Abstract
Androgen dependent induction of the eTs related gene (ERG) expression in more than half of all prostate cancers results from gene fusions involving regulatory sequence of androgen regulated genes (i.e., TMPRSS2, SLC45A3 and NDRG1) and protein coding sequence of the ERG. Emerging studies in experimental models underscore the functions of ERG in prostate tumorigenesis. however, biological and biochemical functions of ERG in prostate cancer (Cap) remain to be elucidated. This study suggests that ERG activation plays a role in prostaglandin signaling because knockdown of ERG expression in TMPRSS2-ERG fusion containing Cap cells leads to altered levels of the 15-hydroxy-prostaglandin dehydrogenase (HPGD), a tumor suppressor and prostaglandin catabolizing enzyme and prostaglandin E2 (PGE2). We demonstrate that HPGD expression is regulated by the binding of the ERG protein to the core promoter of this gene. Moreover, prostaglandin E2 dependent cell growth and urokinase-type plasminogen activator (uPA) expression are also affected by ERG knockdown. Together, these data imply that the ERG oncoprotein in CaP cells positively influence prostaglandin mediated signaling, which may contribute to tumor progression.
Key words: prostate, cancer, ETS, ERG, TMPRSS2, oncogene, HPGD, tumor suppressor, prostaglandin, inflammation
Introduction
Prevalent gene fusions involving regulatory sequences of androgen receptor (AR) regulated prostate associated genes (predominantly TMPRSS2) and protein coding sequences of the nuclear transcription factors in the ETS transcription family (predominantly ERG) result in overexpression of ERG in two-thirds of prostate cancer (CaP) patients.1–13 Emerging studies in experimental models suggest oncogenic functions of ERG and ETV1 in CaP.6,14–17 Our earlier report suggested a regulatory role of the ERG oncoprotein in prostate epithelial differentiation program and activation of C-MYC in CaP cells.5 Recent studies in mouse models show cooperative effects of ERG overexpression and the PI3-Kinase pathway in CaP progression.14,16,18 Thus, a better understanding of ERG functions in CaP biology may lead to rational therapeutic strategies for ERG positive tumors.
During our recent evaluation of ERG downstream transcriptional targets, we noted consistent induction of the 15-hydroxyprostaglandin dehydrogenase gene (HPGD) in response to ERG knockdown.5 HPGD is an important enzyme in prostaglandin metabolism that catalyzes the oxidation of prostaglandins into inactive keto-metabolites. HPGD physiologically antagonizes COX-2, a prostaglandin-synthesizing enzyme, thus playing a critical role in diverse physiological aspects ranging from inflammation to cancer.19,20 Recent studies have indicated that HPGD is downregulated in a majority of lung, colon, breast and bladder cancers. Tumor suppressor functions of HPGD have been demonstrated in cell culture and mouse models.21–26 Moreover, accumulating evidence suggests the involvement of HPGD in chemopreventive effects of non-steroid antiinflammatory drugs (NSAIDs). Several NSAIDs, including Celecoxib, Indomethacin and Flurbiprofen, exert their antiinflammatory effects by inducing HPGD or inhibiting COX2.27–29 However, other studies have shown HPGD involvement with cell differentiation and immune regulation.30,31 Due to these diverse functions of HPGD and the suggested roles of inflammation in prostate cancer,32 we have focused on the regulation of HPGD and related signaling events in the context of TMPRSS2-ERG fusion in prostate cancer cells.
Results
HPGD expression is upregulated in response to ERG inhibition.
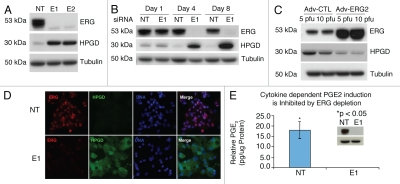
Evaluation of ERG siRNA (E1, E2) treatment in the TMPRSS2-ERG expressing human prostate cancer cell line (VCaP cells) revealed robust upregulation of HPGD (Fig. 1A and B). Consistent with this observation, VCaP cells infected with an adenovirus vector expressing wild type ERG-2 (Adv-E2) inhibited HPGD protein expression (Fig. 1C). Further, immunofluorescence staining showed that cells expressing siRNA to ERG showed a robust reduction of ERG transcription factor in the nuclei of VCaP cells as well as an overexpression of cytoplasmic HPGD (Fig. 1D).
Figure 1.
ERG regulates HPGD expression and PGE2 in VCaP cells. (A) VCaP cells transfected with ERG siRNA (E1, E2) or with non-targeting siRNa (NT) from triplicate experiments were harvested on day 4 post transfection and processed for immunoblot analysis for detecting ERG and HPGD. Tubulin expression was used as the input control. (B) Induction of HPGD expression in response to ERG knockdown in a time dependent fashion. Transfected VCaP cells, as described in the Materials and Methods, were harvested on days 1, 4 and 8 post-transfection and processed for immunoblot assay for detecting ERG and HPGD proteins. (C) Ectopic expression of ERG decreased HPGD protein levels. VCaP cells were infected with either wild type ERG2 adenovirus expression vector (Adv-ERG2) or control adenovirus expression vector (Adv-CTL). (D) Sub-cellular localization of ERG and HPGD in response to ERG siRNa (E1) or non-targeting siRNA (NT) was assessed by immunofluorescence assay in VCaP cells. (E) PGE2 levels were measured in the conditioned medium of VCaP cells transfected with either non-targeting siRNA (NT) or ERG siRNA (E1) in the presence of IL-1β. Cells were harvested after 24 h and total lysate were prepared for PGE2 normalization (insert).
Cytokine mediated PGE2 induction is inhibited by ERG knockdown.
To assess the effect of ERG inhibition on prostaglandin E2 (PGE2), VCaP cells transfected with ERG siRNA (E1) or non-targeting siRNA (NT) were analyzed for Interleukin-1beta (IL-1β) induced PGE2. PGE2 was significantly inhibited in ERG siRNA transfected VCaP cells in comparison to the control NT siRNA transfected VCaP cells (Fig. 1E).
ERG is recruited to the core promoter of HPGD.
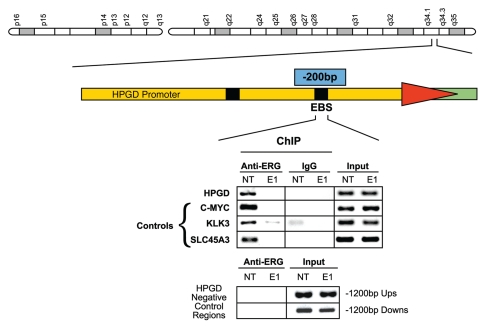
An ETS binding site was identified in the core promoter at −200 bp upstream of the HPGD transcription initiation site by using MatInspector software (Genomatix GmbH, Munich, Germany), consistent with earlier reports showing the presence of ETS transcription factor binding sites within the HPGD promoter upstream sequences.33,34 Chromatin immunoprecipitation assays (ChIP) confirmed the specific recruitment of the ERG oncoprotein to the predicted ETS site of the HPGD core promoter, which was significantly reduced in ERG siRNA treated VCaP cells (Fig. 2). The observations of ERG-induced alterations in HPGD gene expression, along with the recruitment of ERG to the HPGD promoter suggested that ERG directly regulates HPGD expression in prostate tumor cells.
Figure 2.
ERG is recruited to the HPGD core promoter ETS binding site in VCaP cells. ERG recruitment is specific to the core ETS binding site of HPGD and is eliminated by ERG siRNA treatment. Upstream and downstream sequences with no ETS binding element were used as negative controls. In the ChIP assay recruitment of ERG to the KLK3/PSA, C-MYC and SLC45A3 gene promoter upstream regions were also tested as positive controls of ERG binding as similar data have been reported before (controls).5 Input indicates control genomic DNA amplicons.
PGE2 dependent cell growth is abrogated by depletion of ERG.
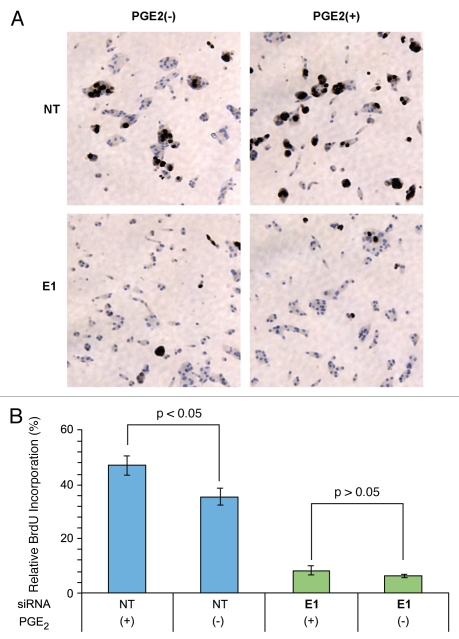
Because ERG knockdown resulted in the overexpression of HPGD, which is known to metabolize PGE2 into inactive keto-metabolites, we hypothesized that ERG knockdown would lead to inhibition of PGE2 associated biological and biochemical functions. PGE2 has been shown to induce growth in prostate cancer cell culture models.35 VCaP cells were transfected with either of ERG siRNA or NT siRNA in the presence or absence of PGE2. As expected, PGE2 treatment increased the incorporation of Bromodeoxyuridine (BrdU) into the nucleus of control NT siRNA transfected VCaP cells. In contrast, significantly less BrdU incorporation was observed in ERG siRNA treated cells (Fig. 3A and B). These findings indicate that PGE2 mediated cell growth is inhibited when ERG is depleted from prostate cancer cells.
Figure 3.
PGE2 induced CaP cell growth is inhibited by ERG knockdown. VCaP cells were evaluated for cell growth in the presence (+) or absence (−) of PGE2 in response to non-targeting siRNA (NT) or ERG siRNA (E1). (A) BrdU nuclear staining of proliferating cells. In the presence of PGE2, VCaP cells with control NT siRNA showed dramatically increased BrdU nuclear staining in response to PGE2 treatment. In contrast, ERG siRNa transfected VCaP cells show significantly decreased BrdU nuclear staining both in the presence or absence of PGE2. (B) Relative percent of BrdU positive cells. In control NT siRNA transfected cells, BrdU incorporation is higher in PGE2 treated cells than in the untreated group. In contrast, BrdU incorporation is low in both ERG siRNA treated groups irrespective to PGE2 treatment.
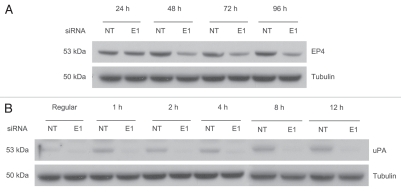
ERG knockdown reduced PGE2 receptor 4 (E prostanoid receptor type 4, EP4) expression.
To further investigate ERG involvement in the prostaglandin signaling, we evaluated the relationship between ERG and the PGE2 receptor, EP4. ERG depletion decreased EP4 protein expression in VCaP cells, indicating that ERG does affect EP4, a key regulator of the prostaglandin pathway (Fig. 4A).
Figure 4.
EP4 levels are reduced and uPA induction is inhibited in response to ERG knockdown. (A) EP4 protein levels were evaluated by immunoblot assays. Result shows that EP4 is downregulated in response to ERG knockdown. (B) expression of upa was assessed by immunoblotting at 1, 2, 4, 8 and 12 hours time points in VCaP cells treated with PGE2 as described in the Methods and Materials. PGE2 induces uPA in NT siRNA treated cells, whereas, ERG knockdown prevents uPA induction.
PGE2 induced urokinase-type plasminogen activator (uPA) expression in prostate cancer cells is inhibited by ERG knockdown.
Urokinase-type plasminogen activator (uPA) mediated signaling has been implicated in tumor cell invasion, survival and metastasis in a variety of cancers.36,37 PGE2 has been reported to increase cell growth, induce uPA expression and enhance tumor metastasis and angiogenesis in prostate cancer.38 Studies evaluating ERG functions have also shown that ERG induces cell invasion associated genes including uPA.15,17 To determine the effect of ERG on PGE2 induced uPA expression, TMPRSS2-ERG harboring VCaP cells were treated with PGE2. Expression of uPA protein in response to PGE2 treatment was inhibited by ERG knockdown (Fig. 4B).
HPGD expression is attenuated in TMPRSS2-ERG fusion positive prostate cancer specimens.
HPGD expression is down-regulated in many solid tumors.22,23 On the basis of our data, we hypothesized that HPGD expression will be decreased in CaP cells harboring the TMPRSS2-ERG fusion. We compared HPGD expression levels between TMPRSS2-ERG positive and negative prostate tumor specimens in 28 patients. For this analysis, we selected well-to-moderately differentiated tumor specimens in order to minimize potentially confounding genetic alterations associating with more advanced tumors with poorly differentiated phenotypes. Laser capture micro-dissection (LCM) derived RNA samples were evaluated for HPGD expression levels. The results, although not reaching statistical significance, revealed a trend towards decreased HPGD RNA expression in TMPRSS2-ERG positive tumors, which is consistent with the reciprocal relationship of HPGD expression observed in response to ERG knockdown in VCaP cells (Fig. 5). A more uniform suppression of the HPGD gene was found in the fusion positive tumor cells while the fusion negative tumor cells showed heterogeneity of expression with most of the samples showing higher HPGD expression.
Figure 5.
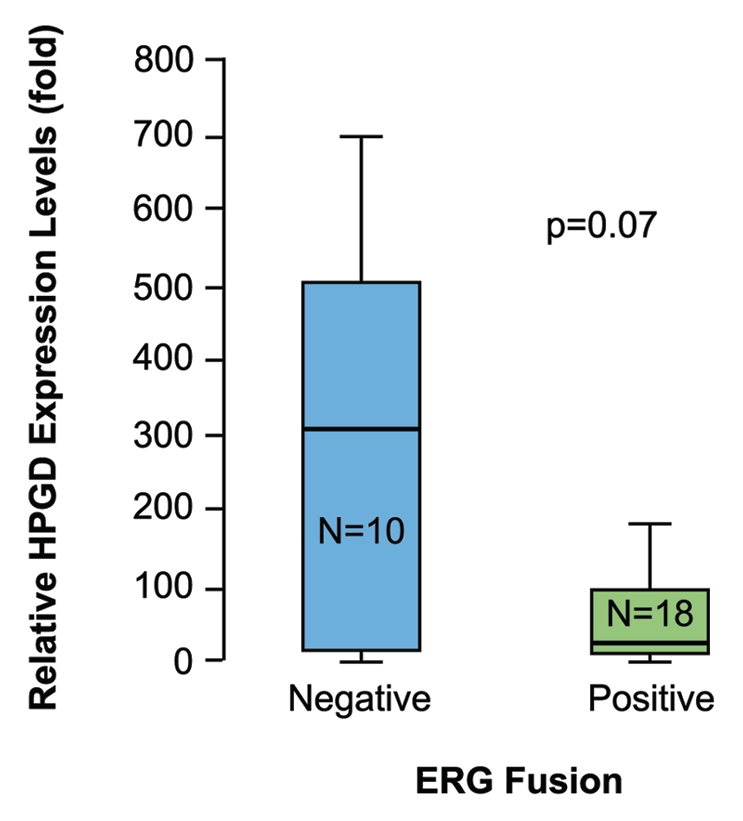
HPGD transcript levels are lower in TMPRSS2-ERG fusion positive prostate tumor epithelial cells than in fusion negative tumor cells. Total RNa isolated from LCM prostate epithelial tumor cells and VCaP cells were used for evaluation of HPGD and GAPDH expression. Tissue expression levels of HPGD normalized for GAPDH are shown in comparison to the normalized expression levels in VCaP cells. Evaluation of HPGD expression between TMPRSS2-ERG fusion positive (+) and negative (−) prostate tumors revealed a trend towards decreased HPGD RNA expression in fusion positive tumors.
Discussion
It is now established that ERG expression in CaP is activated by gene fusions involving androgen regulated promoter sequences, such as, TMPRSS2, SLC45A3, NDRG1 and the ERG protein coding sequence, which represent one of the most common oncogenic alterations in CaP.1,2,8,39,40 However, the biochemical mechanisms by which elevated ERG contributes to the development and/or progression of CaP needs further clarification. Our findings showing that ERG alterations may influence the components of the prostaglandin signaling pathway in CaP cells; HPGD in particular, suggest a new biological function of ERG in prostate cancer cells.
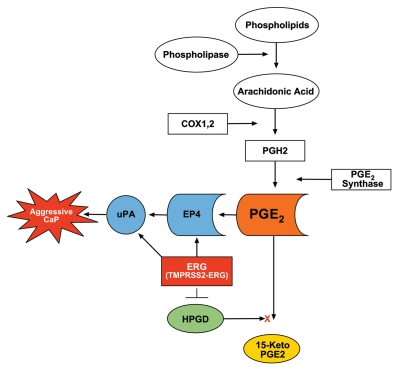
On the basis of data presented in this study we propose that elevated expression of ERG leads to decreased HPGD, increased prostaglandin E2 and its EP4 receptor; the cumulative effects of these changes may contribute to increased tumor cell growth and invasion (Fig. 6). Signal transduction pathways such as C-MYC, the PI3K/PTEN/AKT axis or AR-mediated signaling may also influence ERG functions in CaP progression as defined by recent studies.5,14,16,18,41,42 Our study shows that ERG regulates the expression of HPGD, suggesting that ERG plays a role in prostate tumorigenesis in part through modulation of prostaglandin signaling pathway. We have previously reported that ERG activates C-MYC and abrogates differentiation genes (KLK3/PSA, MSMB, SLC45A3).5 Although the role of HPGD in prostate cells differentiation is unclear, it is intriguing that other reports have shown a role of HPGD regulating cell differentiation.30
Figure 6.
Proposed model for ERG functions in prostaglandin signaling pathway. Inhibition of HPGD as result of TMPRSS2-ERG overexpression prevents PGE2 catabolism, thus accumulation of PGE2 will result in uPA activation and cell growth, contributing to the progression of CaP. ERG directly binds to the promoter of uPA.
Several biological processes that are important in tumorigenesis such as angiogenesis, cell proliferation and motility, inhibition of the immune responses and apoptosis are known to be regulated by prostaglandins, specifically PGE2.43 Steady state cellular levels of PGE2 depend on the relative rates of COX-2/PGE synthase dependent biosynthesis and HPGD dependent degradation.25,43 Inflammation, as well as alterations in enzymes involved in prostaglandin synthesis or degradation have been suggested to play roles in CaP.22,23,32,35,44,45 Our study suggests the biological potential for ERG activation to interfere with prostaglandin signaling and the associated physiological context such as inflammation, suspected of fueling prostate tumorigenesis.32,44 It is also important to point out that in contrast to HPGD, ERG does not affect COX-2 expression in ERG siRNA treated VCaP cells (data not shown).
In summary, this study establishes that ERG knockdown results in overexpression of HPGD, downregulation of EP4, inhibition of PGE2 induction of cell growth and uPA expression, suggesting a role for the ERG oncoprotein in inflammation and prostate tumorigenesis. Given that TMPRSS2-ERG fusion occurs relatively early and is a common oncogenic activation in prostate tumorigenesis, ERG or ERG network components such as HPGD may be further evaluated as a target for prevention, as well as early therapeutic intervention in CaP.
Methods and Materials
Cell culture and treatment.
Human prostate tumor cell line, VCaP, was purchased from American Type Culture Collection (ATCC). Cells were maintained in DMEM (cat.# 30-2002, ATCC), supplemented with 10% of fetal bovine serum (cat.#21640-079, Invitrogen) in a humidified CO2 (5%) incubator at 37°C. Synthetic androgen analog, R1881 (cat.# NLP005005MG, Perkin Elmer) at 0.1 nM concentrations was used for androgen treatment. For prostaglandin E2 treatment, 10 µM of prostaglandin E2 (cat.# 14010, Cayman Chemical) was used. Five pico grams of IL-1β (cat.#19401-5UG, Sigma) was used for IL-1β treatment. Ten millimolar of BrdU was used for BrdU cell proliferation assay (cat.#550891, BD Biosciences).
Inhibition of endogenous ERG by siRNA.
Small interfering RNA (siRNA) oligo duplex (5′-CGA CAU CCU UCU CUC ACA UAU-3′ and 5′-UGA UGU UGA UAA AGC CUU A-3′) against human ERG gene (E1, E2 respectively) (Gene ID: 2078, Accession: NM_004449) was purchased from Dharmacon. Two different siRNA each targeting ERG were used to assure that the effect we observed was specific and was not due to off target or none specific effect of siRNA (Fig. 1A). Since both siRNA showed identical results, one siRNA was selected and used in subsequent experiments. Non-targeting (NT) siRNA duplexes were used as control (D-001206-13-20; Dharmacon). Adenovirus expression vectors encoding the wild type protein products of ERG isoform 2 (Accessions: NM_004449.4) and adenovirus control vector were previously described in reference 5. VCaP cells were cultured in DMEM supplemented with 10% of Charcoal/Dextran stripped serum (cFBS), (cat.#100-119, Gemini Bio-Products) for 72 hours followed by transfection with 50 nM of NT siRNA or ERG siRNA using Lipofectamine 2000 (cat.#11668-109, Invitrogen). Twenty-four hours after transfection, cells were treated with 0.1 nM of R1881. Cells were harvested at days 1, 4 and 8 post treatments for western blot analysis. For ectopic expression of ERG, VCaP cells were infected with adenoviral ERG (Adv-ERG2) or control (Adv-CTL) expression vectors. Infected cells were incubated for 48 hours followed by cell harvesting and western blot analysis.
PGE2 treatment.
VCaP cells were transfected with either NT siRNA or ERG siRNA. Cells were treated with 10 µM of PGE2 (cat.# 14010, Cayman Chemicals) at the time of R1881 treatment and cell growth medium were changed every two days with fresh DMEM containing 0.1 nM of R1881 and 10 µM of PGE2. Cells were then harvested at days 1, 4 and 8 for cell growth assay. For assaying PGE2 mediated signaling read-out, cells were treated with 10 µM of PGE2 and harvested after 1, 2, 4, 8 and 12 hours post treatment.
Western blot assays.
Cells were lysed in Mammalian Protein Extraction Reagent (M-PER) (cat.# 78501, Pierce) containing protease inhibitor cocktail and phosphatase inhibitor cocktails I and II (cat.# P8340, P2850 and P5726, respectively, Sigma). Cell lysates equivalent to 50 µg of protein were separated on 4–12% Bis-Tris Gel (cat.#NP0335BOX, Invitrogen) and transferred to PVDF membrane (cat.# LC2005, Invitrogen). Membranes were incubated with primary antibodies: Anti- ERG monoclonal antibody (CPDR ERG-MAb, generated in our laboratory),46,47 anti-HPGD (cat.# sc-48910, Santa Cruz Biotechnology), anti-α-Tubulin (cat.# sc-5286, Santa Cruz Biotechnology), at 4°C for 12 hours. Membranes were washed three times for five minutes each at room temperature followed by treatment with secondary antibodies: sheep anti-mouse IgG-HRP (cat.# NXA931, GE Health Care) or bovine-anti goat IgG-HRP (cat.# sc-2352, Santa Cruz Biotechnology) at 24°C for one hour. Finally membranes were washed three times and developed with ECL western blot detection reagent (cat.#RPN2209, GE Health Care).
BrdU cell proliferation assay.
VCaP cells were grown in DMEM containing 10% hormone depleted cFBS for 72 hours followed by transfection with 50 nM of NT siRNA or ERG siRNA. Twelve hours post-transfection, cells were treated with 0.1 nM R1881 alone, or with 0.1 nM R1881 and 10 µM PGE2. Bromodeoxyuridine (BrdU) incorporation technique was used to determine the effect of the ERG knockdown and PGE2 treatment on cell proliferation. In brief, forty eight hours post transfection; cells were pulse-label with 10 mM BrdU (cat.#550891, BD Biosciences) for additional 24 h. Cells were then washed twice with 1x PBS, fixed with 4% paraformaldehyde for 20 minutes at room temperature and centrifuged onto silanized slides (cat.#89033-034, VWR, West Chester, PA) with a cytospin centrifuge. Slides were boiled at 90°C for 20 minutes, washed once with PBS after cooling down to 30°C, blocked in H2O2 for 10 minutes followed by ddH2O wash. Biotinylated mouse monoclonal anti-BrdU antibody (Cat.#B8434, Sigma, St. Louis, MO) was added to the slides and were incubated for 30 min at room temperature followed by PBS wash. Visualization of incorporated BrdU was carried out with Peroxidase substrate kit DAB (cat.# SK-4100, Vector Laboratories) as recommended by the supplier. The slides were analyzed under Leica DMLB upright microscope using ×20 objective lens. BrdU incorporation was calculated from the number of stained BrdU-positive cells per 100 cells counted in each field. A total of five fields per experiment were evaluated.
Immuno-fluorescence assay.
VCaP cells transfected with either ERG siRNA or non-targeting siRNA were fixed with 4% paraformaldehyde and centrifuged onto silanized slides (Sigma) with a cytospin centrifuge. Cells were immunostained with anti-HPGD antibody (cat.# 160615, Cayman Chemical), or CPDR ERG-MAb46,47 followed by goat anti-rabbit Alexa-594 or anti-mouse Alexa-594 secondary antibodies (cat.s#A11037 and A11032, respectively, Invitrogen). Images were captured using a 40×/0.65 N-Plan objective on a Leica DMLB upright microscope with a QImaging Retiga-EX CCD camera (Burnaby) controlled by OpenLab software (Improvision). Images were converted into color and merged by using Adobe Photoshop.
PGE2 measurement.
PGE2 was measured in the conditioned medium by using an enzyme linked immunoassay kit (cat.#930001, Assay designs) according to the manufacturer's recommendation. VCaP Cells were grown in DMEM containing 10% of FBS for 48 hours and then transfected with 25 nM of NT siRNA or ERG siRNA. Twenty-four hours post-transfection, cell growth media was replaced with fresh pre-warmed DMEM containing 10% FBS. Seventy-two hours post transfection, the old medium was exchanged with fresh pre-warmed DMEM containing 10% FBS and 5 pg/ml of IL-1β for each groups of cells for 24 hours and the conditioned media were collected for PGE2 analysis and cells were harvested for total protein extraction which is used for PGE2 normalization.
Quantitative reverse transcription-polymerase chain reaction (QRT-PCR).
Radical prostatectomy specimens were obtained under an Institutional Review Board (IRB) approved protocol (#20405). Total RNA from LCM derived tumor epithelial cells were used for QRT-PCR as previously described in reference 10 by using Sybr green PCR Master Mix Kit (cat.#4304437, Applied Biosystems). Relative expression of HPGD (normalized to GAPDH) was determined as described before in reference 48. Primers used for HPGD gene amplifications were as follows: forward primer: 5′-AAC CTC AGA AGA CTC TGT TCA TCC A-3′, and reverse primer: 5′-CCA AAA TGT CCA GTC TTC CAA AGT-3′. GAPDH gene expression was detected using forward primer: 5′-GAG CCA CAT CGC CTC AGA CAC C-3′; and reverse primer: 5′-GTT CTC AGC TTG ACG GTG CC-3′. The HPGD expression level in VCaP prostate cancer cell line was used as the standard for generating relative expression value.
chIP assay.
VCaP cells were seeded in DMEM containing 10% cFBS and were grown for three days. Cells were transfected with 50 nM of ERG siRNA or with 50 nM of control NT siRNA and were further incubated in 10% FBS containing DMEM for 48 hours. Cells were processed for ChIP assay as described before in reference 5 and 49. Amplification reactions were carried out on T-Gradient Thermoblock (Biometra) by using 95°C, 15 s; 55°C 30 s; 72°C 60 s program setting. For detecting genomic input DNA and specific ChIP products 35 and 40 PCR amplification cycles were used, respectively. ETS binding sites within the target regions were identified by matrix match analysis using the MatInspector software (Genomatix GmbH). ERG protein was detected by an anti-ERG monoclonal antibody (CPDR ERG-MAb).46,47 For amplifying the human HPGD gene (Gene ID: 3248, Accession: U63296 NM_000860) core promoter 5′-GCGAGTCCGGAAGGCAAAGAT-3′ ETS binding site (V$ETS1/ELK10.2) the forward 5′-GGGCACTGAAGGAAACTCTTCTT-3′ and reverse 5′-GTTCTGGAGCGCCAAGCTT-3′ primer pair was used. Two sites that lack ETS binding sites of approximately 1,200 bp upstream and other 1,200 pb downstream of the HPGD transcription initiation site were used as negative internal controls. Primers used for the negative controls are upstream forward 5′-GCC AGG GAG GCA GTG TAT AA-3′ and reverse 5′-AGA ACA CGG GGC AAA TTA AA-3′. Downstream negative control primers are: downstream forward 5′-GGG ACT AAG AGA CAT TGC TTG CTC-3′ and reverse 5′-CTG GCA GCT TGG TAAG AATG CA-3′. For assaying the negative control regions, 40 cycles of PCR amplification cycles were used for both ChIP and input amplifications. Amplicons were detected only in input amplification reaction. As another negative control, we used ChIP with IgG controls.
Statistical analysis.
Wilcoxon Rank-sum test was used to compare HPGD transcript expressions between TMPRSS2-ERG fusion positive and fusion negative prostate epithelial samples and data is presented with median ± standard deviation (SD). A Student's t-test was used for the comparison of the effect of prostaglandin E2 induced cell growth, cytokine induced PGE2. All data are presented as means ± standard deviation (SD), n = 3.
Acknowledgements
This research was supported by the Center for Prostate Disease Research Program through an ongoing grant from the US Army Medical Research and Material Command, NIH Grant, RO1 DK065977 to Shiv Srivastava and Gyorgy Petrovics. The authors would like to thank Ms. Tia Morris for editing the manuscript and Mr. Stephen Doyle for graphics design. The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of the Army, Department of Defense or the US Government. Released for publication by the WRAMC Public Affairs Office. The authors declare no competing financial interest.
Abbreviations
- CaP
prostate cancer
- ERG
ETS-related gene
- HPGD
15-hydroxy-prostaglandin dehydrogenase
- AR
androgen receptor
- PGE2
prostaglandin E2
- EP4
E prostanoid receptor type 4
- uPA
urokinase-type plasminogen activator
- COX-2
cyclooxygenase 2
- IL-1β
interleukin 1beta
- ChIP
chromatin immunoprecipitation
- LCM
laser capture micro-dissection
- BrdU
bromodeoxyuridine
- DAB
3′3′-diaminobenzidine
References
- 1.Petrovics G, Liu A, Shaheduzzaman S, Furusato B, Sun C, Chen Y, et al. Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene. 2005;24:3847–3852. doi: 10.1038/sj.onc.1208518. [DOI] [PubMed] [Google Scholar]
- 2.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 3.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8:497–511. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demichelis F, Fall K, Perner S, Andren O, Schmidt F, Setlur SR, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596–4599. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- 5.Sun C, Dobi A, Mohamed A, Li H, Thangapazham RL, Furusato B, et al. TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene. 2008;27:5348–5353. doi: 10.1038/onc.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gopalan A, Leversha MA, Satagopan JM, Zhou Q, Al-Ahmadie HA, Fine SW, et al. TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res. 2009;69:1400–1406. doi: 10.1158/0008-5472.CAN-08-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rostad K, Mannelqvist M, Halvorsen OJ, Oyan AM, Bo TH, Stordrange L, et al. ERG upregulation and related ETS transcription factors in prostate cancer. Int J Oncol. 2007;30:19–32. [PubMed] [Google Scholar]
- 8.Perner S, Mosquera JM, Demichelis F, Hofer MD, Paris PL, Simko J, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882–888. doi: 10.1097/01.pas.0000213424.38503.aa. [DOI] [PubMed] [Google Scholar]
- 9.Mehra R, Tomlins SA, Yu J, Cao X, Wang L, Menon A, et al. Characterization of TMPRSS2-ETS gene aberrations in androgen-independent metastatic prostate cancer. Cancer Res. 2008;68:3584–3590. doi: 10.1158/0008-5472.CAN-07-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y, Dobi A, Sreenath T, Cook C, Tadase AY, Ravindranath L, et al. Delineation of TMPRSS2-ERG splice variants in prostate cancer. Clin Cancer Res. 2008;14:4719–4725. doi: 10.1158/1078-0432.CCR-08-0531. [DOI] [PubMed] [Google Scholar]
- 11.Rickman DS, Pflueger D, Moss B, VanDoren VE, Chen CX, de la Taille A, et al. SLC45A3-ELK4 is a novel and frequent erythroblast transformation-specific fusion transcript in prostate cancer. Cancer Res. 2009;69:2734–2738. doi: 10.1158/0008-5472.CAN-08-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furusato B, Gao CL, Ravindranath L, Chen Y, Cullen J, McLeod DG, et al. Mapping of TMPRSS2-ERG fusions in the context of multi-focal prostate cancer. Mod Pathol. 2008;21:67–75. doi: 10.1038/modpathol.3800981. [DOI] [PubMed] [Google Scholar]
- 13.Pflueger D, Rickman DS, Sboner A, Perner S, LaFargue CJ, Svensson MA, et al. N-myc downstream regulated gene 1 (NDRG1) is fused to ERG in prostate cancer. Neoplasia. 2009;11:804–811. doi: 10.1593/neo.09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carver BS, Tran J, Gopalan A, Chen Z, Shaikh S, Carracedo A, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet. 2009;41:619–624. doi: 10.1038/ng.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klezovitch O, Risk M, Coleman I, Lucas JM, Null M, True LD, et al. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc Natl Acad Sci USA. 2008;105:2105–2110. doi: 10.1073/pnas.0711711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zong Y, Xin L, Goldstein AS, Lawson DA, Teitell MA, Witte ON. ETS family transcription factors collaborate with alternative signaling pathways to induce carcinoma from adult murine prostate cells. Proc Natl Acad Sci USA. 2009;106:12465–12470. doi: 10.1073/pnas.0905931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, Helgeson BE, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King JC, Xu J, Wongvipat J, Hieronymus H, Carver BS, Leung DH, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat Genet. 2009;41:524–526. doi: 10.1038/ng.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai HH, Cho H, Tong M, Ding Y. NAD+-linked 15-hydroxyprostaglandin dehydrogenase: structure and biological functions. Curr Pharm Des. 2006;12:955–962. doi: 10.2174/138161206776055958. [DOI] [PubMed] [Google Scholar]
- 20.Heighway J, Knapp T, Boyce L, Brennand S, Field JK, Betticher DC, et al. Expression profiling of primary non-small cell lung cancer for target identification. Oncogene. 2002;21:7749–7763. doi: 10.1038/sj.onc.1205979. [DOI] [PubMed] [Google Scholar]
- 21.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 22.Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci USA. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf I, O'Kelly J, Rubinek T, Tong M, Nguyen A, Lin BT, et al. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Res. 2006;66:7818–7823. doi: 10.1158/0008-5472.CAN-05-4368. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, DuBois RN. Pro-inflammatory prostaglandins and progression of colorectal cancer. Cancer Lett. 2008;267:197–203. doi: 10.1016/j.canlet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudry AA, Wahle KW, McClinton S, Moffat LE. Arachidonic acid metabolism in benign and malignant prostatic tissue in vitro: effects of fatty acids and cyclooxygenase inhibitors. Int J Cancer. 1994;57:176–180. doi: 10.1002/ijc.2910570208. [DOI] [PubMed] [Google Scholar]
- 26.Ma X, Kundu N, Rifat S, Walser T, Fulton AM. Prostaglandin E receptor EP4 antagonism inhibits breast cancer metastasis. Cancer Res. 2006;66:2923–2927. doi: 10.1158/0008-5472.CAN-05-4348. [DOI] [PubMed] [Google Scholar]
- 27.Quidville V, Segond N, Lausson S, Frenkian M, Cohen R, Jullienne A. 15-Hydroxyprostaglandin-dehydrogenase is involved in anti-proliferative effect of non-steroidal anti-inflammatory drugs COX-1 inhibitors on a human medullary thyroid carcinoma cell line. Prostaglandins Other Lipid Mediat. 2006;81:14–30. doi: 10.1016/j.prostaglandins.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Frenkian M, Segond N, Pidoux E, Cohen R, Jullienne A. Indomethacin, a COX inhibitor, enhances 15-PGDH and decreases human tumoral C cells proliferation. Prostaglandins. 2001;65:11–20. doi: 10.1016/s0090-6980(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 29.Chi X, Freeman BM, Tong M, Zhao Y, Tai HH. 15-Hydroxyprostaglandin dehydrogenase (15-PGDH) is upregulated by flurbiprofen and other non-steroidal anti-inflammatory drugs in human colon cancer HT29 cells. Arch Biochem Biophys. 2009;487:139–145. doi: 10.1016/j.abb.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Tseng-Rogenski S, Lee IL, Gebhardt D, Fischer SM, Wood C, Park JM, et al. Loss of 15-hydroxyprostaglandin dehydrogenase expression disrupts urothelial differentiation. Urology. 2008;71:346–350. doi: 10.1016/j.urology.2007.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eruslanov E, Kaliberov S, Daurkin I, Kaliberova L, Buchsbaum D, Vieweg J, et al. Altered expression of 15-hydroxyprostaglandin dehydrogenase in tumor-infiltrated CD11b myeloid cells: a mechanism for immune evasion in cancer. J Immunol. 2009;182:7548–7557. doi: 10.4049/jimmunol.0802358. [DOI] [PubMed] [Google Scholar]
- 32.De Marzo AM, Nakai Y, Nelson WG. Inflammation, atrophy and prostate carcinogenesis. Urol Oncol. 2007;25:398–400. doi: 10.1016/j.urolonc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Greenland KJ, Jantke I, Jenatschke S, Bracken KE, Vinson C, Gellersen B. The human NAD+-dependent 15-hydroxyprostaglandin dehydrogenase gene promoter is controlled by Ets and activating protein-1 transcription factors and progesterone. Endocrinology. 2000;141:581–597. doi: 10.1210/endo.141.2.7313. [DOI] [PubMed] [Google Scholar]
- 34.Nandy A, Jenatschke S, Hartung B, Milde-Langosch K, Bamberger AM, Gellersen B. Genomic structure and transcriptional regulation of the human NAD+-dependent 15-hydroxyprostaglandin dehydrogenase gene. J Mol Endocrinol. 2003;31:105–121. doi: 10.1677/jme.0.0310105. [DOI] [PubMed] [Google Scholar]
- 35.Lee KS, Lee HJ, Ahn KS, Kim SH, Nam D, Kim DK, et al. Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett. 2009;280:93–100. doi: 10.1016/j.canlet.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad A, Kong D, Wang Z, Sarkar SH, Banerjee S, Sarkar FH. Downregulation of uPA and uPAR by 3,3′-diindolylmethane contributes to the inhibition of cell growth and migration of breast cancer cells. J Cell Biochem. 2009;108:916–925. doi: 10.1002/jcb.22323. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Noskova V, Ahmadi S, Asander E, Casslen B. Ovarian cancer cells stimulate uPA gene expression in fibroblastic stromal cells via multiple paracrine and autocrine mechanisms. Gynecol Oncol. 2009;115:121–126. doi: 10.1016/j.ygyno.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Hughes-Fulford M. Prostaglandin E2 and the protein kinase A pathway mediate arachidonic acid induction of c-fos in human prostate cancer cells. Br J Cancer. 2000;82:2000–2006. doi: 10.1054/bjoc.2000.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perner S, Demichelis F, Beroukhim R, Schmidt FH, Mosquera JM, Setlur S, et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006;66:8337–8341. doi: 10.1158/0008-5472.CAN-06-1482. [DOI] [PubMed] [Google Scholar]
- 40.Nam RK, Sugar L, Wang Z, Yang W, Kitching R, Klotz LH, et al. Expression of TMPRSS2:ERG gene fusion in prostate cancer cells is an important prognostic factor for cancer progression. Cancer Biol Ther. 2007;6:40–45. doi: 10.4161/cbt.6.1.3489. [DOI] [PubMed] [Google Scholar]
- 41.Shin S, Kim TD, Jin F, van Deursen JM, Dehm SM, Tindall DJ, et al. Induction of prostatic intraepithelial neoplasia and modulation of androgen receptor by ETS variant 1/ETS-related protein 81. Cancer Res. 2009;69:8102–8110. doi: 10.1158/0008-5472.CAN-09-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X, et al. An integrated network of androgen receptor, poly-comb and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 17:443–454. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawcroft G, Ko CW, Hull MA. Prostaglandin E2-EP4 receptor signalling promotes tumorigenic behaviour of HT-29 human colorectal cancer cells. Oncogene. 2007;26:3006–3019. doi: 10.1038/sj.onc.1210113. [DOI] [PubMed] [Google Scholar]
- 44.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otani T, Yamaguchi K, Scherl E, Du B, Tai HH, Greifer M, et al. Levels of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase are reduced in inflammatory bowel disease: evidence for involvement of TNFalpha. Am J Physiol Gastrointest Liver Physiol. 2006;290:361–368. doi: 10.1152/ajpgi.00348.2005. [DOI] [PubMed] [Google Scholar]
- 46.Furusato B, Tan SH, Young D, Dobi A, Sun C, Mohamed AA, et al. ERG oncoprotein expression in prostate cancer: clonal progression of ERG-positive tumor cells and potential for ERG-based stratification. Prostate Cancer Prostatic Dis. 2010;13:228–237. doi: 10.1038/pcan.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohamed AA, Tan SH, Mikhalkevich N, Ponniah S, Vasioukhin V, Bieberich CJ, et al. Ets family protein, ERG expression in developing and adult mouse tissues by a highly specific monoclonal antibody. Journal of Cancer. 2010;1:197–208. doi: 10.7150/jca.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 49.Masuda K, Werner T, Maheshwari S, Frisch M, Oh S, Petrovics G, et al. Androgen receptor binding sites identified by a GREF_GATA model. J Mol Biol. 2005;353:763–771. doi: 10.1016/j.jmb.2005.09.009. [DOI] [PubMed] [Google Scholar]