Abstract
Curcumin, a naturally-occurring compound found in the rhizome of Curcuma longa plant, is known for its antitumor activities. However, its clinical efficacy is limited due to poor bioabsorption. A new class of synthetic analogs of curcumin, namely diarylidenylpiperidone (DAP), has been developed with substantially higher anticancer activity than curcumin. However, its cellular uptake and bioabsorption have not been evaluated. In this study we have determined the absorption of a representative DAP compound, HO-3867, using optical and electron paramagnetic resonance spectrometry. The cellular uptake of HO-3867 was measured in a variety of cancer cell lines. HO-3867 was taken in cells within 15 minutes of exposure and its uptake was more than 100-fold higher than curcumin. HO-3867 was also retained in cells in an active form for 72 hours and possibly longer. HO-3867 was substantially cytotoxic to all the cancer cells tested. However, there was no direct correlation between cellular uptake and cytotoxicity suggesting that the cytotoxic mechanisms could be cell-type specific. When administered to rats by intraperitoneal injection, significantly high levels of HO-3867 were found in the liver, kidney, stomach and blood after 3 hours. Also, significant accumulation of HO-3867 was found in murine tumor xenografts with a dose-dependent inhibition of tumor growth. The results suggest that the curcumin analog has substantially higher bioabsorption when compared to curcumin.
Key words: curcumin, ovarian cancer, bioabsorption, HO-3867
Introduction
Curcumin (diferuloylmethane) is a naturally-occurring compound found in the rhizome of the Curcuma longa plant in south and southeast tropical Asia. Curcumin is known to protect against carcinogenesis and prevent tumor development in several types of cancer.1–6 However, its poor bioavailability and potency prevent it from being effective in chemotherapeutic applications in the clinic.7–9 One potential means of circumventing these limitations is the development of synthetic analogs with enhanced bioabsorption and potency.10–13 Recently, a novel class of curcumin analogs, namely diarylidenylpiperidone (DAP), has been synthesized by incorporating a piperidone ring in the beta-diketone backbone structure, as well as fluorinating the phenyl groups.14–16 In a previous study, we compared the anticancer efficacy of four DAPs, named as H-4073, HO-3867, HO-4318 and HO-4200 with curcumin in a number of cancer as well non-cancerous (healthy) cell lines.17,18 All four compounds showed substantially higher antiproliferative activity and induction of apoptosis than curcumin in the cell lines tested.
Interestingly, we observed that HO-3867 was significantly less toxic to non-cancerous cells when compared to cancer cells.17 Many anticancer drugs have undesirable side effects caused by the production of free radicals, which may increase the oxidative stress levels and cause damage to normal cells and tissues.19,20 It has been shown that nitroxides, a class of small-molecular-weight heterocyclic molecules containing “NO” and hydroxylamines, the one-electron-reduced form of nitroxides characterized by “NOH”, preferentially scavenge oxygen radicals in cells that have normal oxygenation or redox status.21,22 HO-3867 incorporates a nitroxide-promoting moiety in its chemical structure (Fig. 1A).
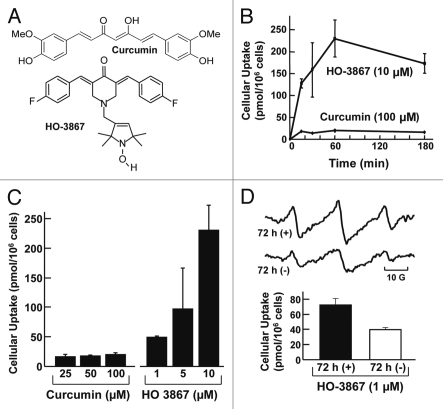
Figure 1.
Cellular absorption of varying concentrations of curcumin and HO-3867 as a function of incubation period. HO-3867 was extracted into methanol from A2780 cells, as described in methods. (A) The structures of curcumin and HO-3867. (B) The effect of incubation time on the cellular absorption of curcumin and HO-3867. (C) The effect of curcumin and HO-3867 concentration on cellular uptake determined after 1 hour of incubation. (D) Cellular uptake of HO-3867 with continuous exposure to HO-3867-containing medium over 72 hours and cellular uptake of HO-3867 with 1-h exposure to HO-3867-containing medium followed by 72 hours exposure to medium containing no HO-3867. Also shown are representative EPR spectra of cells measured after 72 hours of incubation of cells. All values are mean ± SE (N = 3).
The goal of the present study was to determine the cellular absorption of both HO-3867 and curcumin in a variety of cancerous and noncancerous cell lines, as well as to demonstrate the bioavailability of HO-3867 in vivo using a non-cancer-bearing rat model and a murine xenograft ovarian tumor model. HO-3867 was chosen for this study due to the apparent selectivity or targeted effect towards cancerous versus noncancerous cells. While HO-3867 has been shown to have a greater inhibitory effect than curcumin on the proliferation of many cancer cell lines,17 as yet there has been no study demonstrating that HO-3867 or any other DAP compound, is more readily absorbed by cells in any greater capacity than curcumin. As the low bioabsorption of curcumin is one of its greatest drawbacks towards use in clinical practice, the quantification of cellular uptake of curcumin analogs is a necessary step in evaluating usefulness of these drugs. We used optical and electron paramagnetic resonance (EPR) spectrometry to quantify the intracellular and tissue content of HO-3867. The results showed a substantial uptake of HO-3867, both in vitro and in vivo.
Results
Cellular absorption of curcumin and HO-3867 in A2780 ovarian cancer cells.
We examined the time-course of cellular uptake of curcumin and HO-3867 over a period of 3 hours. Cellular uptake of curcumin remained low and constant over the time period examined, whereas cellular uptake of HO-3867 increased up to 60 minutes (Fig. 1B). The cellular uptake of HO-3867 was 10-fold greater than curcumin at 60 min of incubation, at the respective concentrations (10–100 µM) tested. We investigated the uptake of curcumin and HO-3867 by A2780 ovarian cancer cells exposed to different concentration of the drugs for a period of 1 hour. The cellular absorption of curcumin after 1 hour of exposure did not significantly increase as the curcumin concentration in medium increased. In contrast, the absorption of HO-3867 increased significantly as HO-3867 concentration in medium was increased (Fig. 1C). Further, we determined the change in HO-3867 concentration within the cells over a longer period of time of continuous exposure using a minimal concentration (1 µM) of HO-3867. The cells were incubated for 72 hours and the concentration within the cells remained substantial. In addition, we determined the longer-term bioavailability of HO-3867 within cells following a period of exposure. After 1 hour exposure to HO-3867, followed by 72 hours exposure to non-drug-containing medium, the amount of HO-3867 in cells remained substantial (Fig. 1D). In the relevant cases, the identification of intracellular HO-3867 was further supported by EPR spectroscopy (Fig. 1D). The results clearly showed that HO-3867 exhibited a greater cellular absorption than curcumin (up to 100-fold on an equal-concentration basis) in A2780 cells and that HO-3867 was taken up as early as 15 minutes following exposure.
Uptake and cytotoxicity of HO-3867 in various cancer cell lines.
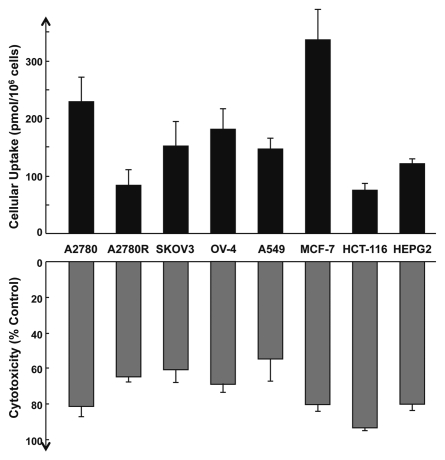
To ensure that the absorption of HO-3867 demonstrated using the A2780 cells was not cell-line specific, we confirmed the uptake of the compound using two additional ovarian cancer cell lines: OV-4, SKOV3 and A2780R, a cisplatin-resistant variant of A2780 cell line. Uptake was also tested in non-ovarian human cancer cell lines, including: A549 (lung), HCT-116 (colon), HepG2 (liver) and MCF-7 (breast). The cells were exposed to 10-µM HO-3867 for 1 hour, after which the cellular uptake was determined. The absorption of HO-3867 was measurable in all cell lines examined (Fig. 2). The results showed variable levels of cellular uptake. We also examined the cytotoxicity of HO-3867 in the same cell lines (Fig. 2). A quick comparison of the cellular uptake with cytotoxicity indicated no significant correlation suggesting that the underlying mechanism(s) of cytotoxicity could be different in the cell lines tested.
Figure 2.
Cellular absorption and cytotoxicity of HO-3867 in several cancer cell lines. Cellular absorption of HO-3867 was measured as in Figure 1. Cytotoxicity was measured by MTT assay. Cancer cell lines include: A2780 (cisplatin-sensitive), A2780R (cisplatin-resistant), OV-4 and SKOV3 (ovarian); A549 (lung); HCT-116 (colon); HepG2 (liver); MCF-7 (breast). Values are mean ± SE (N = 6). The results indicate a variable, but substantial uptake of HO-3867 in cancer cells. There was no correlation between the cellular uptake and cytotoxicity.
In vivo distribution of HO-3867 in rat.
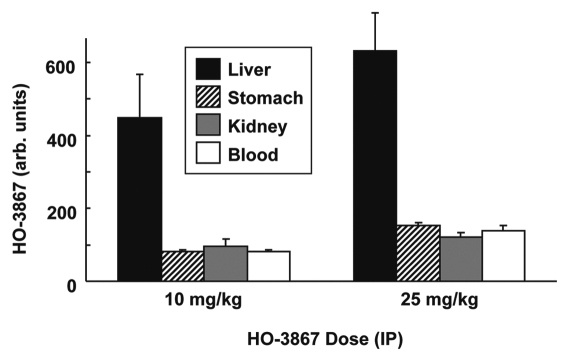
In order to examine the distribution of HO-3867 in vivo after dosage, we administered HO-3867 by IP injection to Wistar rats in doses of either 10 or 25 mg/kg body-weight. After three hours, the rats were euthanized and the liver, kidneys and stomach were collected. Blood samples were also collected and added to vacutainer tubes containing heparin. The samples were analyzed using EPR spectroscopy. There were EPR-detectable levels of HO-3867 in all samples collected (Fig. 3). Untreated control samples produced no detectable EPR signal, indicating that the spectrum obtained is due to the presence of HO-3867, in nitroxide form, in the sample. The signal intensity from the liver samples was almost twice the signal intensity from serum and the other tissues collected.
Figure 3.
Absorption of HO-3867 into rat tissues. Rats were dosed with HO-3867 by IP injection. After 3 hours, the rats were euthanized and blood and tissues collected. HO-3867 levels in the tissue and blood samples were determined using EPR spectrometry as described in the Methods section. Values are mean ± SE (N = 3). The results show variable levels of absorption in the tissues, with a maximum accumulation in the liver.
HO-3867 detected in ovarian tumor xenograft tissues after oral intake.
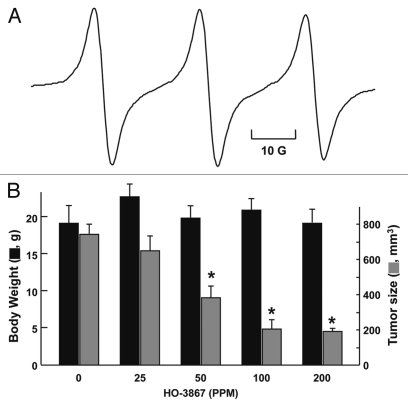
We also determined the amount of HO-3867 in samples of ovarian tumor tissue using a murine xenograft model treated orally by mixing the compound with chow. Based on our in vitro results, which showed significant uptake-induced cytotoxicity of HO-3867 to human ovarian cancer cell lines, we next evaluated the efficacy of HO-3867 in a human ovarian tumor xenograft grown in the back of mice. The mice were treated with HO-3867, the tumor mice were given feed containing 25, 50, 100 or 200 parts-per-million (ppm) HO-3867 for a treatment period of four weeks, after which they were euthanized and the tumor tissues were collected and prepared for analysis as described in Methods. A substantial amount of HO-3867 was detected in the tumor tissues (Fig. 4A). We also observed a significant reduction in the tumor volume in a dose-dependent manner with no apparent effect on body weight.
Figure 4.
Absorption of HO-3867 into tumor tissue. Mice with human ovarian cancer xenograft tumors were administered with varying doses (0–200 PPM) of HO-3867 in the feed four weeks, after which the mice were euthanized and the tumor tissues harvested for analysis of tissue content. (A) A representative EPR spectrum obtained from a tumor tissue biopsy showing the presence on HO-3867 in the oxidized (nitroxide) form. (B) Body weight (left axis) and tumor size (right axis) measured after four weeks as a function of dose of HO-3867 in the feed. Data represent mean ± SD (N = 3). *p < 0.05 versus untreated (0 PPM) group. The results show a significant reduction of tumor volume in a dose-dependent manner with no apparent effect on body weight.
Discussion
The comparatively low bioabsorption and bioavailability of curcumin in vivo are important factors that must be considered when evaluating its potential use as an anticancer agent. HO-3867, examined in this study, is related to curcumin in structure and function. It is reasonable to examine the cellular absorption of the compounds, to ensure that they do not share the same, low levels of uptake. The present study demonstrated that HO-3867 exhibits a much greater absorption than curcumin, supporting the contention that this synthetic analog does not face the same limitations.
The results of the present study clearly showed that HO-3867 has a much greater cellular absorption than curcumin under all conditions examined. Indeed, the cellular uptake of 10 µM HO-3867 was over 10-fold greater than 100 µM curcumin in A2780 cells. Thus, it can be assumed that even a much lower dose of HO-3867 can result in the accumulation of a higher concentration in target cells than a given dose of curcumin. Further, it can be seen that HO-3867 enters into cells quickly, as indicated by detectable levels of HO-3867 in cells after only 15 minutes of exposure. Moreover, once the HO-3867 has entered the cells it remains, in nitroxide form, for long periods of time, whether the cells are subsequently incubated in medium with HO-3867 or without. This suggests that HO-3867 may be effective even if administered intermittently.
In addition, the cellular absorption of HO-3867 remains substantial in cisplatin-resistant cells (A2780R), an important factor given the frequent development of drug-resistant tumors in clinical practice.24–26 While the presence of HO-3867 in drug resistant cells does not indicate that the drug will be equally effective under these conditions, it does indicate that the potential for effectiveness is not necessarily lost. The absorption of HO-3867 in a wide variety of cancer cell lines suggests a wide range of efficacy for the drug. The HO-3867 detected in several rat organs and in blood further supports its use as an anticancer drug, as it demonstrates that HO-3867 is effectively distributed after IP injection. The presence of HO-3867 in the mouse tumor samples further indicates the ability of HO-3867 to be delivered in the body to its target. Further studies using HPLC or other methods may be needed in the future to measure the quantity of HO-3867 in the tissues more precisely, but we consider the indication of HO-3867's presence to be sufficient at this time.
The cytotoxicity data of HO-3867 presented in this study are in agreement with those of other structurally related DAPs measured after 24 hours of treatment with a number of cancer, as well as non-cancerous cells.17 HO-3867 induced G2/M cell cycle arrest in A2780 cells by modulating cell cycle regulatory molecules p53, p21, p27, cdk2 and cyclin and promoted apoptosis by caspase-8 and caspase-3 activation.18 HO-3867 also decreased STAT3 (Tyr705) and JAK1 phosphorylation and increased apoptosis markers cleaved caspase-3 and PARP in ovarian xenograft tumors treated with HO-3867.18
The results of the study clearly indicate that the cellular absorption of HO-3867 is substantially greater than that of curcumin. Further, the distribution throughout the body of HO-3867 after IP injection has been demonstrated. As the low bioabsorption of curcumin is one of the barriers against its clinical use, the demonstration that HO-3867 does not share this weakness is an important factor supporting the effectiveness of this drug in cancer treatment.
Materials and Methods
Chemicals.
HO-3867 was synthesized in the laboratory according to previously-published methods.18,23 Curcumin was obtained from Sigma. Stock solutions of the compounds were freshly prepared in dimethylsulfoxide (DMSO). All other reagents, of analytical grade or higher, were purchased from Sigma-Aldrich, unless otherwise noted.
Cell lines.
The ovarian cancer cell line A2780 was primarily used. Additional ovarian cancer cell lines include: A2780R (cisplatin-resistant strain of A2780), OV-4 and SKOV3. Other cancer cell lines tested include: A549 (lung), HCT-116 (colon), HepG2 (liver) and MCF-7 (breast). Cells were grown in RPMI or DMEM, as appropriate, supplemented with 10% FBS, 2% sodium pyruvate, 1% penicillin and 1% streptomycin. Cells were maintained in 75-cm2 flasks at 37°C in an atmosphere of room air diluted with 5% CO2. Cells were routinely trypsinized (0.05% trypsin/EDTA) and counted using an automated counter (NucleoCounter, New Brunswick Scientific, Edison, NJ).
Measurement of curcumin and HO-3867 in vitro.
HO-3867 was administered to cells grown in 10 cm-diameter dishes until 85–90% confluency. HO-3867 was administered in one of several methods: (i) 85–90% confluent cells were incubated in medium containing either HO-3867 (1, 5 or 10 µM) or curcumin (25, 50 and 100 µM) for up to three hours; (ii) 85–90% confluent cells were treated for 72 hours at 1 µM concentration of HO-3867; (iii) 85–90% confluent cells were exposed to 1 µM of HO-3867 for 1 hour, followed by the removal of the drug-containing medium and the replacement of fresh medium, to which the cells were exposed for 72 hours. In all cases, after their respective treatments, the cells were washed and then recovered using 0.05% trypsin/EDTA. The cells were then centrifuged for 5 minutes at 1,500 rpm and 4°C. The supernatant was removed and then the pellet was suspended in 5 ml cold PBS. A 20 µl aliquot was taken at this time for cell counting. The cells were again centrifuged for 5 minutes at 1,500 rpm and 4°C, after which the supernatant was removed and the pellet was allowed to dry at room temperature. After the pellet had completely dried, it was resuspended in methanol (1 ml) and sonicated for 10 minutes using a Fisher Scientific Sonic Dismembrator (Model 100 set to 7 W), to extract the drug from the cells. While being sonicated, the samples were kept in ice, to avoid excessive heating of the samples. After sonication, the suspension was centrifuged for 5 minutes at 10,000 rpm and 4°C and the supernatant was then collected. For UV/Vis spectrometry, the sample was diluted in methanol (1:1) and scanned using a UV/Visible spectrophotometer (Varian Cary 50). The concentrations of the compounds in the methanol solution were determined by: (i) for curcumin, absorbance at 428 nm, with a molar absorption coefficient of 48,000 M−1cm−1; and (ii) for HO-3867, absorbance at 328 nm, with a molar absorption coefficient of 25,200 M−1cm−1. For EPR spectroscopy, the samples were incubated for 10 minutes with PbO2 (10 mg) to convert the hydroxylamine to nitroxide and measured using an X-band (9.8 GHz) EPR spectrometer (Bruker BioSpin EMX; Billerica, MA). Quantitation of the EPR signal was performed by comparing the double integral of the signal with that of a nitroxide standard.
Cell viability by MTT assay.
Cell viability was determined by a colorimetric assay using MTT. In the mitochondria of living cells, yellow MTT undergoes a reductive conversion to formazan, giving a purple color. Cells, grown to 80% confluence in 75 mm flasks, were trypsinized, counted, seeded in 96-well plates with an average population of 7,000 cells/well, incubated overnight and then treated with the HO-3867 (10 µM) for 48 hours. The dose and time of incubation were determined from a set of preliminary experiments. All experiments were done using six replicates and repeated at least three times. Cell viability was expressed as a percentage of MTT viability of untreated cells.
Animals.
Male Wistar rats (∼300 g body-weight) were used for the first in vivo organ distribution study. In a subsequent series of experiments, we measured the signal of HO-3867 in ovarian tumor tissue xenografts using male 6-week-old BALB/c nude mice (∼25 g body-weight) from the National Cancer Institute.
Measurement of HO-3867 in vivo.
Rats were given intraperitoneal (IP) injections of either 10 or 25 mg/kg HO-3867. The rats were then left undisturbed, under observation for 3 hours, after which they were euthanized using isoflurane. The kidneys, liver and stomach tissues were recovered for analysis. In addition, blood was taken and added to vacutainer tubes containing heparin. The tissues were homogenized as individual samples in PBS, after which they were diluted 1:10 in methanol. The samples were then sonicated for 15 minutes using a Fisher Scientific Sonic Dismembrator, Model 100 set to 7 W. As with the in vitro samples, the samples were kept in ice during sonication. The samples were centrifuged for 5 minutes at 10,000 rpm and 4°C. The supernatant was collected and analyzed in an X-band (9.8 GHz) EPR spectrometer using the same settings that were used for the in vitro samples. For blood, the samples were centrifuged and the upper layer, consisting of the blood plasma, was taken and measured by X-band EPR spectroscopy, as with the tissue samples. The absorbance of the samples was also measured at 328 nm using a UV/Vis spectrophotometer as for cells, but the abundance of light-absorbing molecules in the tissues precluded accurate quantification of drug uptake.
Tumor xenografts in mice.
Cultured A2780 cancer cells (2 × 106 cells in 60 µl PBS) were subcutaneously injected into the back of 6-week-old BALB/c nude mice from the National Cancer Institute. Five to 7 days later, when the tumors reached 3–5 mm in diameter, the mice were divided into groups in a manner to equalize the mean tumor diameter among the groups. The control group was given a normal diet (no treatment), whereas the experimental groups were treated using the HO-3867 compounds mixed with the animal feed (Harlan Teklad) at four different levels (25, 50, 100 or 200 ppm). The doses were chosen based on our previous dose-response study optimized to produce an observable effect on tumor growth.18 The size of the tumor was measured twice per week using a digital Vernier caliper. The tumor volume was determined from the orthogonal dimensions (d1, d2, d3) using the formula (d1 × d2 × d3) × π/6. Body weights were measured weekly once. Twenty-eight days after the beginning of HO-3867 treatment, the mice were sacrificed and the tumors were resected. The tumor tissues were then subjected to EPR analysis for determination of HO-3867.
Data analysis.
Comparisons between sets were made by ANOVA or Student's t-test, as appropriate. A p value of less than 0.05 was considered significant.
Acknowledgements
This work was supported by NIH grant CA102264 (P.K.), The Kaleidoscope of Hope Foundation grant (K.S.) and Hungarian Research Fund Grant OTKA K81123 (K.H.).
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/13250
References
- 1.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr Med Chem. 2010;17:190–197. doi: 10.2174/092986710790149738. [DOI] [PubMed] [Google Scholar]
- 3.Kamat AM, Tharakan ST, Sung B, Aggarwal BB. Curcumin potentiates the antitumor effects of Bacillus Calmette-Guerin against bladder cancer through the downregulation of NFkappaB and upregulation of TRAIL receptors. Cancer Res. 2009;69:8958–8966. doi: 10.1158/0008-5472.CAN-09-2045. [DOI] [PubMed] [Google Scholar]
- 4.Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, et al. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597–601. [PubMed] [Google Scholar]
- 5.Ushida J, Sugie S, Kawabata K, Pham QV, Tanaka T, Fujii K, et al. Chemopreventive effect of curcumin on N-nitrosomethylbenzylamine-induced esophageal carcinogenesis in rats. Jpn J Cancer Res. 2000;91:893–898. doi: 10.1111/j.1349-7006.2000.tb01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weir NM, Selvendiran K, Kutala VK, Tong L, Vishwanath S, Rajaram M, et al. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol Ther. 2007;6:178–184. doi: 10.4161/cbt.6.2.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 8.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52:139–151. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 9.Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea and grape seed extracts. Altern Med Rev. 2009;14:226–246. [PubMed] [Google Scholar]
- 10.Tong QS, Zheng LD, Lu P, Jiang FC, Chen FM, Zeng FQ, et al. Apoptosis-inducing effects of curcumin derivatives in human bladder cancer cells. Anticancer Drugs. 2006;17:279–287. doi: 10.1097/00001813-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari E, Lazzari S, Marverti G, Pignedoli F, Spagnolo F, Saladini M. Synthesis, cytotoxic and combined cDDP activity of new stable curcumin derivatives. Bioorg Med Chem. 2009;17:3043–3052. doi: 10.1016/j.bmc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Lin L, Hutzen B, Ball S, Foust E, Sobo M, Deangelis S, et al. New curcumin analogues exhibit enhanced growth-suppressive activity and inhibit AKT and signal transducer and activator of transcription 3 phosphorylation in breast and prostate cancer cells. Cancer Sci. 2009;100:1719–1727. doi: 10.1111/j.1349-7006.2009.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamvakopoulos C, Dimas K, Sofianos ZD, Hatziantoniou S, Han Z, Liu ZL, et al. Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clin Cancer Res. 2007;13:1269–1277. doi: 10.1158/1078-0432.CCR-06-1839. [DOI] [PubMed] [Google Scholar]
- 14.Selvendiran K, Tong L, Vishwanath S, Bratasz A, Trigg NJ, Kutala VK, et al. EF24 induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by increasing PTEN expression. J Biol Chem. 2007;282:28609–28618. doi: 10.1074/jbc.M703796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas SL, Zhong D, Zhou W, Malik S, Liotta D, Snyder JP, et al. EF24, a novel curcumin analog, disrupts the microtubule cytoskeleton and inhibits HIF-1. Cell Cycle. 2008;7:2409–2417. doi: 10.4161/cc.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selvendiran K, Kuppusamy ML, Bratasz A, Tong L, Rivera BK, Rink C, et al. Inhibition of vascular smooth-muscle cell proliferation and arterial restenosis by HO-3867, a novel synthetic curcuminoid, through upregulation of PTEN expression. J Pharmacol Exp Ther. 2009;329:959–966. doi: 10.1124/jpet.108.150367. [DOI] [PubMed] [Google Scholar]
- 17.Selvendiran K, Ahmed S, Dayton A, Kuppusamy ML, Tazi M, Bratasz A, et al. Safe and targeted anticancer efficacy of a novel class of antioxidant-conjugated difluorodiarylidenyl piperidones: differential cytotoxicity in healthy and cancer cells. Free Radic Biol Med. 2010;48:1228–1235. doi: 10.1016/j.freeradbiomed.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selvendiran K, Tong L, Bratasz A, Kuppusamy ML, Ahmed S, Ravi Y, et al. Anticancer efficacy of a difluorodiarylidenyl piperidone (HO-3867) in human ovarian cancer cells and tumor xenografts. Mol Cancer Ther. 2010;9:1169–1179. doi: 10.1158/1535-7163.MCT-09-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Injac R, Strukelj B. Recent advances in protection against doxorubicin-induced toxicity. Technol Cancer Res Treat. 2008;7:497–516. doi: 10.1177/153303460800700611. [DOI] [PubMed] [Google Scholar]
- 20.Santos NA, Bezerra CS, Martins NM, Curti C, Bianchi ML, Santos AC. Hydroxyl radical scavenger ameliorates cisplatin-induced nephrotoxicity by preventing oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Cancer Chemother Pharmacol. 2008;61:145–155. doi: 10.1007/s00280-007-0459-y. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell JB, Krishna MC, Kuppusamy P, Cook JA, Russo A. Protection against oxidative stress by nitroxides. Exp Biol Med (Maywood) 2001;226:620–621. doi: 10.1177/153537020222600703. [DOI] [PubMed] [Google Scholar]
- 22.Samuni Y, Gamson J, Samuni A, Yamada K, Russo A, Krishna MC, et al. Factors influencing nitroxide reduction and cytotoxicity in vitro. Antioxid Redox Signal. 2004;6:587–595. doi: 10.1089/152308604773934341. [DOI] [PubMed] [Google Scholar]
- 23.Kalai T, Kuppusamy P, Hideg K. Synthesis, characterization and structure-activity studies with a novel class of diarylidenylpiperidones. (to be published) [Google Scholar]
- 24.Yuan F, Qin X, Zhou D, Xiang QY, Wang MT, Zhang ZR, et al. In vitro cytotoxicity, in vivo biodistribution and antitumor activity of HPMA copolymer-5-fluorouracil conjugates. Eur J Pharm Biopharm. 2008;70:770–776. doi: 10.1016/j.ejpb.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Christoffersen T, Guren TK, Spindler KL, Dahl O, Lonning PE, Gjertsen BT. Cancer therapy targeted at cellular signal transduction mechanisms: strategies, clinical results and unresolved issues. Eur J Pharmacol. 2009;625:6–22. doi: 10.1016/j.ejphar.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Soulie P, Raymond E, Brienza S, Cvitkovic E. [Oxaliplatin: the first DACH platinum in clinical practice] Bull Cancer. 1997;84:665–673. [PubMed] [Google Scholar]