffmann SO, Christensen JP, Thomsen AR. Agonistic anti-CD40 antibody profoundly suppresses the immune response to infection with lymphocytic choriomeningitis virus. J Immunol. 2007;178:1662–1670. doi: 10.4049/jimmunol.178.3.1662. [DOI] [PubMed] [Google Scholar]
- 24.Mauri C, Mars LT, Londei M. Therapeutic activity of agonistic monoclonal antibodies against CD40 in a chronic autoimmune inflammatory process. Nat Med. 2000;6:673–679. doi: 10.1038/76251. [DOI] [PubMed] [Google Scholar]
- 25.Hussein MA, Berenson JR, Niesvizky R, Munshi NC, Harrop KL, McDonald M, et al. A phase I humanized anti-CD40 monoclonal antibody (SGN-40) in patients with multiple myeloma. Blood. 2005;1060:2572. doi: 10.3324/haematol.2009.008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Advani R, Forero-Torres A, Furman RR, Rosenblatt JD, Younes A, Ren H, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin's lymphoma. J Clin Oncol. 2009;27:4371–4377. doi: 10.1200/JCO.2008.21.3017. [DOI] [PubMed] [Google Scholar]