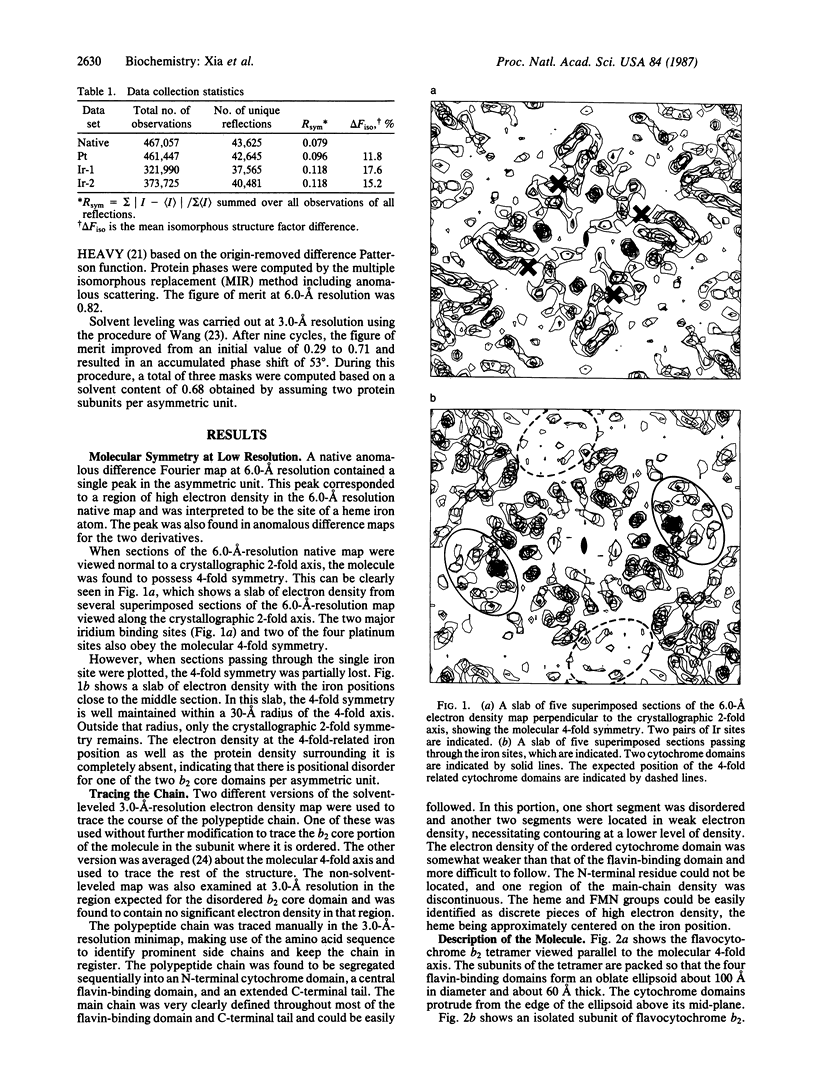
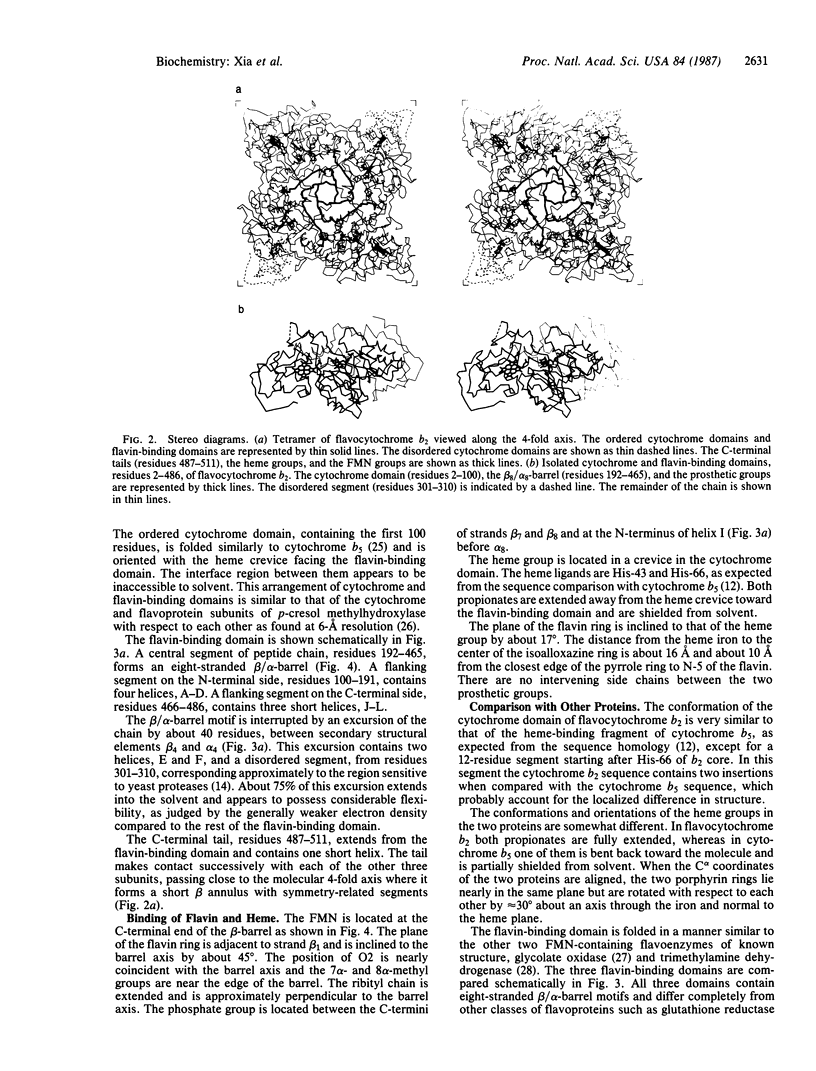
Abstract
The structure of flavocytochrome b2 from baker's yeast was solved at 3.0-A resolution by the multiple isomorphous replacement method combined with solvent leveling procedures, using data collected from an area detector. The tetramer of Mr 230,000 has 4-fold symmetry. Each subunit contains a cytochrome domain consisting of the first 100 residues, a flavin-binding domain containing the next 386 residues, and an extended C-terminal tail of 25 residues. The cytochrome domain closely resembles microsomal cytochrome b5, whereas the flavin-binding domain contains a parallel beta 8/alpha 8 barrel motif similar to glycolate oxidase and trimethylamine dehydrogenase. Two of the four cytochrome domains are disordered in the crystals. The flavin ring and heme group are separated by about 16 A between their centers, and their planes are inclined by about 17 degrees to each other.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEBY C. A., MORTON R. K. Crystalline cytochrome b2 and lactic dehydrogenase of yeast. Nature. 1954 Apr 24;173(4408):749–752. doi: 10.1038/173749a0. [DOI] [PubMed] [Google Scholar]
- Baudras A., Krupa M., Labeyrie F. Molecular complexes between cytochrome b2. (Yeast L(+)lactate: cytochrome c oxidoreductase) and cytochrome c in crystalline state and in solution. Eur J Biochem. 1971 May 11;20(1):58–64. doi: 10.1111/j.1432-1033.1971.tb01362.x. [DOI] [PubMed] [Google Scholar]
- Capeillere-Blandin C. Transient kinetics of the one-electron transfer reaction between reduced flavocytochrome b2 and oxidized cytochrome c. Evidence for the existence of a protein complex in the reaction. Eur J Biochem. 1982 Nov 15;128(2-3):533–542. doi: 10.1111/j.1432-1033.1982.tb06998.x. [DOI] [PubMed] [Google Scholar]
- Capeillère-Blandin C., Bray R. C., Iwatsubo M., Labeyrie F. Flavocytochrome b2: kinetic studies by absorbance and electron-paramagnetic-resonance spectroscopy of electron distribution among prosthetic groups. Eur J Biochem. 1975 Jun;54(2):549–566. doi: 10.1111/j.1432-1033.1975.tb04168.x. [DOI] [PubMed] [Google Scholar]
- Capeillère-Blandin C. Flavocytochrome b2: simulation studies of the electron-transfer reactions among the prosthetic groups. Eur J Biochem. 1975 Aug 1;56(1):91–101. doi: 10.1111/j.1432-1033.1975.tb02210.x. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Ghrir R., Lederer F. Study of a zone highly sensitive to proteases in flavocytochrome b2 from Saccharomyces cerevisiae. Eur J Biochem. 1981 Nov;120(2):279–287. doi: 10.1111/j.1432-1033.1981.tb05701.x. [DOI] [PubMed] [Google Scholar]
- Guiard B., Groudinsky O., Lederer F. Homology between bakers' yeast cytochrome b2 and liver microsomal cytochrome b5. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2539–2543. doi: 10.1073/pnas.71.6.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard B., Lederer F. The "cytochrome b5 fold": structure of a novel protein superfamily. J Mol Biol. 1979 Dec 15;135(3):639–650. doi: 10.1016/0022-2836(79)90169-4. [DOI] [PubMed] [Google Scholar]
- Guiard B. Structure, expression and regulation of a nuclear gene encoding a mitochondrial protein: the yeast L(+)-lactate cytochrome c oxidoreductase (cytochrome b2). EMBO J. 1985 Dec 1;4(12):3265–3272. doi: 10.1002/j.1460-2075.1985.tb04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacq C., Lederer F. Cytochrome b2 from bakers' yeast (L-lactate dehydrogenase). A double-headed enzyme. Eur J Biochem. 1974 Jan 16;41(2):311–320. doi: 10.1111/j.1432-1033.1974.tb03271.x. [DOI] [PubMed] [Google Scholar]
- Lederer F., Cortial S., Becam A. M., Haumont P. Y., Perez L. Complete amino acid sequence of flavocytochrome b2 from baker's yeast. Eur J Biochem. 1985 Oct 15;152(2):419–428. doi: 10.1111/j.1432-1033.1985.tb09213.x. [DOI] [PubMed] [Google Scholar]
- Lim L. W., Shamala N., Mathews F. S., Steenkamp D. J., Hamlin R., Xuong N. H. Three-dimensional structure of the iron-sulfur flavoprotein trimethylamine dehydrogenase at 2.4-A resolution. J Biol Chem. 1986 Nov 15;261(32):15140–15146. [PubMed] [Google Scholar]
- Lindqvist Y., Brändén C. I. Structure of glycolate oxidase from spinach. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6855–6859. doi: 10.1073/pnas.82.20.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K., STURTEVANT J. M. KINETIC INVESTIGATIONS OF YEAST L-LACTATE DEHYDROGENASE (CYTOCHROME B2). I. THE DEHYDROGENATION OF L-LACTATE IN THE PRESENCE AND ABSENCE OF FERRICYANIDE AS ELECTRON ACCEPTOR. J Biol Chem. 1964 May;239:1614–1624. [PubMed] [Google Scholar]
- Mathews F. S., Argos P., Levine M. The structure of cytochrome b 5 at 2.0 Angstrom resolution. Cold Spring Harb Symp Quant Biol. 1972;36:387–395. doi: 10.1101/sqb.1972.036.01.050. [DOI] [PubMed] [Google Scholar]
- Mathews F. S., Lederer F. Crystallographic study of bakers' yeast cytochrome b2. J Mol Biol. 1976 Apr 25;102(4):853–857. doi: 10.1016/0022-2836(76)90295-3. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Determination of molecular weight from protein crystals. J Mol Biol. 1974 Feb 5;82(4):513–526. doi: 10.1016/0022-2836(74)90245-9. [DOI] [PubMed] [Google Scholar]
- Mével-Ninio M. Subunit structure of L-lactate dehydrogenase (cytochrome b2) of Saccharomyces cerevisiae. Ultracentrifugation studies. Eur J Biochem. 1972 Feb 15;25(2):254–261. doi: 10.1111/j.1432-1033.1972.tb01691.x. [DOI] [PubMed] [Google Scholar]
- Pompon D. Flavocytochrome b2 from baker's yeast. Computer-simulation studies of a new scheme for intramolecular electron transfer. Eur J Biochem. 1980 May;106(1):151–159. [PubMed] [Google Scholar]
- Pompon D., Iwatsubo M., Lederer F. Flavocytochrome b2 (Baker's yeast). Deuterium isotope effect studied by rapid-kinetic methods as a probe for the mechanism of electron transfer. Eur J Biochem. 1980 Mar;104(2):479–488. doi: 10.1111/j.1432-1033.1980.tb04450.x. [DOI] [PubMed] [Google Scholar]
- Salemme F. R. An hypothetical structure for an intermolecular electron transfer complex of cytochromes c and b5. J Mol Biol. 1976 Apr 15;102(3):563–568. doi: 10.1016/0022-2836(76)90334-x. [DOI] [PubMed] [Google Scholar]
- Shamala N., Lim L. W., Mathews F. S., McIntire W., Singer T. P., Hopper D. J. Structure of an intermolecular electron-transfer complex: p-cresol methylhydroxylase at 6.0-A resolution. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4626–4630. doi: 10.1073/pnas.83.13.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simondsen R. P., Weber P. C., Salemme F. R., Tollin G. Transient kinetics of electron transfer reactions of flavodoxin: ionic strength dependence of semiquinone oxidation by cytochrome c, ferricyanide, and ferric ethylenediaminetetraacetic acid and computer modeling of reaction complexes. Biochemistry. 1982 Dec 7;21(25):6366–6375. doi: 10.1021/bi00268a008. [DOI] [PubMed] [Google Scholar]
- Strahs G., Kraut J. Low-resolution electron-density and anomalous-scattering-density maps of Chromatium high-potential iron protein. J Mol Biol. 1968 Aug 14;35(3):503–512. doi: 10.1016/s0022-2836(68)80010-5. [DOI] [PubMed] [Google Scholar]
- Tegoni M., Mozzarelli A., Rossi G. L., Labeyrie F. Complex formation and intermolecular electron transfer between flavocytochrome b2 in the crystal and cytochrome c. J Biol Chem. 1983 May 10;258(9):5424–5427. [PubMed] [Google Scholar]
- Thomas M. A., Gervais M., Favaudon V., Valat P. Study of the Hansenula anomala yeast flavocytochrome-b2-cytochrome-c complex 2. Localization of the main association area. Eur J Biochem. 1983 Oct 3;135(3):577–581. doi: 10.1111/j.1432-1033.1983.tb07691.x. [DOI] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Drenth J., Schulz G. E. Comparison of the three-dimensional protein and nucleotide structure of the FAD-binding domain of p-hydroxybenzoate hydroxylase with the FAD- as well as NADPH-binding domains of glutathione reductase. J Mol Biol. 1983 Jul 5;167(3):725–739. doi: 10.1016/s0022-2836(83)80106-5. [DOI] [PubMed] [Google Scholar]


