Abstract
Objectives
We herein assessed the influence of Epidermal Growth Factor Receptor (EGFR) gene mutations on EGFR expression levels, downstream mediators such as Akt or ERK and overall survival in patients with ovarian cancer.
Results
Twenty-nine EGFR gene mutations were detected in 24 of 102 patinets (23.5%). EGFR mutations were observed in 27.9% (19/68) in serous adenocarcinomas, 15.0% (3/20) in clear cell adenocarcinomas and 66.7% (2/3) in mucinous adenocarcinomas, while no mutations were observed in endometrioid adenocarcinomas (0/11). Protein expression of EGFR, pAkt and pERK were detected in 47 (46.1%), 49 (48%) and 17 (16.7%) of patients, respectively. EGFR gene mutations, EGFR and pERK expression were not associated with a poor prognosis. In a multivariate analysis, a High pAkt expression was found to be a significant predictor for both the progression free survival (p = 0.017) and overall survival (p = 0.025).
Study Design
EGFR mutation status was analyzed by direct sequencing in 102 Japanese ovarian cancer patients. The EGFR expression, phosphorylated Akt (pAkt) and phosphorylated ERK (pERK) were determined by immunohistochemistry.
Conclusion
EGFR gene mutations were frequently observed in not only non-small-cell lung cancer (NSCLC), but also in ovarian cancer in Japanese patients. The selective EGFR inhibitor Gefitinib might therefore offer some benefit in patients with EGFR mutations in ovarian cancer. Our results indicate that the Akt, but not necessarily EGFR, is one of the most important target in the response of the platinum-based chemotherapy and prognosis for ovarian cancer patients.
Key words: ovarian cancer, EGFR mutation, pAkt, pERK, EGFR protein, gefitinib, platinum, immunohistochemistry, prognostic factor
Introduction
Ovarian cancer is the most frequent cause of cancer-related deaths among all gynecological cancers. Approximately 70% of all patients with ovarian cancer are diagnosed at an advanced stage. The current management of patients with advanced disease involves optimal surgical debulking followed by chemotherapy. The current standard chemotherapeutic approach for ovarian cancer patients includes platinum-based regimens. Although this treatment is highly effective, 60–80% of women still die of this disease.1 The main reasons for poor prognosis are a high recurrence rate and resistance to second-line chemotherapeutics. Therefore, the development of new therapies is critical for treatment of ovarian cancer patients.
The Epidermal Growth Factor Receptor (EGFR) is involved in many cellular processes including cell proliferation, motility, adhesion and angiogenesis via the activation of principally two pathways: Phosphatidylinositol-3 Kinase (PI3K)/Akt pathway, and the External signal-Regulated Kinase (ERK) pathway. EGFR is widely expressed in a variety of human tumors including head and neck cancer, breast cancer, non-small-cell lung cancer (NSCLC) and ovarian cancer2 and is a promising target for cancer therapy. The EGFR is reported to be present in 33–75% of ovarian cancers3 and has been implicated in both the growth and progression of this disease.4–6 Given the importance of this receptor in both ovarian cancer growth and progression, EGFR therefore represents a good target for anticancer drug development.
Recent several studies showed that in the NSCLC, a kinase domain mutation of the EGFR gene was predictive for significant clinical responses to the selective EGFR inhibitor Gefitinib.7–13 Although Paez et al. reported that EGFR mutations were more frequent in adenocarcinoma than in other NSCLCs, and were more frequent in patients from Japan than those from the United States (28 vs. 2%),10 there were only four mutations of the EGFR gene in ovarian cancer patients worldwide as previously reported.4,6,14–16 In ovarian cancer, a phase II trial to assess Gefitinib as a single agent was well-tolerated but had minimal activity in patients with recurrent ovarian cancer or primary peritoneal carcinoma.16 However, the efficacy of a large number of EGFR gene mutation-positive patients treated with the selective EGFR inhibitor Gefitinib in ovarian cancer remains unclear.
Akt and ERK are important downstream signaling molecules of EGFR.17 However, it remains to be elucidated whether Akt, ERK and EGFR are indeed the most important molecules associated with either the response of anticancer agents or the prognosis of ovarian cancer. In vitro assays have shown that a mutation in the tyrosine kinase domain of the EGFR protein resulted in stronger activation of its signaling cascade.9,11 To date, many investigators extensively studied the associations between EGFR mutation and the downstream molecules such as Akt and ERK in lung cancer cell line and revealed that EGFR mutation is almost always accompanied with enhanced signaling of intracellular cascades in preclinical setting.18–21 We therefore hypothesized that Akt and ERK may be phosphorylated at higher frequencies in tumors with EGFR mutations than in tumors without EGFR mutations, and such activation may correlate with poor prognosis. Therefore, to clarify the clinical significance of EGFR-related molecular markers in ovarian cancers, we investigated EGFR gene mutations and expression as well as activation of downstream molecules using clinical tumor specimens from ovarian tumors of cancer patients. Finally, we assessed mutated EGFR association with clinicopathological factors and treatment outcomes.
Results
Mutation analysis of EGFR gene in ovarian carcinomas.
Clinical and pathological data for the patients are shown in Table 1. Of the 102 investigated patients, 9, 78 and 15 were categorized as Stage II, III and IV, respectively. Of the 102 cases, 68 were histopathologically diagnosed to have serous adenocarcinoma, 20 clear cell carcinoma, 11 endometrioid adenocarcinoma and 3 mucinous adenocarcinoma. All patients underwent adjuvant chemotherapy with platinum-based agents. Twenty-nine EGFR gene mutations were detected in 24 of 102 patients (23.5%); 16 patients had exon 18 mutations, 3 patients had exon 19 mutations and 8 patients had exon 21 mutations. The mutation types are shown in Table 2. In exon 18, 17 point mutations were detected, including 12 missense mutations and 5 silent mutations. Three tumors had the same point mutation at codon 703: CTC to CCC. In exon 19, two tumors had the same type mutation resulting in an in-frame deletion, removing amino acids 746 through 750 (delE746-A750), which is the major type of EGFR mutation in NSCLC. One patient harbored the silent point mutation at codon 744: ATC to ATT. In exon 21, 9 point mutations, including 6 missense mutations and 3 silent mutations, were detected. One tumor had the point mutation at codon 858: CTG to CGG, which is also the major type of EGFR mutation in NSCLC tumors.
Table 1.
Patient characteristics
| Variables | No of patients (%) |
| n = 102 | |
| Histological type | |
| Serous | 68 (65.7) |
| Clear cell | 20 (19.6) |
| Endometrioid | 11 (10.8) |
| Mucinous | 3 (2.9) |
| FIGO stage | |
| II | 9 (8.8) |
| III | 78 (76.5) |
| IV | 15 (14.7) |
| Type of chemotherapy | |
| Platinum based | 95 (93.1) |
| Other regimen | 0 (0.0) |
| No chemotherapy | 7 (6.9) |
Correlation with clinicopathological features and molecular markers.
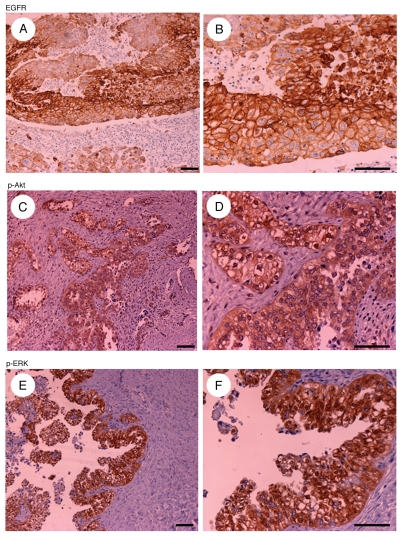
In tumor specimens obtained by surgical resection, EGFR, pAkt and pERK protein expression was positive in 47 (46.1%), 49 (48%) and 17 (16.7%) of the 102 patients. A representative example of the immunostaining analysis is shown in Figure 1. EGFR expression was detected mainly in the membrane and pAkt and pERK expression were detected mainly in the cytoplasm of tumor specimens. We examined the relationships between the clinicopathological factors and the EGFR gene mutation status or the immunohistochemical staining patterns, which are listed in Table 3. No significant associations between EGFR, pAkt or pERK expression status and any clinicopathological factors were observed. However, EGFR gene mutation status was significantly related to histological type (p = 0.04). EGFR mutations were observed 27.9% (19/68) in serous adenocarcinomas, 15.0% (3/20) in clear cell adenocarcinomas and 66.7% (2/3) in mucinous adenocarcinomas, while no mutations were observed in endometrioid adenocarcinomas (0/11).
Figure 1.
Representative examples of immunohistochemically stained sections positive for EGFR (A and B), pAkt (C and D) and pERK (E and F) in tumor specimens ([A, C and E], x20 original magnification; [B, D and F] x40 original magnification). Scale bars represent 100 µm.
Table 3.
Correlation with clinicopathological features and molecular markers
| Variables(%) | EGFR protein | EGFR mutation | pAkt | pERK | ||||||||
| positive | negative | p value | positive | negative | p value | positive | negative | p value | positive | negative | p value | |
| Histology | 0.15 | 0.04 | 0.17 | 0.61 | ||||||||
| serous | 29(61.7) | 39(7039) | 19(79.2) | 49(62.8) | 36(73.5) | 32(60.4) | 10(58.8) | 58(68.2) | ||||
| clear cell | 13(27.7) | 7(12.7) | 3(12.5) | 17(21.8) | 7(14.3) | 13(24.5) | 5(29.4) | 15(17.6) | ||||
| endometrioid | 3(6.4) | 8(14.5) | 0(0.0) | 11(14.1) | 6(12.2) | 5(9.4) | 2(11.8) | 9(10.6) | ||||
| mucinous | 2(4.2) | 1(1.9) | 2(8.3) | 1(1.3) | 0(0.0) | 3(5.7) | 0(0.0) | 3(3.6) | ||||
| Stage | 0.83 | 0.95 | 0.03 | 0.37 | ||||||||
| II | 4(8.5) | 5(9.1) | 2(8.3) | 7(9.0) | 2(4.1) | 7(13.2) | 0(0.0) | 9(10.6) | ||||
| III | 35(74.5) | 43(78.2) | 18(75.0) | 60(76.9) | 43(87.8) | 35(66.0) | 14(82.4) | 64(75.3) | ||||
| IV | 8(17.0) | 7(12.7) | 4(16.7) | 11(14.1) | 4(8.1) | 11(20.8) | 3(17.6) | 12(14.1) | ||||
| IV | 8(17.0) | 7(12.7) | 4(16.7) | 11(14.1) | 4(8.1) | 11(20.8) | 3(17.6) | 12(14.1) | ||||
| Recurrence | 0.52 | 0.24 | 0.10 | 0.64 | ||||||||
| ≤ 6M | 10(21.3) | 10(18.2) | 6(25.0) | 14(17.9) | 12(24.5) | 8(15.1) | 3(17.6) | 17(20.0) | ||||
| >6M | 23(48.9) | 33(60.0) | 15(62.5) | 41(52.6) | 29(59.2) | 27(50.9) | 11(64.8) | 45(52.9) | ||||
| - | 14(29.8) | 12(21.8) | 3(12.5) | 23(29.5) | 8(16.3) | 18(34.0) | 3(17.6) | 23(27.1) | ||||
| 5-year survival | 0.84 | 0.15 | 0.16 | 0.27 | ||||||||
| alive | 17(36.2) | 22(40.0) | 6(25.0) | 33(42.3) | 15(30.6) | 24(45.3) | 4(23.6) | 35(41.2) | ||||
| dead | 30(73.8) | 33(60.0) | 18(75.0) | 45(57.7) | 34(69.4) | 39(54.7) | 13(76.4) | 50(58.8) | ||||
Impact of molecular markers on survival.
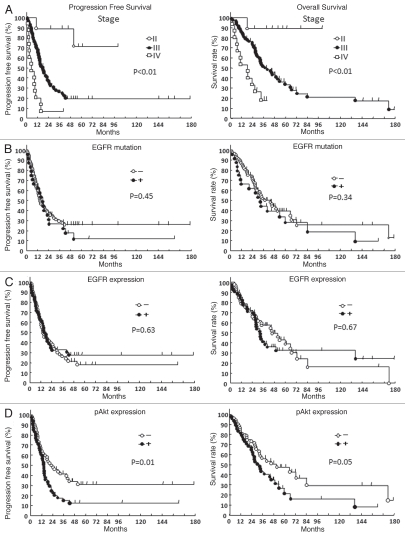
We next examined the staining intensity of various markers and patient survival. The median survival time for all patients was 5.5 years. Overall survival curves were stratified according to FIGO stage, EGFR protein expression, pAkt expression, pERK expression and EGFR gene mutation status (Fig. 2). A univarite analysis revealed a high tumor pAkt expression along with the FIGO stage to be significantly associated with a poor outcome regarding both the progression-free survival (p = 0.01) and overall survival (p = 0.05). However, pERK expression (data not shown), EGFR expression and EGFR gene mutation status was not significantly related to survival time, respectively.
Figure 2.
Survival curves with Kaplan-Meier method of 102 ovarian cancer patients. Progression-free survival and overall survival of patients according to FIGO stage (A), EGFR gene mutation status (B), EGFR expression status (C) and pAkt expression status (D). p values were calculated using the log-rank test.
Multivariate survival analysis.
Using the Cox proportional hazards models, we conducted a multivariate analysis to assess the predictive value of the tumor EGFR gene mutation status. In addition, the EGFR expression, pAkt expression and pERK expression were analyzed for the progression free survival and overall survival. We also included the following known prognostic variables: FIGO stage and initial histological type (serous and endometrioid vs. clear and mucinous). A high pAkt expression (99% CI, 1.11–2.93; p = 0.017) along with the FIGO stage (99% CI, 2.29–6.94; p < 0.001) were found to be significant predictor variables of the progression free survival. Regarding overall survival, a high pAkt expression (99% CI, 1.08–3.14; p = 0.025) along with the FIGO stage (99% CI, 2.20–7.14; p < 0.001) were identified to be significant predictors. The results of multivariate survival analyses are also summarized in Table 4.
Table 4.
Multivariate analysis for survival rates
| Variables | HR | 95% CI | p value |
| Overall survival | |||
| Stage (II/III/IV) | 3.967 | 2.203–7.142 | <0.001 |
| Histology (serous + endometrioid/clear + mucinous) | 1.570 | 0.778–3.166 | 0.208 |
| EGFR mutation (positive/negative) | 1.465 | 0.819–2.622 | 0.198 |
| EGFR expression (positive/negative) | 1.176 | 0.691–2.002 | 0.549 |
| pERK expression (positive/negative) | 1.279 | 0.685–2.391 | 0.439 |
| pAkt expression (positive/negative) | 1.840 | 1.078–3.142 | 0.025 |
| Progression free survival | |||
| Stage (II/III/IV) | 3.989 | 2.293–6.940 | <0.001 |
| Histology (serous + endometrioid/clear + mucinous) | 0.901 | 0.458–1.774 | 0.764 |
| EGFR mutation (positive/negative) | 1.307 | 0.764–2.236 | 0.329 |
| EGFR expression (positive/negative) | 1.041 | 0.639–1.696 | 0.872 |
| pERK expression (positive/negative) | 1.046 | 0.573–1.907 | 0.884 |
| pAkt expression (positive/negative) | 1.805 | 1.113–2.930 | 0.017 |
Discussion
It has been reported that EGFR gene mutations in ovarian cancer present only four mutations in 318 patients (1.26%);4,6,14–16 however, in the present study, we found 29 mutations in 24 Japanese patients (23.5%). In previous studies of ovarian cancer, Schilder et al. reported two multinucleotide in-frame deletions that eliminated four amino acids (LREA) encoded by exon 19 in 57 patients (3.5%).16 Lassus et al. and Stadlmann et al. reported one insertion mutation (codon 772–775; YVMA) in 198 patients (0.51%) and one point mutation (codon 787; ACG to ACT) in 11 patients (9.1%) in exon 20 of EGFR.4,6 In this study, two of the EGFR mutations we observed were the deletion of E746–A750 mutation, and we found that the remaining 27 mutations were nucleotide substitutions in exons 18, 19 and 21, which were not previously described. To our knowledge, this is the first report to describe tumors which harbor various EGFR mutations in ovarian cancer and we have demonstrated that EGFR mutations are frequent in ovarian cancers from Japanese patients. These results might indicate that EGFR mutations are affected by ethnic variations, in line with previous studies on NSCLC.10 Interestingly, histological analysis revealed that no mutations are present in endometrioid adenocarcinoma, suggesting that EGFR mutations might differ across histological types. However, there is little evidence thus far to validate these hypotheses, which will require future studies. Moreover, previous studies on the relationship between EGFR overexpression and clinicopathological characteristics, the patient response to chemotherapy and survival have shown conflicting results in ovarian cancers.5,24,25 In our immunohistochemical analysis, we also did not find any statistically significant associations between EGFR expression and prognosis, or between EGFR mutations and protein expression. Recently, some investigators have reported EGFR gene mutation-positive patients in NSCLC to demonstrate the greatest progression-free survival and overall survival benefit from the selective EGFR inhibitor Gefitinib.7–9 Schilder et al. reported Phase II study data describing that 4 of 27 patients treated with Gefitinib had a progression-free survival of more than 6 months, including one partial responder (4%) in ovarian cancers.16 The authors also reported that the response rate for patients with EGFR-positive tumors was only 9% (1 of 11), and did not find any association between EGFR mutations and Gefitinib sensitivity in ovarian cancers.16 Lacroix et al. also reported that the response of patients with platinum-resistant ovarian cancers to Gefitinib was independent of EGFR mutational status.14 However, these studies could not evaluate the efficacy of a large number of EGFR gene mutation-positive patients treated with the selective EGFR inhibitor Gefitinib in ovarian cancer. We did not find any significant associations between EGFR mutations and survival time in ovarian cancer patients treated with platinum-based chemotherapeutics, although these eligible patients were not treated with the selective EGFR inhibitor Gefitinib. According to the current clinical studies on NSCLC,7–9 Gefitinib might therefore be highly effective for the treatment of ovarian cancer patients with EGFR mutations. Further studies will thus be required to determine whether the response rate of ovarian cancer patients with EGFR gene mutations can increase using the selective EGFR inhibitor Gefitinib.
In this study, the overexpression of pAkt correlated with the progression-free survival for patients with ovarian cancer. Akt is regulated by many factors including Her2/neu, Platelet-Derived Growth Factor Receptor and BCR-ABL in tumor cells.26 These relationships suggest that Akt may be activated by upstream molecules other than EGFR in ovarian cancer. Altomare et al. demonstrated that positive expression of the pAkt protein was related to the degree of differentiation and clinical stage of ovarian cancer.27 Consistent with the Guo et al. study, pAkt was involved in invasion and metastasis of ovarian cancers.28 Moreover, the Akt inhibitor API-2 has recently been described as an effective treatment in animal models of ovarian cancers.29 According to previous studies, Akt activation might be a key step in the development and/or progression of ovarian cancer. We previously reported that Akt is a key molecule for anticancer drug resistance and clarified that EGFR, as well as alterations in Akt, are associated with resistance to platinum- and taxanebased chemotherapy and Akt inactivation sensitized human ovarian cancer cells to Cisplatin and Paclitaxel.30–33 Moreover, Gefitinib inhibited the activation of Akt and ERK in lung cancer cell lines.34 These results indicated that the Akt cascade, but not necessarily EGFR, is a promising target for development of chemotherapeutic drugs for the treatment of ovarian cancer. In the response of the platinum-based chemotherapy and prognosis for ovarian cancer patients, we would like to emphasize the importance of the Akt cascade. Further studies will thus be required to determine whether the response rate of ovarian cancer patients can be increased by using such molecular targeting strategies.
In conclusion, we herein demonstrated that EGFR gene mutations are frequent in not only NSCLC, but also ovarian epithelial cancer in Japanese patients, but they do not correlate with either the EGFR protein expression or clinical outcome. The administration of Gefitinib to patients with ovarian cancer might therefore offer some benefits when selecting patients with EGFR mutations. However, this hypothesis still need to be confirmed in the context of a prospective study. Taken together, the EGFR mutation status was not associated with Akt activation, and the molecular factor which correlates with survival is Akt. The inhibition of the Akt pathway may therefore be a potentially useful molecular target in ovarian cancer.
Material and Methods
Patients.
The inclusion criteria were 102 primary epithelial ovarian cancer patients (FIGO [International Federation of Gynecology and Obstetrics]22 stages II, III and IV) who underwent a surgical resection in the Department of Gynecology of Osaka Medical College Hospital in Japan between 1991 and 2005 and were treated postoperatively with platinum-based chemotherapy. In all cases, an effort was made to perform optimal surgical cytoreduction and adequate staging, which included, at least, total abdominal hysterectomy with bilateral salpingoophrectomy, omentectomy, peritoneal washings and retroperitoneal lymphadenectomy. The histology of all carcinomas was determined by a gynecological pathologist according to the WHO criteria (World Health Organization).23 The Institutional Review Board approved this study and informed consents were obtained from all patients.
PCR amplification and analysis of EGFR gene mutations.
Genomic DNA was extracted from microdissected tissue obtained from paraffin-embedded tissue samples. Nested polymerase chain reaction (PCR) method was performed for the 3 exons (exons 18, 19 and 21) encoding the tyrosine kinase domain of the EGFR. We used primers as follows: exon 18, forward 5′-GAC CCT TGT CTC TGT GTT CTT GT-3′, reverse-1 5′-TAT ACA GCT TGC AAG GAC TCT-3′, reverse-2 5′-CCA GAC CAT GAG AGG CCC TG-3′; exon 19, forward 5′-CAG ATC ACT GGG CAG CAT GT-3′, reverse-1 5′-AGG GTC TAG AGC AGA GCA GC-3′, reverse-2 5′-GCC TGA GGT TCA GAG CCA T-3′; exon21, forward 5′-CAT GAT GAT CTG TCC CTC ACA G-3′, reverse-1 5′-CTG GTC CCT GGT GTC AGG AA-3′ and reverse-2 5′-GCT GGC TGA CCT AAA GCC ACC-3′. PCR conditions were as follows: 94°C for 3 min, 35 cycles of 94°C for 30 sec, 58°C for 30 sec and 72°C for 1 min and a final cycle of 72°C for 5 min. PCR products were purified and directly sequenced using the BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems) with ABI PRISM 3130 Genetic Analyzer (Applied Biosystems).
Immunohistochemistry.
Tumor samples were formalin-fixed and embedded in paraffin. EGFR expression was analyzed using the EGFR pharm Dx kit (Dako Cytomation). pAkt and pERK were analyzed as follows: briefly, Tumor sections were incubated at 4°C for 18 h with the phospho-AKT specific antibodies phospho-Akt(Ser473)(736E11), at a 1:50 dilution (Cell signaling Technology) and Phospho-p44/42(Thr202)(Tyr204), at a 1:200 dilution (Cell signaling Technology). Evaluation of the immunohistochemical data was performed by two independent pathologists who were blinded to clinicopathological data. An overexpression of EGFR, pAkt and pERK was defined to exist if 70% or more of the tumor cells exhibited the cytoplasmic and/or nuclear staining or membranous staining.
Statistical analysis.
Statistical analyses in this study were carried out with the StatView statistical software package (SAS Institute, Cary, NC USA). Fisher's exact probability test was used for evaluating correlations between immunohistochemical and clinical data. The end points investigated were the progression-free and overall survivals (PFS and OS). The progression-free survival was defined as the time from the first day of chemotherapy until the first of either death from any cause or disease progression (based on an increase in the CA 125 levels and/or on the findings of imaging studies). Overall survival was defined as time from the first day of chemotherapy to death from any cause.
Univariate and multivariate analyses of the progression free survival and overall survival were determined with the Kaplan-Meier method using a log-rank test and the Cox proportional hazards model, respectively. Differences with p values of less than 0.05 were considered to be statistically significant.
Table 2.
Type of EGFR gene mutations
| No | Exon | Type of sequence alteration | Nucleotide alteration | Amino acid alteration |
| 1 | 18 | Substitution | 2174C→G | 725(ACG-ATG) Thr-Met |
| 2 | 18 | Substitution | 2073T→C | 691(CCT-CCC) Pro-Pro |
| 3 | 21 | Substitution | 2573T→G | 858(CTG-CGG) Leu-Arg |
| 4 | 18 | Substitution | 2091A→G | 697(GAA-GAG) Glu-Glu |
| 5 | 21 | Substitution | 2494C→T | 832(CGC-TGC) Arg-Cys |
| 21 | Substitution | 2604A→T | 868(GAA-GAT) Glu-Asp | |
| 6 | 18 | Substitution | 2160C→A | 720(TCC-TCA) Ser-Ser |
| 21 | Substitution | 2556G→T | 852(AAG-AAT) Thr-Met | |
| 7 | 18 | Substitution | 2173A→G | 725(ACG-GCG) Thr-Ala |
| 8 | 19 | Deletion | 2235–2249del GGA ATT AAG AGA AGC | E746-A750 deletion |
| 9 | 19 | Deletion | 2235–2249del GGA ATT AAG AGA AGC | E746-A750 deletion |
| 10 | 18 | Substitution | 2112G→A | 704(TTG-TTA) Leu-Leu |
| 11 | 18 | Substitution | 2108T→C | 703(CTC-CCC) Leu-Pro |
| 18 | Substitution | 2159C→T | 720(TCC-TTC) Se-Phe | |
| 12 | 18 | Substitution | 2099A→G | 700(AAC-AGC) Asn-Ser |
| 13 | 21 | Substitution | 2506C→A | 836(CGC-AGC) Arg-Ser |
| 14 | 18 | Substitution | 2123A→G | 708(AAG-AGG) Lys-Arg |
| 15 | 18 | Substitution | 2165C→A | 722(GCG-GTG) Ala-Val |
| 16 | 18 | Substitution | 2159C→T | 720(TCC-TTC) Ser-Phe |
| 19 | Substitution | 2232C→A | 744(ATC-ATT) Ile-Ile | |
| 17 | 18 | Substitution | 2161G→A | 721(GGT-AGT) Gly-Ser |
| 21 | Substitution | 2597A→G | 866(GAG-GGG) Glu-Gly | |
| 18 | 18 | Substitution | 2108T→C | 703(CTC-CCC) Leu-Pro |
| 19 | 21 | Substitution | 2559C→T | 853(ATC-ATT) Ile-Ile |
| 20 | 18 | Substitution | 2122A→G | 708(AAG-GAG) Lys-Glu |
| 21 | 18 | Substitution | 2108T→C | 703(CTC-CCC) Leu-Pro |
| 22 | 21 | Substitution | 2472C→A | 824(GGC-GGA) Gly-Gly |
| 23 | 18 | Substitution | 2136C→T | 712(TTC-TTT) Phe-Phe |
| 24 | 21 | Substitution | 2481C→T | 827(TAC-TAT) Tyr-Tyr |
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas, No.19591946 (to Y.T.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 3.Berchuck A, Rodriguez GC, Kamel A, Dodge RK, Soper JT, Clarke-Pearson DL, et al. Epidermal growth factor receptor expression in normal ovarian epithelium and ovarian cancer. I. Correlation of receptor expression with prognostic factors in patients with ovarian cancer. Am J Obstet Gynecol. 1991;164:669–674. doi: 10.1016/s0002-9378(11)80044-x. [DOI] [PubMed] [Google Scholar]
- 4.Lassus H, Sihto H, Leminen A, Joensuu H, Isola J, Nupponen NN, et al. Gene amplification, mutation and protein expression of EGFR and mutations of ERBB2 in serous ovarian carcinoma. J Mol Med. 2006;84:671–681. doi: 10.1007/s00109-006-0054-4. [DOI] [PubMed] [Google Scholar]
- 5.Psyrri A, Kassar M, Yu Z, Bamias A, Weinberger PM, Markakis S, et al. Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clin Cancer Res. 2005;11:8637–8643. doi: 10.1158/1078-0432.CCR-05-1436. [DOI] [PubMed] [Google Scholar]
- 6.Stadlmann S, Gueth U, Reiser U, Diener PA, Zeimet AG, Wight E, et al. Epithelial growth factor receptor status in primary and recurrent ovarian cancer. Mod Pathol. 2006;19:607–610. doi: 10.1038/modpathol.3800575. [DOI] [PubMed] [Google Scholar]
- 7.Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, Gervais R, et al. Molecular predictors of outcome with Gefitinib and Docetaxel in previously treated nonsmall-cell lung cancer: data from the randomized phase III interest trial. J Clin Oncol. 2010;28:744–752. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 8.Lara-Guerra H, Waddell TK, Salvarrey MA, Joshua AM, Chung CT, Paul N, et al. Phase II study of preoperative gefitinib in clinical stage I non-small-cell lung cancer. J Clin Oncol. 2009;27:6229–6236. doi: 10.1200/JCO.2009.22.3370. [DOI] [PubMed] [Google Scholar]
- 9.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 10.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 11.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosokawa S, Toyooka S, Fujiwara Y, Tokumo M, Soh J, Takigawa N, et al. Comprehemsive analysis of EGFR signaling pathways in Japanese patients with non-small cell lung cancer. Lung Cancer. 2009;66:107–113. doi: 10.1016/j.lungcan.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix L, Pautier P, Duvillard P, Motté N, Saulnier P, Bidart JM, et al. Response of ovarian carcinomas to gefitinib-carboplatin-paclitaxel combination is not associated with EGFR kinase domain somatic mutations. Int J Cancer. 2006;118:1068–1069. doi: 10.1002/ijc.21460. [DOI] [PubMed] [Google Scholar]
- 15.Mustea A, Sehouli J, Fabjani G, Koensgen D, Möbus V, Braicu EI, et al. Epidermal growth factor receptor (EGFR) mutation does not correlate with platinum resistance in ovarian carcinoma. Results of a prospective pilot study. Anticancer Res. 2007;27:1527–1530. [PubMed] [Google Scholar]
- 16.Schilder RJ, Sill MW, Chen X, Darcy KM, Decesare SL, Lewandowski G, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group Study. Clin Cancer Res. 2005;11:5539–5548. doi: 10.1158/1078-0432.CCR-05-0462. [DOI] [PubMed] [Google Scholar]
- 17.Bunn PA, Jr, Franklin W. Epidermal growth factor receptor expression, signal pathway and inhibitors in nonsmall cell lung cancer. Semin Oncol. 2002;29:38–44. doi: 10.1053/sonc.2002.35646. [DOI] [PubMed] [Google Scholar]
- 18.Cappuzzo F, Magrini E, Ceresoli GL, Bartolini S, Rossi E, Ludovini V, et al. Akt phosphorylation and gefitinib efficacy in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2004;96:1133–1141. doi: 10.1093/jnci/djh217. [DOI] [PubMed] [Google Scholar]
- 19.Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005;23:2493–2501. doi: 10.1200/JCO.2005.01.388. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, Franklin WA, Dziadziuszko R, Thatcher N, et al. Molecular predictors of outcome with gefitinib in a phase III palacebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5034–5042. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- 21.Cappuzzo F, Ligorio C, Jänne PA, Toschi L, Rossi E, Trisolini R, et al. Prospective study of gefitinib in epidermal growth factor receptor fluorescence in situ hybridization-positive/phospho-Akt-positive or never smoker patients with advanced non-small-cell lung cancer: the ONCOBELL trial. J Clin Oncol. 2007;25:2248–2255. doi: 10.1200/JCO.2006.09.4300. [DOI] [PubMed] [Google Scholar]
- 22.International Federation of Gynaecology and Obstetrics, author. Changes in definitions of clinical staging for carcinoma of the cervix and ovary. Am J Obstet Gynecol. 1987;156:263–264. [PubMed] [Google Scholar]
- 23.Scully RE, editor. International Histological Classification of Tumours. Berlin, Springer: World Health Organization; 2004. Histological typing of ovarian tumours; pp. 11–19. [Google Scholar]
- 24.Elie C, Geay JF, Morcos M, Le Tourneau A, Girre V, Broët P, et al. Lack of relationship between EGFR-1 immunohistochemical expression and prognosis in a multicentre clinical trial of 93 patients with advanced primary ovarian epithelial cancer (GINECO group) Br J Cancer. 2004;91:470–475. doi: 10.1038/sj.bjc.6601961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen JS, Jakobsen E, Holund B, Bertelsen K, Jakobsen A. Prognostic significance of p53, Her-2 and EGFR overexpression in borderline and epithelial ovarian cancer. Int J Gynecol Cancer. 2004;14:1086–1096. doi: 10.1111/j.1048-891X.2004.14606.x. [DOI] [PubMed] [Google Scholar]
- 26.Klapper LN, Kirschbaum MH, Sela M, Yarden Y. Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv Cancer Res. 2000;77:25–79. [PubMed] [Google Scholar]
- 27.Altomare DA, Wang HQ, Skele KL, De Rienzo A, Klein-Szanto AJ, Godwin AK, et al. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23:5853–5857. doi: 10.1038/sj.onc.1207721. [DOI] [PubMed] [Google Scholar]
- 28.Guo RX, Qiao YH, Zhou Y, Li LX, Shi HR, Chen KS. Increased staining for phosphorylated AKT and nuclear factor-kappaB p65 and their relationship with prognosis in epithelial ovarian cancer. Pathol Int. 2008;58:749–756. doi: 10.1111/j.1440-1827.2008.02306.x. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 30.Mabuchi S, Ohmichi M, Kimura A, Hisamoto K, Hayakawa J, Nishio Y, et al. Inhibition of phosphorylation of BAD and Raf-1 by Akt sensitizes human ovarian cancer cells to paclitaxel. J Biol Chem. 2002;277:33490–33500. doi: 10.1074/jbc.M204042200. [DOI] [PubMed] [Google Scholar]
- 31.Mabuchi S, Ohmichi M, Nishio Y, Hayasaka T, Kimura A, Ohta T, et al. Inhibition of inhibitor of nuclear factor-kappaB phosphorylation increases the efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Clin Cancer Res. 2004;10:7645–7654. doi: 10.1158/1078-0432.CCR-04-0958. [DOI] [PubMed] [Google Scholar]
- 32.Ohmichi M, Hayakawa J, Tasaka K, Kurachi H, Murata Y. Mechanisms of platinum drug resistance. Trends Pharmacol Sci. 2005;26:113–116. doi: 10.1016/j.tips.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Ohta T, Ohmichi M, Hayasaka T, Mabuchi S, Saitoh M, Kawagoe J, et al. Inhibition of phosphatidylinositol 3-kinase increases efficacy of cisplatin in in vivo ovarian cancer models. Endocrinology. 2006;147:1761–1769. doi: 10.1210/en.2005-1450. [DOI] [PubMed] [Google Scholar]
- 34.Janmaat ML, Kruyt FA, Rodriguez JA, Giaccone G. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells: limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin Cancer Res. 2003;9:2316–2326. [PubMed] [Google Scholar]