Abstract
Primary effusion lymphoma (PEL) is an aggressive form of lymphoma that is associated with infection by Kaposi sarcoma-associated herpesvirus (KSHV). One of the KSHV genes expressed in PEL cells is K13, a potent activator of the NFκB pathway. K13 transgenic mice develop lymphomas, but after a long period of latency. A possible candidate that could cooperate with K13 in the development of PEL is c-Myc, whose expression is frequently dysregulated in PEL cells. To study the cooperative interaction between K13 and c-Myc in the pathogenesis of PEL, we crossed the K13 transgenic mice to iMycEµ transgenic mice that overexpress Myc. We report that lymphomas in the K13/iMycEµ double transgenic mice developed with shorter latency and were histologically distinct from those observed in the iMycEµ mice. Lymphomas in the K13/iMycEµ mice also lacked the expression of B- and T-cell markers, thus resembling the immunophenotype of PEL. The accelerated development of lymphoma in the K13/iMycEµ mice was associated with increased expression of K13, elevated NFκB activity and decrease in apoptosis. Taken collectively, our results demonstrate a cooperative interaction between the NFκB and Myc pathways in lymphomagenesis.
Key words: vFLIP, K13, NFκB, PEL, Myc, KSHV, lymphoma
Introduction
Primary effusion lymphoma (PEL), also known as body cavity lymphoma, is an aggressive form of lymphoma that typically grows as lymphomatous effusions in the body cavities without a contiguous tumor mass.1,2 PEL accounts for about 4% of all HIV-associated non-Hodgkin lymphomas (NHLs) and is associated with infection by Kaposi sarcoma-associated herpesvirus (KSHV; also known as Human Herpesvirus 8 or HHV 8).3–5 In addition to KSHV, some PEL cells also carry the Epstein-Barr virus genome, but generally lack genetic lesions of c-Myc, Bcl-2 and p53.1,2 Although the exact maturation stage of PEL is still controversial, it is believed to be a malignancy of B cells of a mature, post-germinal center, preterminal differentiation stage.1,2 A characteristic feature of PEL cells is that they lack the expression of most B-cell markers, such as CD20, CD22 and CD19 and surface immunoglobulin.6
The exact mechanism by which KSHV infection contributes to the PEL is not clear. PEL cells express a limited repertoire of KSHV genes, including K13 (ORF71), vCyclin (ORF72), latency-associated nuclear antigen-1, LANA (ORF73), Kaposin (K12), vIRF-3/LANA2 and vIRF-2.4 Based on its sequence homology to the prodomain of caspase 8/FLICE, the K13 protein was originally believed to be an inhibitor of caspase 8 and was classified as a viral FLICE inhibitory protein (vFLIP).7,8 However, subsequent work revealed that K13 does not act as a vFLIP and instead interacts with a 700 kDa IkappaB kinase (IKK) complex to activate the NFκB pathway.9–12 K13 uses the NFκB pathway to protect cells against growth factor withdrawal-induced apoptosis, promote cytokine secretion, transform rodent fibroblasts and promote KSHV latency.13–17 K13 is also primarily responsible for constitutive NFκB activation observed in PEL cells and inhibition of this activity by genetic and pharmacological means has been shown to block cellular proliferation, induce lytic reactivation of the virus and trigger apoptosis.10,12,18–21 Consistent with the in vitro studies, lymphocytes from K13 transgenic mice displayed constitutive NFκB activation and enhanced response to mitogenic stimuli.22 Although K13 transgenic mice developed lymphomas at an increased rate, their incidence was relatively low and they developed after a long period of latency, suggesting that K13-induced NFκB may require cooperative interactions with other cellular and/or viral genes for lymphomagenesis.22
A possible candidate that could cooperate with K13 in the development of PEL is c-Myc.23 The c-Myc gene codes for a basic helix-loop-helix transcription factor that controls cellular growth, proliferation, differentiation and apoptosis.23 c-Myc expression is frequently deregulated in lymphomas due to chromosomal translocations (e.g., Burkitt lymphomas), gene amplifications (e.g., non-Hodgkin lymphomas) and/or mutations in its N-terminal domains that affect protein stability.24–27 Although structural abnormalities involving the c-Myc gene are not seen in PEL,2,28 recent studies suggest that the c-Myc protein is frequently deregulated in PEL due to expression of KSHV-encoded proteins, such as LANA and viral interferon regulatory factor 3 (vIRF3).28–30
To study the cooperative interaction between K13 and Myc in the pathogenesis of PEL, we crossed the K13 transgenic mice to iMycEµ transgenic mice in which a His6-tagged mouse Myc cDNA is inserted in the JH-EA intervening region of mouse Ig heavy-chain locus.31 We report that lymphomas in the K13/iMycEµ double transgenic mice not only develop with shorter latency, but also lack the expression of most B- and T-cell markers, thus resembling the immunophenotype of PEL.
Results
Generation of K13-iMycEµ double transgenic mice.
We had previously described K13 transgenic mice on the ICR background that express the K13 transgene under the H2Kb promoter and immunoglobulin heavy chain heavy chain (IgH) enhancer.22 The K13 transgene in these animals is tagged at its carboxy terminus with three copies of a FLAG epitope tag and is widely expressed in the hemato-lymphoid organs, including spleen, lymph node, thymus and bone marrow.22 The K13 transgenic mice demonstrate constitutive activation of the NFκB pathway and increased incidence of lymphoma, albeit after a long latency period of more than one year.22 In the iMycEµ mice, a His6-tagged mouse Myc cDNA has been inserted into the mouse immunoglobulin heavy-chain locus, Igh, just 5′ of the intronic enhancer, Eµ, to mimic the Myc-activating chromosomal t(8;14)(q24;q32) translocation most commonly observed in human endemic Burkitt lymphoma.31 The heterozygous iMycEµ mice on the C57BL/6 (B6) background develop a spectrum of B-cell tumors, including Burkitt-like lymphoblastic B-cell lymphoma and diffuse large B-cell lymphoma.31 To study the cooperative interaction between Myc and K13-induced NFκB pathway in the lymphomagenesis, we generated K13 mice on the B6 and Balb/c backgrounds and then crossed them with the iMycEµ mice on the corresponding backgrounds to generate K13/iMycEµ double transgenic mice. The results of breeding showed that all four genotypes (i.e., wild type, K13, iMycEµ and K13/iMycEµ) were observed in the Mendelian ratio (1:1:1:1) as determined by Chi square analysis.
Incidence of tumors and survival in single and double transgenic mice.
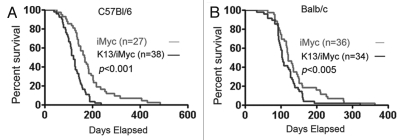
Wild-type and single and double transgenic mice were followed for the development of tumors and survival for 20 months. On the B6 background, unlike the wild-type and K13 transgenic mice, the iMycEµ and the K13/iMycEµ mice developed significant lymphadenopathy and splenomegaly (not shown). However, the rate of these complications was higher in the K13/iMycEµ mice, which translated into a significant difference in their survival rate (Fig. 1). Thus, the K13/iMycEµ mice started to die as early as 2 months of age as opposed to 3 months of age for the iMycEµ mice (Fig. 1A). Moreover, more than 80% of the K13/iMycEµ mice had died by 6 months of age; the corresponding figure for the iMyc mice was 8 months (Fig. 1A). The median survival of K13/iMycEµ and iMycEµ animals was 4 and 5 months, respectively. A similar survival trend was also observed in the Balb/c background, however, both iMycEµ and double transgenic K13/iMycEµ mice on this background had shorter lifespan as compared to the B6 background (Fig. 1B). The median life span in Balb/c background was 4 months for iMycEµ and 3 months for K13/iMycEµ mice, respectively. Collectively, these results demonstrate increased incidence of lymphomas in the K13/iMycEµ mice, which translates into their reduced survival.
Figure 1.
Kaplan-Meier graph showing survival of iMycEµ and K13/iMycEµ mice on B6 (A) and Balb/c (B) backgrounds. Mice were monitored for 1 year. Mice that were found dead due to tumors and the mice that were euthanized due to tumor development were included in the survival curve. Log-rank test was used to determine the difference between the two genotypes.
Histological and immunohistochemical analysis of tumors in the iMycEµ and K13/iMycEµ mice.
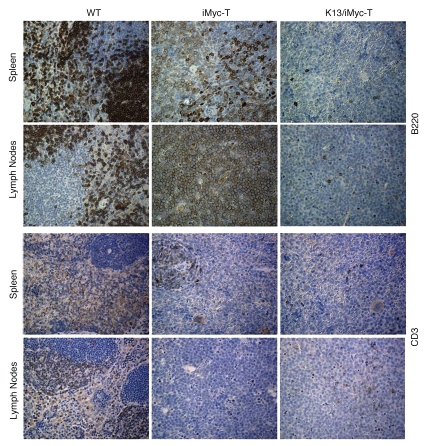
We compared the tumors developing in the iMycEµ and the K13/iMycEµ mice on the B6 background by histological examination. Consistent with the published reports, a majority of the lymphoid neoplasms in the iMycEµ mice were Burkitt-like lymphoblastic B-cell lymphomas.31 A representative example of such a tumor involving the lymph nodes and spleen of an iMycEµ mouse is provided in Figure 2. The lymph node architecture is effaced by an infiltrate of monotonous, intermediate-size lymphoid cells with a high nuclear-cytoplasmic ratio. Mitotic figures are frequent, and there are many histiocytes containing the debris of ingested apoptotic tumor cells, resulting in a ‘starry-sky’ appearance. The atypical neoplastic cells also infiltrate the spleen. Immunohistochemical staining showed the neoplastic cells to be positive with/for the B-cell marker B220 (Fig. 3). Few CD3-positive T cells were found in the lymph nodes and spleen (Fig. 3). In the K13/iMycEµ double transgenic mice, tumors are seen that show histologic features distinct from those in the iMycEµ mice (Fig. 2). In these tumors, the lymph node is effaced by sheets of larger, more pleomorphic cells, though these again show a high nuclear-cytoplasmic ratio. Similar cells also infiltrate the spleen. Mitotic figures are readily apparent and some apoptotic cells are found, but histiocytes with cellular debris are not prominent and thus the prominent “starry-sky” background is lacking. In contrast to the lymphomas in the iMycEµ mice, these tumor cells were negative for both the B-cell and T-cell markers tested (B220 and CD3), although residual small T-cells and B-cells stained positive (Fig. 3).
Figure 2.
Histology of lymphomas in iMycEµ and K13/iMycEµ mice. Tissue sections of representative spleen and lymph nodes were prepared from WT and tumor (T)-bearing iMycEµ and K13/ iMycEµ mice and stained with H&E. The scale shown is ×200.
Figure 3.
Immunohistochemical staining of lymphomas involving the spleen and lymph nodes in iMycEµ and K13/iMycEµ. Tissue sections were prepared from spleens and lymph nodes of WT and tumor (T)-bearing iMycEµ and K13/iMycEµ mice and stained with B220 and CD3 antibodies as described in the materials and methods. The lymphoma-infiltrated spleens and lymph nodes from K13/iMycEµ mice appear negative for B220 and CD3. The scale shown is ×200.
Expression of K13 is elevated in lymphomas developing in the K13/iMycEµ double transgenic mice.
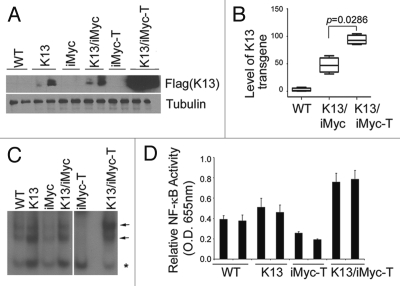
To characterize the contribution of K13 to the pathogenesis of lymphomas developing in the K13/iMycEµ double transgenic mice, splenic extracts from the single and double transgenic mice were analyzed by immunoblotting using a monoclonal antibody against the Flag epitope tag present in the K13 transgene. As shown in Figure 4A, K13 was expressed at comparable levels in the spleens of tumorfree K13 and K13/iMycEµ animals, thereby demonstrating that iMycEµ does not directly affect K13 expression. However, K13 expression was significantly elevated in the lymphomas arising in the K13/iMycEµ animals (Fig. 4A). A real-time PCR (qPCR) analysis revealed that elevated K13 expression in the tumor-bearing K13/iMycEµ mice is associated with amplification of the K13 transgene (Fig. 4B). Collectively, the above results suggest that cells with high K13 expression are selected during lymphomagenesis, supporting its key role in the process.
Figure 4.
Elevated expression of K13 and increased NFκB activity in K13/iMycEµ lymphomas. (A) Western blot showing increased expression of Flag-K13 in the lymphomas developing in the K13/iMycEµ mice. Cell extracts were prepared from spleens of tumor-free and tumor-bearing mice of the indicated genotypes and immunoblotting was done with an antibody against the Flag epitope tag present in the K13 transgene. Tubulin was used as a loading control. (B) A box whisker plot showing median (vertical line in the box), first and third quartiles (box), lowest and highest values for level of K13 transgene (whiskers) as determined by qPCR analysis on genomic DNA. The level of K13 was significantly higher in the tumor (T)-bearing K13/iMycEµ as compared to the tumor-free K13/iMycEµ mice (p = 0.0286; Mann-Whitney U test). (C) Nuclear extracts were prepared from spleens of the wild-type, K13 and tumor-free and tumor (T)-bearing iMycEµ and K13/iMycEµ mice and the NFκB-binding activity measured using an electrophoretic mobility shift assay. The NFκB binding activity is elevated in a lymphoma developing in a K13/iMycEµ double transgenic mice as compared to a lymphoma developing in an iMyc miceEµ. Positions of the NFκB complexes are shown by arrows, while an asterisk marks the position of a constitutive complex. (D) ELISA -based NFκB-binding assay. Nuclear extracts were prepared from spleens of the wild-type, K13 and tumorbearing iMycEµ and K13/iMycEµ mice and the NFκB-binding activity was measured using the TransFactor kit (Clontech). The p65/NFκB activity in nuclear extracts of lymphomas developing in the K13/iMycEµ double-transgenic mice is elevated as compared to the lymphomas developing in the iMycEµ mice or the spleens of wild-type mice. The suffix “T” denotes tumor bearing animals.
Status of the NFκB pathway in single and double transgenic mice with and without tumors.
K13 is a strong activator of the NFκB pathway and uses this pathway to promote cellular survival, proliferation, transformation and cytokine secretion.12,19–21 To determine if the upregulation of K13 expression in the lymphomas developing in the K13/iMycEµ transgenic mice is associated with increased activity of the NFκB pathway, we examined the status of this pathway using an electrophoretic mobility shift assay (EMSA). Consistent with the known ability of K13 to activate the NFκB pathway, nuclear extracts derived from spleens of K13 and tumor free K13/iMycEµ transgenic mice showed modest and equivalent increase in NFκB activity as compared to the wild-type mice (Fig. 4C). However, the NFκB DNA-binding activity was significantly increased in the splenic nuclear extracts from tumor-bearing K13/iMycEµ animals (Fig. 4C; denoted by K13/iMycEµ-T). In contrast, the NFκB activity was diminished in the splenic nuclear extracts from tumor-bearing iMycEµ mice, thereby suggesting that the elevated NFκB activity observed in the tumor-bearing K13/iMycEµ mice is linked to K13 expression and not simply a consequence of increased cellular proliferation accompanying tumor development (Fig. 4C). The above results were confirmed using an ELISA-based NFκB DNA binding assay. This assay measures the recruitment of the p65/RelA subunit of NFκB to a synthetic oligonucleotide duplex containing a consensus NFκB binding motif. As shown in Figure 4D, this assay revealed significant increase in the NFκB DNA-binding activity in the splenic nuclear extracts from tumor-bearing K13/iMycEµ mice as compared to the tumor-free wild-type and K13 mice or the tumor-bearing iMycEµ mice. Thus, the elevated K13 expression in the lymphomas of the K13/iMycEµ mice is associated with the upregulation of the NFκB pathway.
Decreased apoptosis in lymphomas developing in K13/iMycEµ mice.
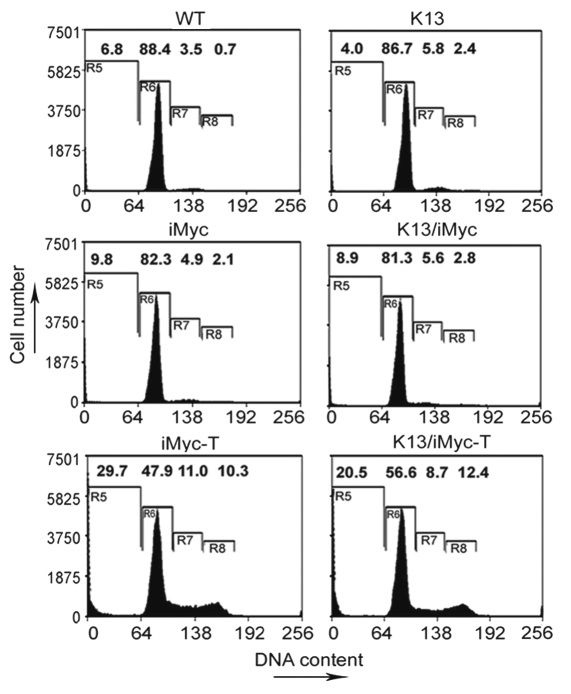
Dysregulated expression of Myc is not only a powerful stimulator of cell cycle progression, but is also known to sensitize cell to apoptosis.23 On the other hand, NFκB pathway is known to both stimulate cell cycle and protect cells against apoptosis.32 We used flow cytometry analysis to examine the status of apoptosis and cell-proliferation in the splenic cells from tumor-bearing and non-tumor-bearing single and double transgenic mice. This analysis revealed that the percentages of cells in the sub-G0/G1 phase, which usually represents apoptotic cells, in the non-tumor bearing WT, K13, iMycEµ and K13/iMycEµ mice were 6.8, 4.0, 9.8 and 8.9%, respectively (Fig. 5). On the other hand, the percentage of cycling splenocytes, as represented by cells in S + G2/M phase, among the above animals were 4.2, 8.2, 7.0 and 8.4%, respectively (Fig. 5). Thus, as compared to the wild-type mice, the apoptotic splenocytes were reduced in the K13 and increased in the iMycEµ and the K13/iMycEµ transgenic mice, while the proliferating splenocytes were increased in all three transgenic mice.
Figure 5.
DNA content analyses showing the percentage of apoptotic and proliferating cells in the tumor-bearing (T) and tumor-free single and double transgenic mice. Cell pellets were fixed in 70% ethanol, resuspended in 0.5 ml of 0.05 mg/ml propidium iodide plus 0.2 mg/ml RNase A and incubated at 37°C for 30 min. Cell cycle distribution was analyzed using a flow cytometer. The numbers represent the percentage of cells in the different phases of cell cycle. R5, Sub-G0/G1; R6, G0/G1; R7, S and R8, G2/M phases.
We next examined the percentage of apoptotic and cycling cells in the spleens of tumor-bearing iMycEµ and K13/iMycEµ mice. Consistent with the known ability of Myc to promote both cell proliferation and apoptosis, we observed significant increase in apoptotic (29.73%) and cycling (21.3%) cells in the spleens of tumor-bearing iMycEµ mice as compared to the wild-type mice (Fig. 5). Interestingly, while the tumor-bearing K13/iMycEµ mice demonstrated a similar increase in the percentage of cycling cells (21.1%) in the spleen, the percentage of apoptotic cells (20.5%) was significantly less as compared to the iMycEµ mice (Fig. 5). These results are consistent with the results of the histological examination and support the hypothesis that the upregulated NFκB pathway accelerates the development of lymphoma in the K13/iMycEµ mice by blocking Myc-induced apoptosis.
Discussion
K13 is one of the few KSHV-encoded proteins that are consistently expressed in PEL cells and gene silencing studies have demonstrated that it is essential for the survival and proliferation of PEL cells.10,12,19–21 K13 is also a powerful activator of the NFκB pathway,9 a pathway known to promote lymphoma development.33 Nevertheless, the incidence of lymphomas in K13 transgenic mice was relatively low and they developed after a long latency period, suggesting that K13 has limited tumorigenic potential on its own and requires cooperative interaction with other oncogenes in lymphomagenesis.22 In this report, we demonstrate increased incidence of lymphoma in the K13/iMycEµ transgenic mice, suggesting that K13 functionally cooperates with Myc to promote lymphomagenesis. A central role of K13 in promoting Myc-induced lymphomas is supported by a significant increase in the K13 expression in the lymphomas developing in the K13/iMycEµ mice. Furthermore, lymphoma cells in the K13/iMycEµ mice demonstrated markedly elevated NFκB activity as compared to the splenocytes from non-tumor bearing animals. Taken collectively, the above results support the hypothesis that cells with elevated K13 expression and NFκB activity are selected during lymphoma development in the K13/iMycEµ mice.
Although structural abnormalities involving the Myc gene are infrequent in PEL,2,28 our results, nonetheless, have important implications for a better understanding of PEL pathogenesis since expression of Myc is frequently dysregulated in PEL cells due to the expression of KSHV-encoded proteins.28–30 Thus, two recent studies reported that although c-Myc is wild-type in PEL, it is abnormally stabilized due to the expression of KSHV LANA protein.28,29 Similar to K13, LANA is one of the KSHV latent proteins that are consistently expressed in KSHV-infected PEL cells. LANA was shown to stabilize Myc by inactivating nuclear GSK3β, which resulted in reduced phosphorylation of c-Myc at Thr58 and decreased c-Myc ubiquitination and degradation.28,29 LANA was also shown to stabilize c-Myc by direct interaction and to stimulate c-Myc transcriptional activity by inducing phosphorylation of its Ser62 residue via ERK activation.29 Not surprisingly, a high proportion of genes modulated by LANA are those regulated by Myc.29,34 Another mechanism for deregulation of c-Myc in PEL was recently described and involved another KSHV latent protein that is constitutively expressed in PEL cells, namely viral interferon regulator factor 3 (vIRF3) or LANA-2.30 The transcriptional activity of Myc is suppressed by Myc modulator-1 (MM-1). vIRF3/LANA-2 was found to bind to MM-1α, which resulted in the release of Myc from MM-1α mediated transcriptional repression and stimulated its transcriptional activity.30 vIRF3/LANA-2 was also shown to directly participate in the activation of c-Myc-mediated transcription independent of its interaction with MM-1α.30 Taken collectively, our results suggest that three of the KSHV latent proteins, K13, LANA-1 and vIRF3/LANA-2, which are constitutively expressed in PEL, can functionally cooperate to promote lymphomagenesis.
What is the mechanism by which K13 promote Myc-induced lymphomas? As discussed above, K13 is a powerful activator of the NFκB pathway, a pathway that is frequently abnormally activated in human lymphomas. For example, abnormal NFκB activation is frequently observed in the activated B cell-like (ABC), primary mediastinal and marginal-zone lymphoma of mucosa-associated lymphatic tissue (MALT) subgroups of diffuse large B-cell lymphomas due to upstream activation of the IKK complex via CARMA1, Bcl10 and MALT1, and has been implicated in their poor response to chemotherapy.35,36 Constitutive NFκB activation has been also linked to the pathogenesis of Adult T-cell lymphoma/leukemia due to the activity of Human T-cell leukemia virus-1 encoded Tax protein.37 Therefore, similar to NHL and ATL, constitutive NFκB activation may promote Myc-induced lymphomas in our double transgenic mice. A more direct evidence for the involvement of the NFκB pathway in promoting lymphomas in the K13/iMycEµ mice comes from our observation that these lymphomas demonstrated markedly elevated NFκB activity as compared to the normal splenocytes or the splenocytes from non-tumor bearing animals.
How does NFκB activation promote Myc-induced lymphomas? Although, Myc is known to stimulate cell growth and proliferation in the presence of growth factors, it is also known to trigger apoptosis, especially under conditions of stress, genotoxic damage or depleted survival factors.23 Indeed, this has led to the hypothesis that the innate apoptotic potential of Myc serves as an in-built foil to its oncogenic capacity.38–41 A remarkable proof of this hypothesis was provided by the ability of Bcl-2 to accelerate formation of B cell lymphomas in Myc transgenic mice by enhancing lymphocyte survival rather than further stimulating their Myc-induced proliferation.42,43 Similarly, another recent study reported accelerated development of plasma cell tumors in double transgenic Myc/Bcl-XL mice, which was accompanied by reduced apoptosis.44 Consistent with the above results, we also observed reduced apoptosis in lymphomas developing in the K13/iMycEµ double transgenic mice as compared to those developing in the iMycEµ single transgenic mice. As the NFκB pathway is known to upregulate the expression of a number of anti-apoptotic genes, such as Bcl2, Mcl-1, c-FLIP and cIAPs,45 it is conceivable that elevated expression of these genes promote the development of lymphomas in the K13/iMycEµ double transgenic mice by protecting against Myc-induced apoptosis. Since the NFκB pathway also upregulates the expression of a number of pro-survival cytokines, it is also conceivable that it protect cells against Myc-induced apoptosis by providing the necessary survival factors.46,47 Other potential mechanisms by which K13-induced NFκB may promote Myc-induced lymphomas include inhibition of autophagy, protection against genotoxic stress and stimulation of cell cycle.32,48,49
K13 not only accelerated the development of Myc-induced lymphomas, it also affected their immunophenotype. Thus, unlike lymphomas in the iMycEµ mice that were B220 positive, the K13/iMycEµ double transgenic mice had tumors that lacked the expression of mature B cell as well as T-cell markers. Since PEL cells are also known to lack the expression of most B-cell markers,1,6 it is conceivable that constitutive expression of K13 may contribute to their null-phenotype. Although the exact mechanism by which K13 promotes the development of lymphomas lacking B-cell markers is not clear at the present, it is noteworthy that loss of B-cell markers is not unique to PEL but is also observed in classical Hodgkin disease. Indeed, strong constitutive activation of NFκB was found to be a characteristic of both Hodgkin lymphoma cell lines50 and Reed-Sternberg cells.51 Since constitutive activation of the NFκB pathway is shared by both PEL and classical Hodgkin disease,10,18,50,51 it is possible that this pathway is functionally involved in the downregulation of B cell markers in the lymphomas developing in our K13/iMycEµ double transgenic mice and in the “null-phenotype” of PEL. However, it is important to point out that the lymphomas in the K13/iMycEµ double transgenic mice do not recapitulate all pathological features of PEL, such as the involvement of body cavities, suggesting the involvement of alterations in additional viral and cellular genes in the pathogenesis of PEL.
The significance of our results, however, is not limited to PEL. Although abnormalities involving the NFκB and the Myc pathways are two of the most frequently described abnormalities in human lymphomas,36,52,53 the functional interaction between these two pathways has not been studied to date. The K13/iMycEµ double transgenic model described here may not only provide new insights into the pathogenesis of human lymphomas but also serve as a useful animal model for the testing novel preventive and therapeutic strategies.
Materials and Methods
Mice.
Transgenic K13 mice have been described previously.22 Transgenic iMycEµ mice have been described previously31 and were obtained from Dr. Siegfried Janz through the NCI Mouse Models of Human Cancers Consortium (MMHCC). K13 and iMycEµ genotypes were maintained in the heterozygous conditions by crossing with wild-type animals in C57BL/6 and BALB/c backgrounds. Double transgenic K13/iMycEµ (KI) mice were produced by cross-breeding the single transgenic K13 and iMycEµ mice. Genotyping of mice was carried out by PCR, as described previously.22,31 All animal procedures were conducted according to an IACUC-approved protocol in the Hillman Cancer Center animal facility.
Flow cytometry and cell cycle analysis.
Spleens and lymph nodes were used to make single cell suspensions as described previously.22 Single cell suspensions of spleen were fixed in 70% ethanol, stained with propidium iodide and then analyzed for cell cycle as described previously.54
Histological analysis and immunohistochemistry.
Spleens and lymph nodes were fixed with 10% formalin and embedded in paraffin. Five-micrometer sections were cut and stained with hematoxylin and eosin. Immunostaining of tissues with B220/CD45R and CD3 antibodies was done as described previously.22
NFκB assay.
DNA binding activity of the p65/RelA NFκB subunit was measured in triplicate in the nuclear extracts using the ELISA-based TransFactor kit (Clontech) following the manufacturer's recommendations.
Electrophoretic mobility shift assay (EMSA).
An EMSA for determining the presence of nuclear NFκB activity was performed as described previously.9
Western blots.
Splenocytes were washed once in ice-cold PBS and lysed on ice for 30 min in RIPA lysis buffer (50 mM Tris, pH 8/0.5% Na deoxycholate/150 mM NaCl/1% Triton X-100/0.1% SDS) supplemented with one protease inhibitor mixture tablet (Roche Applied Science, Indianapolis) per 10 ml of lysis buffer. Lysates were cleared of cellular debris by centrifugation at 20,000x g at 4°C for 10 min. Protein concentration was determined by using the Bio-Rad Protein Assay. Thirty micrograms of protein was separated on SDS/PAGE and western blot was performed essentially as described.22 Antibodies were used at the following dilutions: anti-FLAG (M2) horseradish peroxidase (1:10,000; Sigma) and anti-tubulin (1:10,000; Sigma).
Real-time quantitative PCR (qPCR).
Real-time PCR reactions were performed, on genomic DNA isolated from indicated animals, in triplicate using Step one plus system (Applied Biosystem) and SYBR green-Taq polymerase mix to determine the relative change in the level of K13 transgene. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was used as a normalization control. The PCR thermoprofile consisted of initial activation of the polymerase at 95°C for 10 min followed by 40 PCR cycles of 95°C for 15 s, 60°C for 1 min followed by a melting curve to verify the presence of a single amplification product Real-time PCR data (Ct values) was analyzed using the 2−ΔΔC method and the data presented as fold change in target gene expression ± standard error of mean. Primers used for real-time PCR are K13 Forward: GGA TGC CCT AAT GTC AAT GC, K13 Reverse: GGC GAT AGT GTT GGA GT GT, G3PDH Forward: TAC TAG CGG TTT TAC GGG CG G3PDH Reverse: TCG AAC AGG AGG AGC AGA GAG CGA. The statistical significance of the level of K13 transgene was determined by nonparametric Mann-Whitney U test using Graph Pad Prism 5.02 (Graph Pad software, La Jolla). All tests were two-tailed and p values less than 0.05 were considered significant.
Acknowledgements
We thank Marie Acquafondata (Tissue and Research Pathology Services, University of Pittsburgh) for help with immunohistochemistry, William Grubb (University of Pittsburgh) for help with data collection, William Chow (Carnegie Mellon University) for mouse genotyping and Yanqiang Yang for helpful discussions. This work was supported by grants from the National Institutes of Health (CA85177 and HL085189), the Leukemia & Lymphoma Society and the Multiple Myeloma Research Foundations.
Abbreviations
- PEL
primary effusion lymphoma
- KSHV
Kaposi sarcoma-associated herpesvirus
- HHV8
human herpesvirus 8
- IKK
I-kappaB kinase
- vFLIP
viral flice inhibitory protein
- NFκB
nuclear factor kappaB
- LANA
latent nuclear antigen
- vIRF3
viral interferon regulatory factor 3
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/13291
References
- 1.Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569–576. doi: 10.1634/theoncologist.12-5-569. [DOI] [PubMed] [Google Scholar]
- 2.Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J, et al. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- 3.Simonelli C, Spina M, Cinelli R, Talamini R, Tedeschi R, Gloghini A, et al. Clinical features and outcome of primary effusion lymphoma in HIV-infected patients: a single-institution study. J Clin Oncol. 2003;21:3948–3954. doi: 10.1200/JCO.2003.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 5.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 6.Drexler HG, Uphoff CC, Gaidano G, Carbone A. Lymphoma cell lines: in vitro models for the study of HHV-8+ primary effusion lymphomas (body cavity-based lymphomas) Leukemia. 1998;12:1507–1517. doi: 10.1038/sj.leu.2401160. [DOI] [PubMed] [Google Scholar]
- 7.Sturzl M, Hohenadl C, Zietz C, Castanos-Velez E, Wunderlich A, Ascherl G, et al. Expression of K13/v-FLIP gene of human herpesvirus 8 and apoptosis in Kaposi's sarcoma spindle cells. J Natl Cancer Inst. 1999;91:1725–1733. doi: 10.1093/jnci/91.20.1725. [DOI] [PubMed] [Google Scholar]
- 8.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary PM, Jasmin A, Eby MT, Hood L. Modulation of the NFkappaB pathway by virally encoded death effector domains-containing proteins. Oncogene. 1999;18:5738–5746. doi: 10.1038/sj.onc.1202976. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Eby MT, Rathore N, Sinha SK, Kumar A, Chaudhary PM. The human herpes virus 8-encoded viral flice inhibitory protein physically associates with and persistently activates the ikappab kinase complex. J Biol Chem. 2002;277:13745–13751. doi: 10.1074/jbc.M110480200. [DOI] [PubMed] [Google Scholar]
- 11.Field N, Low W, Daniels M, Howell S, Daviet L, Boshoff C, et al. KSHV vFLIP binds to IKK-{gamma} to activate IKK. J Cell Sci. 2003;116:3721–3728. doi: 10.1242/jcs.00691. [DOI] [PubMed] [Google Scholar]
- 12.Matta H, Chaudhary PM. Activation of alternative NFkappaB pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1beta-converting enzyme inhibitory protein (vFLIP) Proc Natl Acad Sci USA. 2004;101:9399–9404. doi: 10.1073/pnas.0308016101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Q, Matta H, Chaudhary PM. The human herpes virus 8-encoded viral FLICE inhibitory protein protects against growth factor withdrawal-induced apoptosis via NFkappaB activation. Blood. 2003;101:1956–1961. doi: 10.1182/blood-2002-07-2072. [DOI] [PubMed] [Google Scholar]
- 14.Sun Q, Zachariah S, Chaudhary PM. The human herpes virus 8-encoded viral FLICE-inhibitory protein induces cellular transformation via NFkappaB activation. Journal of Biological Chemistry. 2003;278:52437–52445. doi: 10.1074/jbc.M304199200. [DOI] [PubMed] [Google Scholar]
- 15.Sun Q, Matta H, Lu G, Chaudhary PM. Induction of IL-8 expression by human herpesvirus 8 encoded vFLIP K13 via NFkappaB activation. Oncogene. 2006;25:2717–2726. doi: 10.1038/sj.onc.1209298. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Ganem D. Induction of chemokine production by latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells. J Gen Virol. 2007;88:46–50. doi: 10.1099/vir.0.82375-0. [DOI] [PubMed] [Google Scholar]
- 17.Grossmann C, Podgrabinska S, Skobe M, Ganem D. Activation of NFkappaB by the latent vFLIP gene of Kaposi's sarcoma-associated herpesvirus is required for the spindle shape of virus-infected endothelial cells and contributes to their proinflammatory phenotype. J Virol. 2006;80:7179–7185. doi: 10.1128/JVI.01603-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller SA, Schattner EJ, Cesarman E. Inhibition of NFkappaB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood. 2000;96:2537–2542. [PubMed] [Google Scholar]
- 19.Guasparri I, Keller SA, Cesarman E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J Exp Med. 2004;199:993–1003. doi: 10.1084/jem.20031467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Godfrey A, Anderson J, Papanastasiou A, Takeuchi Y, Boshoff C. Inhibiting primary effusion lymphoma by lentiviral vectors encoding short hairpin RNA. Blood. 2005;105:2510–2518. doi: 10.1182/blood-2004-08-3052. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Punj V, Matta H, Mazzacurati L, Schamus S, Yang Y, et al. K13 blocks kshv lytic replication and deregulates vil6 and hil6 expression: a model of lytic replication induced clonal selection in viral oncogenesis. PLoS ONE. 2007;2:1067. doi: 10.1371/journal.pone.0001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chugh P, Matta H, Schamus S, Zachariah S, Kumar A, Richardson JA, et al. Constitutive NFkappaB activation, normal Fas-induced apoptosis and increased incidence of lymphoma in human herpes virus 8 K13 transgenic mice. Proc Natl Acad Sci USA. 2005;102:12885–12890. doi: 10.1073/pnas.0408577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 24.Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci USA. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao PH, Houldsworth J, Dyomina K, Parsa NZ, Cigudosa JC, Louie DC, et al. Chromosomal and gene amplification in diffuse large B-cell lymphoma. Blood. 1998;92:234–240. [PubMed] [Google Scholar]
- 26.Bhatia K, Huppi K, Spangler G, Siwarski D, Iyer R, Magrath I. Point mutations in the c-Myc transactivation domain are common in Burkitt's lymphoma and mouse plasmacytomas. Nat Genet. 1993;5:56–61. doi: 10.1038/ng0993-56. [DOI] [PubMed] [Google Scholar]
- 27.Yano T, Sander CA, Clark HM, Dolezal MV, Jaffe ES, Raffeld M. Clustered mutations in the second exon of the MYC gene in sporadic Burkitt's lymphoma. Oncogene. 1993;8:2741–2748. [PubMed] [Google Scholar]
- 28.Bubman D, Guasparri I, Cesarman E. Deregulation of c-Myc in primary effusion lymphoma by Kaposi's sarcoma herpesvirus latency-associated nuclear antigen. Oncogene. 2007;26:4979–4986. doi: 10.1038/sj.onc.1210299. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Martin HJ, Liao G, Hayward SD. The kaposi sarcoma associated herpesvirus lana protein stabilizes and activates c-myc. J Virol. 2007;81:10451–10459. doi: 10.1128/JVI.00804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lubyova B, Kellum MJ, Frisancho JA, Pitha PM. Stimulation of c-Myc transcriptional activity by vIRF-3 of Kaposi sarcoma-associated herpesvirus. J Biol Chem. 2007;282:31944–31953. doi: 10.1074/jbc.M706430200. [DOI] [PubMed] [Google Scholar]
- 31.Park SS, Kim JS, Tessarollo L, Owens JD, Peng L, Han SS, et al. Insertion of c-Myc into Igh induces B-cell and plasma-cell neoplasms in mice. Cancer Res. 2005;65:1306–1315. doi: 10.1158/0008-5472.CAN-04-0268. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Karin M, Cao Y, Greten FR, Li ZW. NFkappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 34.An FQ, Compitello N, Horwitz E, Sramkoski M, Knudsen ES, Renne R. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from p16INK4A-induced cell cycle arrest. J Biol Chem. 2005;280:3862–3874. doi: 10.1074/jbc.M407435200. [DOI] [PubMed] [Google Scholar]
- 35.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jost PJ, Ruland J. Aberrant NFkappaB signaling in lymphoma: mechanisms, consequences and therapeutic implications. Blood. 2007;109:2700–2707. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- 37.Ratner L. Human T cell lymphotropic virus-associated leukemia/lymphoma. Curr Opin Oncol. 2005;17:469–473. doi: 10.1097/01.cco.0000174037.84903.fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 39.Evan G, Harrington E, Fanidi A, Land H, Amati B, Bennett M. Integrated control of cell proliferation and cell death by the c-myc oncogene. Philos Trans R Soc Lond B Biol Sci. 1994;345:269–275. doi: 10.1098/rstb.1994.0105. [DOI] [PubMed] [Google Scholar]
- 40.Amati B, Alevizopoulos K, Vlach J. Myc and the cell cycle. Front Biosci. 1998;3:250–268. doi: 10.2741/a239. [DOI] [PubMed] [Google Scholar]
- 41.Prendergast GC. Mechanisms of apoptosis by c-Myc. Oncogene. 1999;18:2967–2987. doi: 10.1038/sj.onc.1202727. [DOI] [PubMed] [Google Scholar]
- 42.Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 43.Strasser A, O'Connor L, Huang DC, O'Reilly LA, Stanley ML, Bath ML, et al. Lessons from bcl-2 transgenic mice for immunology, cancer biology and cell death research. Behring Inst Mitt. 1996:101–117. [PubMed] [Google Scholar]
- 44.Cheung WC, Kim JS, Linden M, Peng L, Van Ness B, Polakiewicz RD, et al. Novel targeted deregulation of c-Myc cooperates with Bcl-X(L) to cause plasma cell neoplasms in mice. J Clin Invest. 2004;113:1763–1773. doi: 10.1172/JCI20369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turco MC, Romano MF, Petrella A, Bisogni R, Tassone P, Venuta S. NFkappaB/Rel-mediated regulation of apoptosis in hematologic malignancies and normal hematopoietic progenitors. Leukemia. 2004;18:11–17. doi: 10.1038/sj.leu.2403171. [DOI] [PubMed] [Google Scholar]
- 46.Karin M, Delhase M. The IkappaB kinase (IKK) and NFkappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 47.Karin M. Nuclear factor-[kappa]B in cancer development and progression. Nature. 2006;441:431. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 48.Lee JS, Li Q, Lee JY, Lee SH, Jeong JH, Lee HR, et al. FLIP-mediated autophagy regulation in cell death control. Nat Cell Biol. 2009;11:1355–1362. doi: 10.1038/ncb1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thurau M, Marquardt G, Gonin-Laurent N, Weinlander K, Naschberger E, Jochmann R, et al. Viral inhibitor of apoptosis vFLIP/K13 protects endothelial cells against superoxide-induced cell death. J Virol. 2009;83:598–611. doi: 10.1128/JVI.00629-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bargou RC, Emmerich F, Krappmann D, Bommert K, Mapara MY, Arnold W, et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Invest. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bargou RC, Leng C, Krappmann D, Emmerich F, Mapara MY, Bommert K, et al. High-level nuclear NFkappaB and Oct-2 is a common feature of cultured Hodgkin/Reed-Sternberg cells. Blood. 1996;87:4340–4347. [PubMed] [Google Scholar]
- 52.Packham G. The role of NFkappaB in lymphoid malignancies. Br J Haematol. 2008;143:3–15. doi: 10.1111/j.1365-2141.2008.07284.x. [DOI] [PubMed] [Google Scholar]
- 53.Rui L, Goodnow CC. Lymphoma and the control of B cell growth and differentiation. Curr Mol Med. 2006;6:291–308. doi: 10.2174/156652406776894563. [DOI] [PubMed] [Google Scholar]
- 54.Lu G, Punj V, Chaudhary PM. Proteasome inhibitor Bortezomib induces cell cycle arrest and apoptosis in cell lines derived from Ewing's sarcoma family of tumors and synergizes with TRAIL. Cancer Biol Ther. 2008;7:603–608. doi: 10.4161/cbt.7.4.5564. [DOI] [PubMed] [Google Scholar]