Abstract
Glioblastomas continue to carry poor prognoses for patients despite advances in surgical, chemotherapeutic and radiation regimens. One feature of glioblastoma associated with poor prognosis is the degree of hypoxia and expression levels of hypoxia-inducible factor-1α (HIF-1α). HIF-1α expression allows metabolic adaptation to low oxygen availability, partly through upregulation of VEGF and increased tumor angiogenesis. Here, we demonstrate an induced level of astrocyte-elevated gene-1 (AEG-1) by hypoxia in glioblastoma cells. AEG-1 has the capacity to promote anchorage-independent growth and cooperates with Ha-ras in malignant transformation. In addition, AEG-1 was recently demonstrated to serve as an oncogene and can induce angiogenesis in glioblastoma. Results from in vitro studies show that hypoxic induction of AEG-1 is dependent on HIF-1α stabilization during hypoxia and that PI3K inhibition abrogates AEG-1 induction during hypoxia through loss of HIF-1α stability. Furthermore, we show that AEG-1 is induced by glucose deprivation and that prevention of intracellular reactive oxygen species (ROS) production prevents this induction. Additionally, AEG-1 knockdown results in increased ROS production and increased glucose deprivation-induced cytotoxicity. On the other hand, AEG-1 overexpression prevents ROS production and decreases glucose deprivation-induced cytotoxicity, indicating that AEG-1 induction is necessary for cells to survive this type of cell stress. These observations link AEG-1 overexpression in glioblastoma with hypoxia and glucose deprivation and targeting these physiological pathways may lead to therapeutic advances in the treatment of glioblastoma in the future.
Key words: AEG-1, glioblastoma, hypoxia, glucose deprivation, necrosis
Introduction
Glioblastomas continue to rank among the most lethal primary human tumors. Despite treatment with the most rigorous surgical interventions along with the most optimal chemotherapeutic and radiation regimens, the median survival is just 12–15 months for patients with glioblastoma. Among the histological hallmarks of glioblastoma, necrosis has been demonstrated to be a powerful predictor of poor patient prognosis. Necrosis is induced within glioblastoma subpopulations by oxygen and nutrient deprivation, which result in cellular migration away from oxygen- and nutrient-deprived areas and necrotic death in remaining cells. Both hypoxia and glucose deprivation have been demonstrated to induce necrotic cell death in vitro.1,2 Over the years, there have been many advances in our understanding of the molecular mechanisms underlying glioblastoma formation, yet the mechanisms that lead to tumor necrosis remain unclear.
Recent studies show that the TNFα-inducible gene, astrocyte-elevated gene-1 (AEG-1), is overexpressed in more than 95% of human brain tumors3 and is involved in many features of oncogenesis, including increased tumor proliferation,4 invasion4 and augmentation of cellular transformation.4 Furthermore, AEG-1 has been shown to be significantly upregulated and to correlate with decreased patient prognosis in esophageal cancer,5 breast cancer,6 prostate cancer,7 non-small cell lung cancer8 and hepatocellular carcinoma.9 Recently, AEG-1 has also been shown to be pro-angiogenic both in vitro and in vivo, and can also augment expression of key angiogenesis molecules, such as angiopoietin-1 (Ang1), matrix metalloprotease (MMP)-2 and HIF-1α.4 In addition, it was recently shown that AEG-1 expression is increased in high-grade astrocytomas,10 which are marked by focal areas of hypoxia and necrosis. These studies indicate that the increased expression of AEG-1 observed in glioblastoma may provide these tumors with a significant growth potential and may lead to decreased patient survival.
Though there are many studies that have investigated the effect of AEG-1 on signaling pathways, cell survival and oncogenesis, there have not been many studies investigating regulation of endogenous AEG-1 expression. In light of previous studies in glioblastoma, we investigated the regulation of AEG-1 in glioblastoma by the physiological processes of hypoxia and glucose deprivation. We found that AEG-1 is induced by both hypoxia and glucose deprivation in glioblastoma cells and that hypoxic induction of AEG-1 depends on the stabilization of HIF-1α through signaling of the phosphatidylinositol 3-kinase (PI3K) pathway. Furthermore, we found that AEG-1 induction by glucose deprivation depends on reactive oxygen species (ROS) production and that AEG-1 itself can limit ROS accumulation. In addition, we have found that AEG-1 plays a key role in survival from glucose deprivation-induced cytotoxicity, implicating AEG-1 as a pivotal mediator of cell survival during glucose starvation.
Because tumor hypoxia and subsequent necrosis remain strong indicators of poor prognosis in glioblastoma, the role of oncogenes in these glioblastoma processes warrants further investigation. As a gene involved in oncogenesis, anti-apoptosis and chemoresistance, AEG-1 represents a significant target in the treatment of glioblastoma.11 Since the many molecular and functional pathways involving AEG-1 activation may converge on the necrosis cascade, AEG-1 overexpression may portend poor prognosis in glioblastoma. By better understanding the role of AEG-1 in glioblastoma pathogenesis, it may be possible to investigate the role of AEG-1 as a novel biomarker in glioblastoma and to identify therapeutic targets of the AEG-1 pathway to improve patient prognosis in the future.
Results
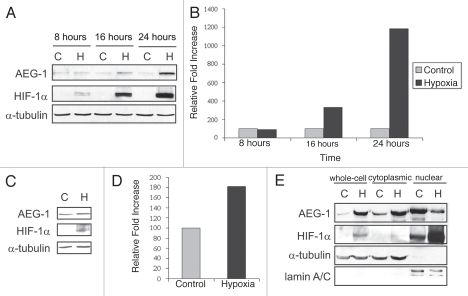
Astrocyte-elevated gene-1 (AEG-1) is an oncogene involved in many aspects of tumorigenesis, including enhanced anchorage-independent growth,12 angiogenesis,4 protection from serum starvation-induced apoptosis13 and downregulation of pro-apoptotic factors13 and AEG-1 has also been shown to be induced by HIF-1α in vitro.4 However, there have been few studies examining the regulation of endogenous AEG-1 under various pathophysiological conditions and the impact of hypoxia on AEG-1 expression remains unknown. To investigate the effect of hypoxia on AEG-1 expression in glioblastoma, we exposed the glioblastoma cell line, U87-MG, as well as a non-established mixed primary glioblastoma cell culture, GLI87, to a 24-hour time-course of hypoxia. We found that AEG-1 was significantly upregulated following 16- and 24-hour hypoxia in U87-MG cells and following 24-hour hypoxia in GLI87 cells (Fig. 1A–D). We found similar results in the T98-G and U118-MG cell lines (unpublished results). This upregulation appeared to occur mostly at the post-transcriptional level since levels of AEG-1 mRNA were unchanged under hypoxic conditions (unpublished results).
Figure 1.
Hypoxia induces AEG-1 expression in vitro. (A) AEG-1 expression during 24-hour time-course of hypoxia in vitro. U87MG cells were exposed to 8, 16 or 24 hours of hypoxia or normoxia. Hypoxia was achieved by placing cells in a sealed chamber and then flushing the chamber with a mixture of 95% N2, 5% CO2 mixture for 15 minutes. Cells were then placed in 37°C for the indicated time-points, after which whole-cell extracts were collected. HIF-1α staining was used to ensure that hypoxia was achieved. (B) Quantification of hypoxic induction of AEG-1. (C) The primary, non-established mixed glioblastoma cell culture, GLI87, was exposed to hypoxia or normoxia for 24 hours, and the expression of AEG-1 was assessed. (D) Quantification of hypoxic induction of AEG-1 in GLI87 cells. (E) Analysis of the sub-cellular localization of AEG-1 during hypoxia. U87MG cells were treated with hypoxia or normoxia as before, and whole-cell, cytoplasmic and nuclear extracts were collected. Lamin A/C expression was used to confirm nuclear extract purity.
We also investigated the cellular localization of AEG-1 during hypoxia to determine whether hypoxia alters AEG-1 trafficking. AEG-1 has been shown to be localized predominantly in a perinuclear distribution and associated with the ER membrane,3 though some groups have also found AEG-1 in the nucleus.14 Though the nuclear import of AEG-1 is stimulated by TNFα, a downstream target of hypoxia, through three putative nuclear localization signals,15 we found that hypoxia induces cytoplasmic accumulation of AEG-1 rather than increased AEG-1 nuclear import (Fig. 1E). This corresponded to an increase in nuclear HIF-1α, which binds to promoters containing hypoxia response elements (HREs). Interestingly, it has been demonstrated that cytoplasmic accumulation of AEG-1 is significantly associated with tumorigenic and metastatic disease.16 Therefore, it appears that cytoplasmic AEG-1 may play a role in the response to cellular hypoxia and may indicate poor prognosis in glioblastoma patients as well.
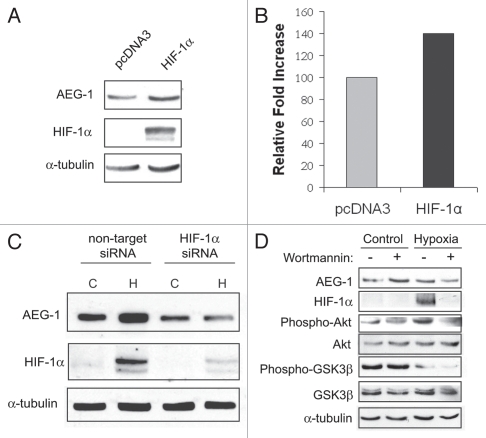
We then sought to identify some of the mediators of hypoxia-induced AEG-1 expression. One feature of glioblastoma associated with poor prognosis is the degree of hypoxia and expression levels of hypoxia-inducible factor-1α (HIF-1α). HIF-1α expression allows metabolic adaptation to low oxygen availability, partly through upregulation of VEGF and increased tumor angiogenesis. HIF-1α is one of the best studied downstream effectors of hypoxia and HIF-1α has been associated with necrosis in a variety of tumors. Therefore, we analyzed the expression of AEG-1 after transient transfection of U87-MG cells with HIF-1α. We found that AEG-1 is slightly upregulated following 24-hour transfection with HIF-1α (Fig. 2A and B). We then sought to determine whether HIF-1α is required for AEG-1 upregulation following hypoxia. For these experiments, we transfected U87-MG cells with HIF-1α siRNA or non-targeting siRNA and after 24-hour treatment, we exposed these cells to hypoxia for 24 hours. We found that HIF-1α is required for AEG-1 induction upon hypoxia, since HIF-1α siRNA transfection prevented AEG-1 upregulation following hypoxia (Fig. 2C). These data indicate that HIF-1α may not be sufficient but is necessary for AEG-1 induction following hypoxia. Since the PI3K pathway has been demonstrated to regulate AEG-1 expression in glioblastoma cells10 and because inhibition of this pathway prevents HIF-1α stabilization during hypoxia,17 we investigated the role of the PI3K pathway in AEG-1 induction during hypoxia. Incubation of U87-MG cells with a specific inhibitor of PI3K, wortmannin (25 uM), during hypoxia partially prevented its induction and completely prevented HIF-1α induction (Fig. 2D). Therefore, it may be possible that hypoxic induction of AEG-1 depends on either HIF-1α stabilization or alternative HIF-1α-independent, PI3K-dependent pathways.
Figure 2.
HIF-1α is necessary but not sufficient for AEG-1 induction during hypoxia. (A) U87-MG cells were transfected with HIF-1α or empty vector for 24 hours, and the expression of AEG-1 was analyzed by western blot. (B) U87-MG cells were transfected with either non-target siRNA or HIF-1α siRNA and after 24 hours were placed in normoxia (C) or hypoxia (H) for an additional 24 hours. (C) U87-MG cells were incubated with the PI3K inhibitor, wortmannin (25 uM), just before 24 hour hypoxia or normoxia. AEG-1 expression as well as the phosphorylated and total levels of Akt and GSK3β were evaluated. HIF-1α expression was used to monitor hypoxic induction.
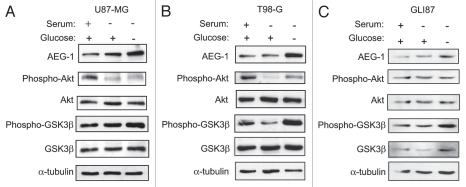
Since hypoxic cells utilize glucose at an increased rate through upregulation of membrane glucose transporters, we hypothesized that hypoxic upregulation of AEG-1 may be mediated through alterations in glucose metabolism in glioblastoma cells. To investigate this hypothesis, we treated U87-MG cells, T98-G cells and GLI87 glioblastoma cells with medium lacking serum or with medium lacking both serum and glucose. We found that AEG-1 was significantly upregulated in each of these cell types by glucose deprivation but not to a large extent by serum starvation (Fig. 3A–C). These data indicate that AEG-1 expression may be influenced by shifts in energy metabolism due to glucose deprivation. Furthermore, HIF-1α stabilization during hypoxia may activate these pathways through increased utilization of cellular glucose stores.
Figure 3.
AEG-1 is induced by glucose deprivation in glioblastoma cells. (A) U87-MG cells, (B) T98-G cells and (C) GLI87 cells were treated with DMEM containing 1 g/L glucose and 10% FBS or no FBS or DMEM without FBS and without glucose for 8 hours. AEG-1 expression was assessed in each cell line as well as the phosphorylated and total amounts of the members of the PI3K signaling pathway, Akt and GSK3β.
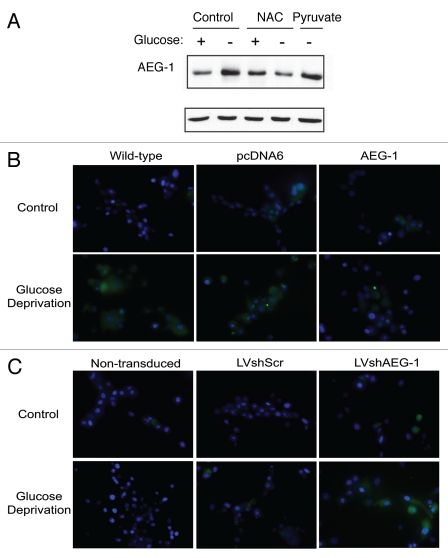
Because HIF-1α is not stabilized by glucose deprivation alone, AEG-1 induction by low glucose must occur through alternate mechanisms. It is well known that glucose deprivation induces production of reactive oxygen species (ROS) through decreased production of NADPH, the reduced form of nicotinamide adenine dinucleotide phosphate (NADP+) and a loss of reductive potential in the cell.18 Therefore, we hypothesized that ROS production during glucose deprivation may play a role in AEG-1 induction. Treatment of U87-MG cells with the thiol antioxidant, N-acetylcysteine (NAC), completely prevented AEG-1 upregulation from glucose deprivation (Fig. 4A). However, treatment of these cells with pyruvate, which has also been shown to act as an intracellular scavenger of ROS,19 did not prevent AEG-1 induction. Pyruvate is likely a weaker anti-oxidant than NAC and thus may not reduce ROS production to the same extent. These data demonstrate that the induction of ROS during glucose deprivation is critical for the observed increase in AEG-1 expression.
Figure 4.
AEG-1 upregulation during glucose deprivation is dependent on reactive oxygen species production. (A) U87-MG cells were incubated with 24 mM N-acetylcysteine (NAC), 1 mM pyruvate or were untreated and then exposed to glucose deprivation or control medium for 8 hours. The expression of AEG-1 was assessed in whole-cell extracts. (B) Wild-type U87-MG cells or U87-MG cells stably expressing AEG-1 or empty vector were exposed to glucose deprivation or control medium for 16 hours and ROS production was measured using the fluorescent dye, 25 µM carboxy-H2-DCFDA. Hoechst staining was also performed to label nuclei. (C) Non-transduced, lentiviral scrambled shRNA (LVshScr)-transduced or lentiviral AEG-1 shRNA (LVshAEG-1)-transduced U87-MG cells were exposed to 9 hours of glucose deprivation, and ROS production was measured as in (B).
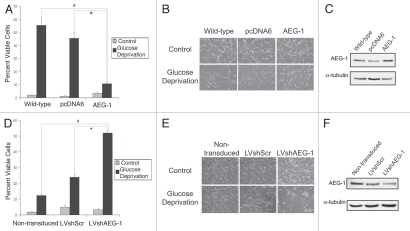
To examine the importance of AEG-1 expression on ROS production during glucose deprivation, we exposed U87-MG cells stably overexpressing AEG-1 to glucose deprivation and measured resultant ROS production. Using these cells, we found that ROS production was significantly reduced as compared to wild-type or empty vector-transfected cells (Fig. 4B). In addition, in U87-MG cells with stable expression of lentiviral AEG-1 shRNA, we found increased ROS production as compared to non-transduced and lentiviral scrambled-transduced controls (Fig. 4C). These findings suggest that AEG-1 induction during glucose deprivation serves to prevent ROS accumulation that would lead to subsequent cytotoxicity.
We next wanted to investigate the functional significance of glucose deprivation on glioblastoma cell survival. Since glucose deprivation induces rapid cytotoxicity because of the resulting lack of reductive capacity within the cell, we investigated the impact of AEG-1 modulation on glucose deprivation-induced cell death. We found that U87-MG cells stably overexpressing AEG-1 exhibited significantly less glucose deprivation-mediated cytotoxicity than empty vector-transfected or wild-type cells (Fig. 5A–C). In addition, AEG-1 knockdown using lentiviral AEG-1 shRNA significantly increased cytotoxicity as compared to non-transduced and scrambled shRNA-transduced cells (Fig. 5D–F). These findings suggest that AEG-1 induction during glucose deprivation may be protective against rapid cell death and may select for highly resistant glioblastoma cells that are able to withstand various cellular stressors.
Figure 5.
AEG-1 overexpression prevents glucose deprivation-induced cytotoxicity. (A) Wild-type U87-MG cells or U87-MG cells stably expressing either AEG-1 or empty vector were exposed to glucose deprivation for 16 hours and were then counted by trypan blue exclusion assay. The total number of cells as well as the number of non-viable cells were counted in each field. Four hundred cells were counted in three separate fields per plate, and each experiment was conducted in triplicate. Values are presented as the number of viable cells divided by the total number of cells for each field. Asterisks indicate statistically significant differences where indicated (p < 0.05). (B) Phase-contrast images of wild-type, empty vector-expressing and AEG-1-expressing U87-MG cells exposed to glucose deprivation in (A). (C) Western blot for AEG-1 expression in wild-type, empty vector-expressing and AEG-1-expressing U87-MG cells. (D) Non-transduced U87-MG cells or U87-MG cells stably expressing either lentiviral scrambled shRNA (LVshScr) or lentiviral AEG-1 shRNA (LVshAEG-1) were exposed to glucose deprivation for 10 hours and were then counted by trypan blue exclusion assay as in (A). (E) Phase-contrast images of non-transduced, LVshScr-transduced and LVshAEG-1-transduced U87-MG cells exposed to glucose deprivation in (D). (F) Western blot of AEG-1 expression in non-transduced, LVshScr-transduced and LV-shAEG-1-transduced U87-MG cells.
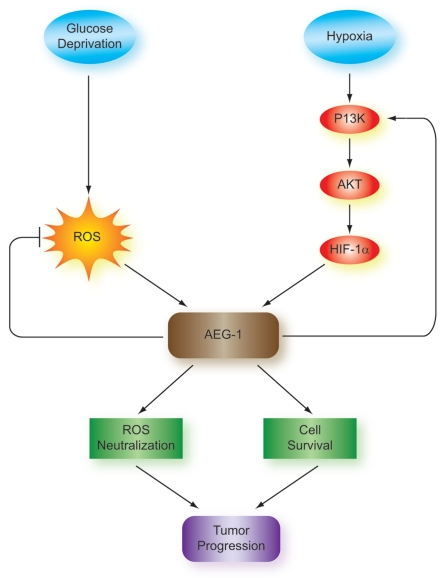
Given findings of AEG-1 overexpression in highly malignant glioblastoma samples and in many other tumor types, AEG-1 may serve as a poor prognostic indicator in glioblastoma. In addition, AEG-1 induction by hypoxia in a HIF-1α-dependent manner and by glucose deprivation in vitro suggests that tumoral hypoxia, increased HIF-1α expression and nutrient deprivation within developing areas of necrosis may lead to AEG-1 induction and tumor progression (Fig. 6). Therefore, this process may induce increased cell survival as a result of AEG-1 upregulation, selecting for highly resistant cell subpopulations able to withstand stress signals within the local tumor microenvironment. Future experiments will further assess the mechanism of AEG-1 upregulation during hypoxia and glucose deprivation and the biological significance of this AEG-1 upregulation and its correlation with necrosis in glioblastoma.
Figure 6.
Mechanism and significance of AEG-1 induction by hypoxia and glucose deprivation in glioblastoma. Glucose deprivation and hypoxia lead to AEG-1 induction in glioblastoma. AEG-1 induction by glucose deprivation depends on the formation of reactive oxygen species (ROS), which are formed during periods of low supplies of NADPH, the reduced form of nicotinamide adenine dinucleotide phosphate (NADP+). Hypoxic induction of AEG-1 acts through the PI3K/Akt pathway to stabilize HIF-1α and AEG-1 feeds back to activate PI3K and create a positive feedback loop. The result of AEG-1 induction is enhanced cell survival during periods of glucose deprivation as well as prevention of ROS induction, which contribute to tumor progression and poor prognosis in glioblastoma.
Discussion
The oncogenic role of AEG-1 has been indicated by its activation of NFκB signaling,15 induction by Ha-Ras through the phosphatidylinositol 3-kinase/Akt pathway20 and activation of angiogenesis.4 On a functional level, AEG-1 has been shown to mediate cell survival under serum starvation conditions,13 prevent chemo-sensitivity to chemotherapeutic agents21 and induce migration, anchorage-independent growth and tumor formation in nude mice.4 We now show that hypoxia, another process associated with tumorigenesis and malignant transformation, leads to AEG-1 induction in glioblastoma cells. Our data demonstrate that hypoxia induces enhanced AEG-1 expression in glioblastoma cells, a process that is dependent on HIF-1α stabilization. Furthermore, since tumoral hypoxia leads to rapid utilization of glucose stores, we show that glucose deprivation likewise leads to AEG-1 induction. Therefore, in our model, AEG-1 induction in glioblastoma likely occurs through coordinated control by hypoxia-regulated gene expression as well as by key intermediates in glucose metabolism during periods of glucose starvation. Since AEG-1 was recently shown to induce HIF-1α as well as Tie2 and VEGF,4 it is likely that AEG-1 overexpression can further enhance the effects of hypoxia in glioblastoma cells, thereby creating a positive feedback loop. As a result, AEG-1 expression may allow survival and continued cell growth in the midst of diminishing oxygen, nutrient and energy supplies, processes which would render cells without induced AEG-1 expression more susceptible to necrosis.
Critical to the physiological process of hypoxia is stabilization of HIF-1α. During hypoxia, HIF-1α is stabilized and translocates to the nucleus. Within the nucleus, HIF-1α binds to hypoxia response elements (HREs) in the promoters of target genes and promotes enhanced tumor growth, angiogenesis, migration and alterations in energy metabolism. Our findings showing that HIF-1α activity is crucial for AEG-1 induction during hypoxia place HIF-1α at the center of this pathway. Previous studies have shown that knockdown of HIF-1α prevents migration of glioblastoma cells.22 Therefore, the association of AEG-1 with enhanced migration and invasion may be due to activation by HIF-1α. As a result, this pathway of AEG-1 induction may be a pivotal mediator of the aggressive phenotype of glioblastoma and could represent a strong therapeutic target as well.
The central role of phosphatidylinositol 3-kinase (PI3K) in AEG-1 induction during hypoxia lends further support to the importance of this pathway in the regulation of AEG-1 expression. This pathway has been shown to be involved in AEG-1-mediated survival pathways13 as well as the transformed phenotype.4 Studies demonstrating that PI3K activity is essential for HIF-1α stabilization also suggest that in our studies, either PI3K independently stabilizes AEG-1 during hypoxia or PI3K-dependent stabilization of HIF-1α is critical for AEG-1 induction. Nonetheless, PI3K appears to be a key mediator of AEG-1 induction during hypoxia and supports previous suggestions that targeting this pathway may help to reduce AEG-1 activation in glioblastoma as well. However, during glucose deprivation, it is likely that there are pathways other than the PI3K signaling pathway involved in the regulation of AEG-1. During glucose deprivation, we observed AEG-1 induction as well as enhanced phosphorylation of GSK3β, a process that would shift glucose away from glycogen formation and towards immediate utilization. The loss of phosphorylated Akt during this process indicates that this pathway may be independent of PI3K and may likely involve other upstream activators of GSK3β, perhaps intermediates in anaerobic glycolysis. Since we also observe ROS-dependent induction of AEG-1 upon glucose deprivation, it is also likely that there are other enzymes that can regulate AEG-1 activity, such as those involved in ROS neutralization and mitochondrial functioning.
The functional significance of AEG-1 activation during glucose deprivation may be that this pathway is necessary for cells to survive periods of glucose withdrawal. To support this possibility, we have found that knockdown of AEG-1 potentiates whereas overexpression reduces cytotoxicity induced by glucose depletion. Therefore, it is likely that AEG-1 induction may serve several purposes. For example, AEG-1 may simply activate growth signaling pathways that serve to resist glucose deprivation-mediated shutdown. Alternatively, in light of the connection of AEG-1 with apoptosis resistance,11 AEG-1 may prevent the induction of apoptosis that may be activated during glucose depletion. However, AEG-1 may itself alter the way that tumor cells utilize glucose and may even possess anti-oxidant activity or interact directly with anti-oxidant molecules. Interestingly, in its initial characterization, AEG-1 was shown to be associated with the endoplasmic reticulum (ER) membrane,3 the location of many glycolytic enzymes, including the glucose-6-phosphate transporter (G6PT). Because G6PT has been shown to regulate glioblastoma cell survival and to function as a bioswitch between apoptosis and necrosis,23 it is possible that AEG-1 may interact with or regulate such an enzyme during hypoxia and glucose deprivation. This modulation may be important in a variety of tumor-associated processes, such as the switch from aerobic to anaerobic metabolism or the tumoral demand for increased NADPH production through activation of the pentose phosphate shunt pathway.
Necrosis continues to be one of the strongest predictors of poor prognosis in glioblastoma, yet few studies have examined the genetic alterations underlying this cellular process. Because AEG-1 is induced by hypoxia and glucose deprivation, two necrosis-associated processes, in glioblastoma cells, AEG-1 may represent a novel regulator of necrosis and a prognostic biomarker in glioblastoma. Furthermore, AEG-1-mediated downregulation of the promoter for the excitatory amino acid transporter 2 (EAAT2), may cause glutamate excitotoxicity in glioblastoma, a process which itself may induce necrosis.24 Therefore, this molecule holds much potential impact as a novel target for the treatment of glioblastoma. By better understanding the mechanism of AEG-1 induction by hypoxia and glucose deprivation and the biological significance of this AEG-1 upregulation, it may be possible to conduct high-throughput chemical screens for small molecule inhibitors of the AEG-1 pathway to improve the overall prognosis of patients diagnosed with glioblastoma.
Materials and Methods
Cell culture.
The human glioblastoma cell lines, U87-MG and T98-G, were maintained in Dulbecco's modified Eagle's medium (DMEM) + 10% FBS + penicillin/streptomycin. Primary glioblastoma cell cultures were created as previously described. Briefly, fresh glioblastoma tissue was separated with mechanical disruption using sterile scalpels. After tissue dissociation, cells were subjected to further separation using cell culture membrane inserts and by passing cells through fire-polished Pasteur pipettes. Cells were cultured in 1:1 DMEM/F12 mix (Gibco) supplemented with 10% FBS, L-glutamine, insulin, gentamycin and amphotericin-B. Astrocytic lineage was verified by immunofluorescent detection of GFAP (DAKO).
Plasmids, reagents and preparation of stable cells.
CMV-HIF-1α was a kind gift from Dr. Steve McKnight (University of Texas Southwestern Medical Center, Dallas, TX). Dr. D.E. Britt (Brown University, Providence, RI) kindly provided CMV-AEG-1, which was then subcloned into pcDNA6 under the CMV promoter with a blasticidin resistance cassette. U87-MG cells stably overexpressing AEG-1 were prepared by transient transfection of these cells with pcDNA6/AEG-1 and selection of single cell clones using selection with 5 ug/mL blasticidin (Sigma). AEG-1 shRNA lentiviral plasmids were purchased from Sigma and lentiviral particles were produced by transfection of HEK293T cells with 5 ug of scrambled shRNA plasmid or AEG-1 shRNA plasmid and 7.5 ug of lentiviral packaging mix containing pLP1, pLP2 and pLP/VSVG. Lentiviral supernatants were then collected after 48 hours. U87-MG cells with stable knockdown of AEG-1 were prepared by transduction of these cells with a ratio of 1:8 lentiviral AEG-1 shRNA supernatants to normal medium. Cells were then selected with 1 ug/mL puromycin (Sigma). Wortmannin (EMD Biosciences) was used at a final concentration of 25 uM. N-acetylcysteine (Sigma) was used at a final concentration of 24 mM and pyruvate (Gibco) was used at a final concentration of 1 mM.
Hypoxia treatment and glucose deprivation.
To achieve hypoxia, cells were placed in a modular incubator chamber (Billups Rothenberg Inc., Del Mar, CA) and flushed for 15 minutes with a gas mixture of 5% CO2/95% N2 at a flow rate of 3 L/minute as previously described.25 The chamber was then sealed and placed at 37°C in an incubator with 7% CO2. For serum starvation and glucose deprivation, cells were first washed three times with PBS. For experiments using no serum or glucose, DMEM lacking only serum or lacking both serum and glucose was added to the cells. For experiments with glucose deprivation alone, DMEM containing 10% dialyzed FBS with either 1 g/L glucose or no glucose was added to the cells.
Cell extraction and western blot analysis.
Whole-cell, cytoplasmic and nuclear protein extraction and western blot were performed as previously described.26 Antibodies used were as follows: AEG-1 antibody (Sigma), Phospho-Akt (Cell Signal), Akt (Cell Signal), Phospho-GSK-3β (Cell Signal), GSK-3β (Cell Signal), α-tubulin (Sigma), HIF-1α (BD Biosciences), lamin A/C (Cell Signal). Immunoblots were developed using the appropriate secondary horseradish peroxidase-coupled antibodies and an enhanced chemiluminescence plus (ECL) kit (Pierce).
Reactive oxygen species (ROS) detection.
Cells were plated in chamber slides in either control media or glucose deprivation media as described before, washed, and then labeled with 25 µM carboxy-H2-DCFDA for 30 minutes at 37°C according to the manufacturer's instructions (Molecular Probes). Cells were counterstained with Hoechst 33342 dye during the last 5 minutes of incubation. In the presence of ROS, the reduced carboxy-DCFH is converted to carboxy-DCF and fluoresces green. Cells were then mounted in warm buffer and analyzed by fluorescence microscopy.
Trypan blue exclusion.
Floating cells in medium were collected, and remaining cells were then trypsinized. Approximately 400 cells were counted in three separate fields per treatment condition. The total number of cells as well as the total number of non-viable cells was counted. Each experiment was conducted in triplicate.
Human subjects.
Cultures of primary human brain tumor tissues were prepared from fresh surgical resections obtained under approval of the Temple University Institutional Review Board (IRB). The study was classified as exempt (category 4) by the IRB.
Acknowledgements
The authors wish to thank past and present members of the Department of Neuroscience/Center for Neurovirology for their support and sharing of ideas and reagents. We also thank G. Tuszynski and C. Schriver for editorial assistance. This work was made possible by research grants awarded to K.K. and a training grant supporting E.N. This investigation was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award (number). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- AEG-1
astrocyte-elevated gene-1
- Hif-1α
hypoxia-inducible factor-1
- MMP-2
matrix metalloprotease 2
- Ang1
angiopoietin 1
- HREs
hypoxia response elements
- ROS
reactive oxygen species
- NADP
nicotinamide adenine dinucleotide phosphate
References
- 1.Oliver L, Olivier C, Marhuenda FB, Campone M, Vallette FM. Hypoxia and the malignant glioma microenvironment: regulation and implications for therapy. Curr Mol Pharmacol. 2009;2:263–284. doi: 10.2174/1874467210902030263. [DOI] [PubMed] [Google Scholar]
- 2.Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69:7986–7993. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Emdad L, Lee SG, Su ZZ, Jeon HY, Boukerche H, Sarkar D, et al. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc Natl Acad Sci USA. 2009;106:21300–21305. doi: 10.1073/pnas.0910936106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu C, Chen K, Zheng H, Guo X, Jia W, Li M, et al. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis. 2009;30:894–901. doi: 10.1093/carcin/bgp064. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Zhang N, Song LB, Liao WT, Jiang LL, Gong LY, et al. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clin Cancer Res. 2008;14:3319–3326. doi: 10.1158/1078-0432.CCR-07-4054. [DOI] [PubMed] [Google Scholar]
- 7.Ash SC, Yang DQ, Britt DE. LYRIC/AEG-1 overexpression modulates BCCIPalpha protein levels in prostate tumor cells. Biochem Biophys Res Comm. 2008;371:333–338. doi: 10.1016/j.bbrc.2008.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song L, Li W, Zhang H, Liao W, Dai T, Yu C, et al. Overexpression of AEG-1 significantly associates with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. J Pathol. 2009;219:317–326. doi: 10.1002/path.2595. [DOI] [PubMed] [Google Scholar]
- 9.Yoo BK, Gredler R, Vozhilla N, Su ZZ, Chen D, Forcier T, et al. Identification of genes conferring resistance to 5-fluorouracil. Proc Natl Acad Sci USA. 2009;106:12938–12943. doi: 10.1073/pnas.0901451106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emdad L, Sarkar D, Lee SG, Su ZZ, Yoo BK, Dash R, et al. Astrocyte elevated gene-1: a novel target for human glioma therapy. Molecular Cancer Ther. 9:79–88. doi: 10.1158/1535-7163.MCT-09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu G, Wei Y, Kang Y. The multifaceted role of MTDH/AEG-1 in cancer progression. Clin Cancer Res. 2009;15:5615–5620. doi: 10.1158/1078-0432.CCR-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emdad L, Sarkar D, Su ZZ, Lee SG, Kang DC, Bruce JN, et al. Astrocyte elevated gene-1: recent insights into a novel gene involved in tumor progression, metastasis and neurodegeneration. Pharmacol Ther. 2007;114:155–170. doi: 10.1016/j.pharmthera.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–1121. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 14.Song H, Li C, Li R, Geng J. Prognostic significance of AEG-1 expression in colorectal carcinoma. Int J Colorectal Dis. 2010;25:1201–1209. doi: 10.1007/s00384-010-1009-3. [DOI] [PubMed] [Google Scholar]
- 15.Emdad L, Sarkar D, Su ZZ, Randolph A, Boukerche H, Valerie K, et al. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: implications for tumor progression and metastasis. Cancer Res. 2006;66:1509–1516. doi: 10.1158/0008-5472.CAN-05-3029. [DOI] [PubMed] [Google Scholar]
- 16.Thirkettle HJ, Girling J, Warren AY, Mills IG, Sahadevan K, Leung H, et al. LYRIC/AEG-1 is targeted to different subcellular compartments by ubiquitinylation and intrinsic nuclear localization signals. Clin Cancer Res. 2009;15:3003–3013. doi: 10.1158/1078-0432.CCR-08-2046. [DOI] [PubMed] [Google Scholar]
- 17.Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, et al. Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem. 2003;278:31277–31285. doi: 10.1074/jbc.M300763200. [DOI] [PubMed] [Google Scholar]
- 18.Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann NY Acad Sci. 2000;899:349–362. doi: 10.1111/j.1749-6632.2000.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 19.Nath KA, Ngo EO, Hebbel RP, Croatt AJ, Zhou B, Nutter LM. Alpha-Ketoacids scavenge H2O2 in vitro and in vivo and reduce menadione-induced DNA injury and cytotoxicity. Am J Physiol. 1995;268:227–236. doi: 10.1152/ajpcell.1995.268.1.C227. [DOI] [PubMed] [Google Scholar]
- 20.Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci USA. 2006;103:17390–17395. doi: 10.1073/pnas.0608386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo BK, Chen D, Su ZZ, Gredler R, Yoo J, Shah K, et al. Molecular mechanism of chemoresistance by astrocyte elevated gene-1. Cancer Res. 70:3249–3258. doi: 10.1158/0008-5472.CAN-09-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendez O, Zavadil J, Esencay M, Lukyanov Y, Santovasi D, Wang SC, et al. Knock down of HIF-1alpha in glioma cells reduces migration in vitro and invasion in vivo and impairs their ability to form tumor spheres. Mol Cancer. 9:133. doi: 10.1186/1476-4598-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belkaid A, Fortier S, Cao J, Annabi B. Necrosis induction in glioblastoma cells reveals a new “bioswitch” function for the MT1-MMP/G6PT signaling axis in proMMP-2 activation versus cell death decision. Neoplasia. 2007;9:332–340. doi: 10.1593/neo.07142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noch E, Khalili K. Molecular mechanisms of necrosis in glioblastoma: the role of gutamate excitotoxicity. Cancer Biol Ther. 2009;8:1791–1797. doi: 10.4161/cbt.8.19.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tendler DS, Bao C, Wang T, Huang EL, Ratovitski EA, Pardoll DA, Lowenstein CJ. Intersection of interferon and hypoxia signal transduction pathways in nitric oxide-induced tumor apoptosis. Cancer Res. 2001;61:3682–3688. [PubMed] [Google Scholar]
- 26.Gentilella A, Khalili K. Autoregulation of co-chaper-one BAG3 gene transcription. J Cell Biochem. 2009;108:1117–1124. doi: 10.1002/jcb.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]