Abstract
Metastatic melanoma is a skin cancer with poor prognosis. In situ photoimmunotherapy (ISPI) is a promising modality for the treatment of metastatic melanoma that combines local, selective photothermal therapy with immunological stimulation. A preliminary clinical study was conducted to evaluate the safety and therapeutic effects of ISPI for latestage melanoma patients using imiquimod as the immune modifier. Eleven patients received ISPI in one or multiple 6-week treatment cycles applied to a 200-cm2 treatment site, which usually contained multiple cutaneous metastases. ISPI consisted of three main components applied directly to the cutaneous metastases: 1) local application of topical imiquimod; 2) injection of indocyanine green (ICG); and 3) an 805 nm laser for local irradiation. All patients completed at least one cycle of treatment. The most common adverse effects were rash and pruritus at the treatment sites. No grade 4 toxicity was observed. Complete response was observed in six patients. All lesions in the treatment area of the patients responded to ISPI, eight of which achieved complete local response (CLR). CLR was observed in the nontreatment site (regional) lesions in four patients. Five patients were still alive at the time of last follow-up. The probability of 12-month overall survival was 70%. This study demonstrates that ISPI with imiquimod is safe and well tolerated. The patient response rate is promising. ISPI can be easily applied on an outpatient basis and can be combined with other modalities to improve the therapeutic response of metastatic melanoma.
Key words: in situ photoimmunotherapy, melanoma, imiquimod, toll-like receptor agonists
Introduction
Metastatic melanoma is a skin cancer with a poor prognosis. It is the most lethal form of skin malignancy because of its aggressive behavior and its ability to spread to lymphatics and visceral organs.1 When diagnosed early, melanoma is curable by surgery alone, with 80% of patients relapse-free 10 years after surgery. Patients with stage III melanomas and cutaneous involvement have a significantly worse 5-year survival (63% for IIIA and 27% for IIIC, respectively).1 When melanoma has spread to regional lymph nodes or metastasized to distant sites or vital organs, many conventional treatment methods such as chemotherapy and radiation therapy seem to contribute very little to prolonged survival.2,3 Moreover, the adverse effects of current therapies seem unacceptable given such poor efficacy.
Immunotherapy offers the hope of improved outcomes with fewer and less severe side effects. Much has been learned about the potential of the immune system to control cancer, and this has stimulated the invention of many new therapeutic methods.4 To date however, most methods applied in clinical practice have had limited success in patients with melanoma.5 Currently, interleukin-2 and interferon-alfa 2b are the only approved immunotherapeutic agents for melanoma in the United States.
In situ photoimmunotherapy (ISPI), combining local selective photothermal therapy (PTT) and immunological stimulation using immunoadjuvant, was proposed in 1997.6 It uses near-infrared laser energy to induce temperature increases in target tissue, killing tumor cells directly and releasing tumor antigens for the generation of antitumor immunity.7 Tumor cells swell and are disrupted due to temperature increase, releasing tumor-associated antigens, thermally induced heat shock proteins (HSPs), and a large number of self-antigens. Antigen presenting cells (APCs), particularly dendritic cells (DCs), can capture these antigens and migrate to lymph nodes. DCs present the antigens to T cells that can induce effective immune responses against tumor cells. Evidence from experimental animal models has demonstrated ISPI to be a useful modality to treat cancer.8–10
To facilitate immunological stimulation in the protocol of photoimmunotherapy, imiquimod, a unique toll-like receptor agonist, was selected as the immunoadjuvant. It has been approved by the FDA for treatment of warts, actinic keratoses and superficial basal cell carcinoma.11,12 Topical imiquimod has demonstrated some degree of effectiveness in cases of advanced cutaneous melanoma, even when used as monotherapy.13,14 Its strong immunological stimulating effect made imiquimod a good candidate for use in combination with photothermal therapy.
We have previously reported two cases of metastatic melanoma treated by ISPI.15 These two patients, one stage IV-M1b and one stage IIIC, had complete responses and are still alive at the time of this writing. In the present study, we report preliminary clinical results for the first series of eleven metastatic melanoma patients, including the original two patients, in terms of safety and efficacy of ISPI.
Results
Demographics.
Eleven patients were enrolled in this study between June 2004 and August 2008, with demographic characteristics as shown in Table 1. Median age was 69 years (range: 46–87 years). Seven patients were male, and four were female. All patients were Caucasians and ECOG performance status of all patients was less than or equal to 1 at enrollment. Two patients had AJCC disease stage IIIB, three had IIIC and six patients had stage IV (Mla 1, Mlb 1 and M1c 4). All 11 patients had prior surgery. Three patients had received prior systemic chemotherapy therapy for metastatic disease, three patients had received radiation therapy and two patients received isolated limb perfusion therapy.
Table 1.
Patient demographics and baseline disease characteristics
| Characteristics | Number of patients | |
| Age (years) | ||
| Median | 69 | |
| Range | 46–87 | |
| Sex | ||
| Male | 7 | |
| Female | 4 | |
| ECOG* Performance Status | ||
| 0 | 11 | |
| 1 | 0 | |
| AJCC** Stage | ||
| IIIB | 2 | |
| IIIC | 3 | |
| IV | 6 | |
| M1a | 1 | |
| M1b | 1 | |
| M1c | 4 | |
| Prior Treatment | ||
| Surgery | 11 | |
| Chemotherapy | 3 | |
| Radiation therapy | 3 | |
| Isolated limb perfusion | 2 | |
ECOG, Eastern Cooperative Oncology Group.
AJCC, American Joint Committee on Cancer.
Toxicity.
Local irritation due to application of imiquimod and laser irradiation was seen in all patients and was generally well-tolerated. Table 2 summarizes the most frequently reported adverse events by severity. The most frequently reported adverse events were rash (90.9%), pruritus (81.8%), pain (54.5%), fatigue (54.5%), anorexia (54.5%), nausea (36.4%), weight loss (36.4%), fever (18.2%), chills (9.1%), vomiting (9.1%) and cellulitis (9.1%). At least one grade 3 AE occurred in 25% of the patients, including fatigue (one patient), nausea (two patients), anorexia (two patients), pain (one patient), dyspnea (one patient) and cellulitis (one patient). The most severe side effects generally occurred during the first treatment cycle. Significant pain with the laser treatment was seen in about 20% of patients and usually responded to the addition of oral premedication with narcotics, although one patient required conscious sedation. No grade 4 AEs were noted in this study.
Table 2.
Summary of treatment-related adverse events
| All grades | Grade 3 | |||
| No. of patients | Percent | No. of patients | Percent | |
| Constitutional | ||||
| Fever | 2 | 18.2% | 0 | 0% |
| Chills, rigors | 1 | 9.1% | 0 | 0% |
| Fatigue, lethargy | 6 | 54.5% | 1 | 9.1% |
| Weight loss | 4 | 36.4% | 0 | 0% |
| Cardiovascular | ||||
| Tachycardia | 0 | 0% | 0 | 0% |
| Prolonged QTc* (>0.48 s) | 0 | 0% | 0 | 0% |
| Edema | 0 | 0% | 0 | 0% |
| Pulmonary | ||||
| Dyspnea | 1 | 9.1% | 1 | 9.1% |
| Hypoxia | 0 | 0% | 0 | 0% |
| Gastrointestinal | ||||
| Nausea | 4 | 36.4% | 2 | 18.2% |
| Vomiting | 1 | 9.1% | 0 | 0% |
| Anorexia | 6 | 54.5% | 2 | 18.2% |
| Diarrhea | 0 | 0% | 0 | 0% |
| Renal | ||||
| Increased creatinine | 0 | 0% | 0 | 0% |
| Hepatic | ||||
| Increased bilirubin | 0 | 0% | 0 | 0% |
| Increased LDH** | 0 | 0% | 0 | 0% |
| Skin | ||||
| Rash | 10 | 90.9% | 0 | 0% |
| Pruritus | 9 | 81.8% | 0 | 0% |
| Pain | 6 | 54.5% | 1 | 9.1% |
| Cellulitis | 1 | 9.1% | 1 | 9.1% |
QTc, QT interval corrected for heart rate.
LDH, lactate dehydrogenase.
Clinical response to treatment and survival.
All the treatment area lesions of enrolled patients responded to ISPI. All subjects noted substantial palliative improvement in the region of the treatment site (Table 3). Eight patients had complete disappearance of tumor from the regional lymphatic drainage area, which is defined as complete local response (CLR) in Table 4. In this series, complete response (CR) was observed in six patients. This includes two patients that were previously reported.15 Since one patient had his single systemic metastasis treated by cyberknife, the CR was not exclusively due to ISPI, although he has remained tumor free for more than a year at the time of this writing (patient 10, Table 3). CLR was observed in the non-treatment site regional lesions solely as a distant, presumably immunological effect, in 4fourpatients.
Table 3.
Clinical characteristics of patients with melanoma and objective response
| No. | Age | Sex | AJCC stage | Primary site | Initial metastatic sites* | Cycles of treatment | Treatment site response | Non-treatment site regional response | Best overall response | Time to response (months) | Response duration (months) | Overall survival (months) |
| 1 | 64 | F | IV M1b | Left forearm | Bilateral lower lobe lung | 3 | CLR | CLR | CR | 1 | 12 | 66.4 |
| 2 | 67 | M | IIIC | Left side of head | - | 3 | CLR | CLR | CR | 4 | 7 | 64.2+ |
| 3 | 46 | F | IV M1c | Back | perihilar region of lungs, liver, uterus | 2 | PLR | N/A | PR | 1 | 2 | 8.5 |
| 4 | 63 | F | IIIB N2c | Left leg | - | 2 | PLR | PLR | PR | 3 | 4 | 44.9+ |
| 5 | 60 | F | IV M1A | Right frontal scalp | Distal skin site | 2 | CLR | CLR | CR | 2 | 4 | 6.6 |
| 6 | 87 | M | IIIC N2c | Right foot (plantar) | - | 6 | PLR | SD | SD | 1 | 0 | 15.5 |
| 7 | 71 | M | IV M1c | Eye | Liver | 1 | CLR | N/A | PD | 1 | 2 | 2.3 |
| 8 | 74 | M | IIIC N3 | Left lower back | - | 1 | CLR | N/A | CR | 8 | 2 | 38.7+ |
| 9 | 84 | M | IIIB N2c | Left foot | - | 1 | CLR | SD | CR | 6 | 1 | 6.0** |
| 10 | 85 | M | IV M1c | Left arm | Anterior mediastinum | 1 | CLR | CLR | CR*** | 1 | 8 | 22.5+ |
| 11 | 69 | M | IV M1c | Right forehead | Bone, lung | 1 | CLR | N/A | CR | 1 | 6 | 20.6+ |
Abbreviations: M, male; F, female; AJCC, American Joint Committee on Cancer; CLR, complete local response; PLR, partial local response; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; N/A, not available or not applicable.
In addition to skin, present at the time of study entry.
This patient died of progression of myelodysplasia to leukemia (unrelated tumor death).
The metastatic lesion was treated with cyberknife.
Table 4.
Local response criteria for evaluation of lesions
| For lesions at the treatment site | |
| Complete Local Response (CLR) | Disappearance of all treatment site lesions |
| Partial Local Response (PLR) | At least a 30% decrease in the sum of the local disease (LD) of treatment site lesions, taking as reference the baseline sum LD |
| Progressive Local Disease (PLD) | At least a 20% increase in the sum of the LD of target lesions, taking as reference the smallest sum LD recorded since the treatment started or the appearance of one or more new lesions |
| Stable Local Disease (SLD) | Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum LD since the treatment started |
| For lesions at the regional, non-treatment site* | |
| Complete Local Response (CLR) | Disappearance of all non-target regional lesions |
| Progressive Local Disease (PLD) | Appearance of one or more new regional lesions and/or unequivocal progression of existing non-treatment site regional lesions** |
| Incomplete Local Response/Stable Local Disease (SLD) | Persistence of one or more non-treatment site regional lesion(s) |
Refers to the regional lymphatic drainage of the treatment site, but not included in the imiquimod/laser treated site.
At least a 20% increase in the sum of the LD of the regional lesions, taking as reference the smallest sum LD recorded since the treatment started.
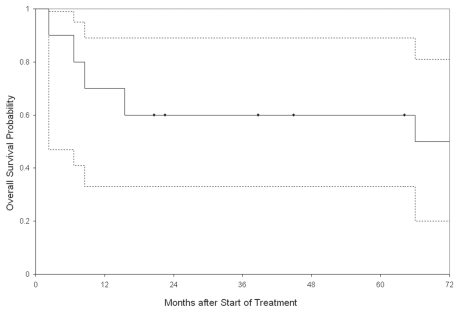
There were altogether ten evaluable patients since one patient died of progression of myelodysplasia to leukemia (patient 9, Table 3). Figure 1 shows the Kaplan-Meier plot of probability of overall survival (OS). Five patients were still alive and no indication of recurrence was observed at recent follow-up (Fig. 1). The median survival time could not be computed, because the survival distribution never fell below 50%. The probability of 12-month OS was 70%.
Figure 1.
Kaplan-Meier plot of the probability of overall survival. Dotted lines are the 95% confidence interval (95% CI) bands. Solid dots at 20.6, 22.5, 38.7, 44.9 and 64.2 months denote times for the six patients who were still alive at the time of last follow-up. The median survival time could not be computed, because the survival distribution never fell below 50%. The probability of 12 month overall survival was 70%.
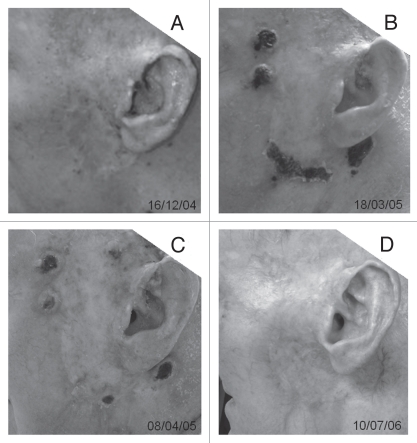
The photos of one IIIC melanoma patient's treatment area before, during and after ISPI are shown in Figure 2. This patient had failed radiation therapy as well as surgery. Figure 2A shows dozens of small black metastatic nodules with diameters of 1–2 mm, which are located on the left side of his head infiltrating the skin and subcutaneous tissues. The extensive acute radiation damage can be seen in this area. The nodules at the edge of lesion were irradiated by laser, in combination with the applications of imiquimod. Bullae can be seen after laser treatment. Figure 2B and C shows the changes of these nodules during the second cycle of ISPI. Figure 2D shows the disappearance of the nodules including those without laser irradiation after three cycles of ISPI.
Figure 2.
Photos of lesion of one patient with stage III C melanoma around the left ear treated by in situ photoimmunotherapy (ISPI). (A) Before ISPI, showing extensive acute radiation damage and numerous small black metastases. (B) Immediately after the first laser treatment in the second cycle of ISPI, bullae can be seen after laser treatment. (C) Immediately after the second laser treatment in the second cycle of ISPI. (D) All treatment-related ulcers have healed and the subject is free of clinically and radiologically detectable tumors after completion of three cycles of ISPI.
Discussion
The present study reports the preliminary clinical outcomes of the first series of 11 patients with advanced melanoma treated with in situ photoimmunotherapy. The results show ISPI to be a safe and useful palliative modality with a favorable toxicity profile compared with current methods of treatment. Less than 30% of the patients suffered grade 3 toxicity in one or more categories, primarily on the first treatment cycle and this level of severity did not recur with subsequent cycles of treatment. No grade 4 toxicity was observed. Complete response was observed in six patients. All the treatment site lesions of enrolled patients responded to ISPI, eight of which achieved CLR. Furthermore, CLR was observed in the non-treatment site (regional) lesions in four patients. The probability of 12-month OS was 70%.
As the immune systems of cancer patients are often compromised, tumor debris generated by laser photothermal therapy may not be sufficient to induce a potent antitumor response.16 Additional immunological stimulations are required to invoke the immune system to achieve an effective and protective immune response against residual tumor cells. Imiquimod is a FDA-approved prescription medication that acts as an immune response modifier. In clinical practice it is commonly used for the treatment of certain neoplastic and infectious diseases of the skin, including skin cancers as well as genital and common warts.11,12 Imiquimod activates immune cells through a toll-like receptor (TLR7), which are commonly involved in pathogen recognition.17 Cells activated by imiquimod via TLR7 secrete cytokines, including interferonα (IFN-α), interleukin-6 (IL-6) and tumor necrosis factorα (TNF-α).18 Imiquimod can also induce localization and activation of Langerhans cells in treated skin, allowing them to subsequently migrate to local lymph nodes to activate the adaptive immune system.19 Natural killer cells, macrophages and B lymphocytes are also activated by imiquimod.19
Immune related adverse events (IR-AEs) were observed in all patients in this study. The main side-effect appeared to be irritation and intermittent itching in the treatment site, which can occasionally be intense. Nausea may be a dose-limiting side-effect of intense topical imiquimod therapy. The grade 3 IR-AEs in this study, which were medically manageable, tended to occur in the first cycle of therapy and were reversible without sequelae. Previous ipilimumab studies suggested an association of IR-AEs with objective response.20–22 However, due to the limited number of patients, such an association was not observed in this study.
Lesimple et al. studied the immunologic effect of injecting mature peptide-loaded dendritic cells by intralymphatic and intranodal routes in 14 metastatic melanoma patients.23 Neither complete response nor partial response was observed and only two patients were stabilized for more than 3 months. They assumed that the particular type, the number and the mode of dendritic cells and the administration route might be the explanation for the limited success. The authors also thought that conventional oncologic criteria for clinical tumor response should be expanded for immunotherapy and in particular disease stabilization could be a sign of clinical benefit.23 Two other phase II clinical trials for metastatic melanoma reported the clinical efficiency of two different anti-CTLA-4 monoclonal antibodies, ipilimumab and tremelimumab.24,25 The objective response rate was 4.6% (4 of 88 patients) using ipilimumab and 10% (8 of 84 patients) using tremelimumab, respectively. All these results demonstrate that immunotherapy still faces challenges in the treatment of latestage melanoma.
Although this study only included a limited number of subjects with a relatively short post-treatment observation period, these patients fared well as a group, particularly the stage IIIC subjects with good initial treatment responses. Several patients have remained tumor free for more than 12 months. Patients in this group usually had small, slow growing and treatable recurrences, suggesting that ISPI therapy had favorably altered the host/tumor interaction. These specific cases strongly indicate the feasibility and efficacy of the combination of thermal therapy and immunological stimulation for the treatment of metastatic tumors.15
Therapeutic efficacy depends ultimately on the balance between disease progression and the immune effect induced by photoimmunotherapy. The general immune system status of melanoma patients is likely to play an important role in determining the final treatment outcome. The immune system of latestage cancer patients is generally thought to be less responsive than early stage patients, which may help explain the disparity in clinical outcomes between early and late stage patients.4 Many patients with late-stage diseases have been treated previously with chemotherapy and radiation therapy, creating selection pressure for the development of resistant tumor cells. Previous chemotherapy may also attenuate or degrade the ability of the immune system to respond, making immune tolerance and immunologic escape of tumor cells more likely.26 These general considerations of tumor biology may explain some of the ISPI failures seen in this study, similar to those seen with other immunotherapies, but they do not preclude other mechanisms.
Patients previously treated with chemotherapy reported that ISPI was more tolerable with less adverse impact on quality of life; they generally preferred ISPI to chemotherapy. In contrast to chemotherapy and isolated limb perfusion treatments, ISPI can be used repeatedly with relative safety in patients with significant comorbid diseases or conditions such as diabetes, advanced age or fragile cardiovascular status, as evidence by the observation of safety (Table 2). This form of therapy can be easily made available through outpatient treatment facilities, unlike some of the more technologically advanced experimental modalities for melanoma.
In summary, ISPI, a novel immunotherapeutic modality that has clinical benefits for a subset of late-stage melanoma patients. It is well tolerated compared with other treatments for metastatic melanoma. These data support further clinical development of ISPI for advanced melanoma and other late-stage, metastatic tumors. In melanoma patients with cutaneous metastases, ISPI should be considered as a viable treatment alternative to isolated limb perfusion and chemotherapy and may even be the treatment of choice if isolated limb perfusion is unavailable or for any reason is considered inappropriate for patients with stage IIIC melanoma.
Patients and Methods
Patients.
Patients were eligible if they were more than 18 years of age and had histologically confirmed stage III or IV melanoma according to the criteria of the 2001 modified American Joint Commission on Cancer (AJCC) staging system with at least one cutaneous lesion.27 All participants were required to comprehend and sign an informed consent form approved by the Institutional Review Board. Patients had to have an eastern Cooperative Oncology Group (ECOG) performance status of no more than 2. Eligibility criteria also included stable hematologic, renal and hepatic functions. Known HIV positive patients were excluded with additional exclusions for active autoimmune disease or corticosteroid dependence.
This protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved both by the Scientific Review Committee and the University of Oklahoma Institutional Review Board, which maintains a Federalwide Assurance (FWA) of Compliance with the DHHS regulations for research involving human participants. Informed written consent was obtained from all patients before treatment.
In situ photoimmunotherapy.
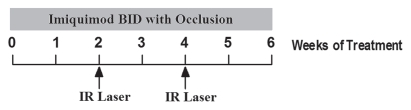
A 6-week cycle of ISPI was carried out for each designated 200 cm2 treatment area with the following four steps. (1) Two weeks of pretreatment with twice daily topical applications of imiquimod (Aldara™, 3M Pharmaceuticals, St. Paul, MN, 5% cream under plastic occlusion). (2) First laser treatment session at the beginning of week 2. Following local administration of anesthetic (lidocaine 1% with adrenaline), hypopigmented and nonpigmented melanoma nodules were injected with a solution of 0.25% indocyanine green (ICG) at a dose of 0.5 mL/cm3, prior to laser irradiation. The target lesions were then irradiated with a near-infrared 805-nm diode laser (Power density = 1.0 W/cm2, applied for 10 min per treated tumor). (3) Second laser treatment session at the beginning of week 4, assuming that target lesions were still present. (4) Two weeks of imiquimod application after second laser session. The timeline for each cycle of photothermal immunotherapy treatment is shown in Figure 3.
Figure 3.
Treatment cycle of photothermal immunotherapy for late-stage melanoma patients. The six-week treatment cycle was carried out on a designated 200 cm2 area of skin and consisted of six weeks of topical imiquimod treatment started two weeks before the first laser treatment session. Two laser treatment sessions were performed for each treatment cycle at the beginnings of week 2 and 4. Additional treatment cycles were carried out in the same treatment area or in different areas, if the response to the treatment is not complete.
Assessment of safety.
Weekly visits included adverse events (AEs) review and analysis of hematology, blood chemistry and baseline coagulation. Toxicity was assessed according to National Cancer Institute Common Toxicity Criteria, version 3.0.28 Baseline laboratory studies were performed at the beginning and at the end of the first treatment cycle.
Assessment of efficacy.
The primary efficacy parameter was the best overall response by investigator's assessment using the Response Evaluation Criteria in Solid Tumors (RECIST). The best overall response was assessed 4 weeks after the first 6-week cycle if only one cycle was accomplished or after every third cycle if multiple treatment cycles were carried out.
In order to evaluate the palliative local effects of ISPI in addition to its systemic effects, additional guidelines based on RECIST principles were developed for evaluating responses in the regional (local) lymphatic drainage of the treatment sites. RECIST response criteria for target (systemic) lesions and non-target (systemic) lesions were extended to include similar evaluations for local responses in treatment site (regional) lesions and non-treatment site (regional) lesions. Therefore, in addition to the usual RECIST criteria (CR or complete systemic response, PR or partial systemic response, PD or progressive systemic disease and SD or stable systemic disease), we applied similar criteria for local responses (CLR or complete local response, PLR or partial local response, PLD or progressive local disease and SLD or stable local disease). See Table 4 for a complete listing of the definitions of the local responses.
Statistical analyses.
This preliminary clinical trial was a single-center, open-label, single-arm phase I/II study in patients with metastatic melanoma. Survival rates with 95% confidence intervals were estimated using the Kaplan-Meier method.29
Acknowledgements
This research is supported in part by grants from the American Cancer Society (IRG-05-066-01 ACS Institutional Research Seed Grant), from the US National Institutes of Health (P20 RR016478 from the INBRE Program of the National Center for Research Resources) and from the National Natural Science Foundation of China (No. 81000994).
References
- 1.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 2.Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, et al. Malignant melanoma in the 21st century, part 2: staging, prognosis and treatment. Mayo Clin Proc. 2007;82:364–380. doi: 10.4065/82.4.490. [DOI] [PubMed] [Google Scholar]
- 3.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. Nat Clin Pract Oncol. 2008;5:4–5. [Google Scholar]
- 4.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood JM, Tarhini AA, Panelli MC, Moschos SJ, Zarour HM, Butterfield LH, et al. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26:3445–3455. doi: 10.1200/JCO.2007.14.6423. [DOI] [PubMed] [Google Scholar]
- 6.Chen WR, Adams RL, Carubelli R, Nordquist RE. Laser-photosensitizer assisted immunotherapy: A novel modality in cancer treatment. Cancer Lett. 1997;115:25–30. doi: 10.1016/s0304-3835(97)04707-1. [DOI] [PubMed] [Google Scholar]
- 7.den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–4029. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 8.Chen WR, Zhu WG, Dynlacht JR, Liu H, Nordquist RE. Long-term tumor resistance induced by laser photo-immunotherapy. Int J Cancer. 1999;81:808–812. doi: 10.1002/(sici)1097-0215(19990531)81:5<808::aid-ijc23>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Chen WR, Singhal AK, Liu H, Nordquist RE. Antitumor immunity induced by laser immunotherapy and its adoptive transfer. Cancer Res. 2001;61:459–461. [PubMed] [Google Scholar]
- 10.Chen WR, Jeong SW, Lucroy MD, Wolf RF, Howard EW, Liu H, et al. Induced antitumor immunity against DMBA-4 metastatic mammary tumors in rats using laser immunotherapy. Int J Cancer. 2003;107:1053–1057. doi: 10.1002/ijc.11501. [DOI] [PubMed] [Google Scholar]
- 11.Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, et al. Taking a toll on human disease: toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther. 2004;4:1129–1138. doi: 10.1517/14712598.4.7.1129. [DOI] [PubMed] [Google Scholar]
- 12.Hesling C, D'Incan M, Mansard S, Franck F, Corbin-Duval A, Chèvenet C, et al. In vivo and in situ modulation of the expression of genes involved in metastasis and angiogenesis in a patient treated with topical imiquimod for melanoma skin metastases. Br J Dermatol. 2004;150:761–767. doi: 10.1111/j.0007-0963.2004.05898.x. [DOI] [PubMed] [Google Scholar]
- 13.Steinmann A, Funk JO, Schuler G, von den Driesch P. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555–556. [PubMed] [Google Scholar]
- 14.Bong AB, Bonnekoh B, Franke I, Schön MP, Ulrich J, Gollnick H. Imiquimod, a topical immune response modifier, in the treatment of cutaneous metastases of malignant melanoma. Dermatology. 2002;205:135–138. doi: 10.1159/000063904. [DOI] [PubMed] [Google Scholar]
- 15.Naylor MF, Chen WR, Teague TK, Perry LA, Nordquist RE. In situ photoimmunotherapy: a tumour-directed treatment for melanoma. Br J Dermatol. 2006;155:1287–1292. doi: 10.1111/j.1365-2133.2006.07514.x. [DOI] [PubMed] [Google Scholar]
- 16.Jäger E, Jäger D, Knuth A. Antigen-specific immunotherapy and cancer vaccines. Int J Cancer. 2003;106:817–820. doi: 10.1002/ijc.11292. [DOI] [PubMed] [Google Scholar]
- 17.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 18.Bilu D, Sauder DN. Imiquimod: modes of action. Br J Dermatol. 2003;149:5–8. doi: 10.1046/j.0366-077x.2003.05628.x. [DOI] [PubMed] [Google Scholar]
- 19.Gerster JF, Owens ML, Slade HB, Tomai MA. Imiquimod applied topically: a novel immune response modifier and new class of drug. Int J Immunopharmacol. 1999;21:1–14. doi: 10.1016/s0192-0561(98)00068-x. [DOI] [PubMed] [Google Scholar]
- 20.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 22.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anticytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesimple T, Neidhard EM, Vignard V, Lefeuvre C, Adamski H, Labarrière N, et al. Immunologic and clinical effects of injecting mature peptide-loaded dendritic cells by intralymphatic and intranodal routes in metastatic melanoma patients. Clin Cancer Res. 2006;12:7380–7388. doi: 10.1158/1078-0432.CCR-06-1879. [DOI] [PubMed] [Google Scholar]
- 24.Camacho LH, Antonia S, Sosman J, Kirkwood JM, Gajewski TF, Redman B, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27:1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 25.Weber JS, O'Day S, Urba W, Powderly J, Nichol G, Yellin M, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–5956. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 26.Prestwich RJ, Errington F, Hatfield P, Merrick AE, Ilett EJ, Selby PJ, et al. The immune system—is it relevant to cancer development, progression and treatment? Clin Oncol (R Coll Radiol) 2008;20:101–112. doi: 10.1016/j.clon.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, author. Common terminology criteria for adverse events v3.0 (CTCAE) [Sep 28, 2009]. Available: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 29.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]