Abstract
The expression of the rabbit intestinal brushborder Na/glucose cotransporter has been studied in Xenopus oocytes. Poly(A)+ RNA isolated from the intestinal mucosa was injected into oocytes, and the expression of the transporter in the oocyte plasma membrane was assayed by measuring the Na-dependent phlorizin-sensitive uptake of methyl alpha-D-[14C]glucopyranoside (MeGlc). Expression of the glucose carrier was detected 3-7 days after mRNA injection, and the rate of glucose transport was proportional to the amount of mRNA injected. mRNA (50 ng) increased the maximum velocity (Vmax) of MeGlc uptake by as much as 10-fold over background. The total mRNA was fractionated by preparative agarose gel electrophoresis and each fraction was assayed for its ability to induce transport activity. The mRNA encoding the Na/glucose cotransporter was found in a single fraction of approximately 2.3 kilobases (kb), which contained 3% of the total mRNA. A similar mRNA fraction (2.0-2.6 kb) isolated from colon did not induce expression of this transporter. In vitro translation of the fractionated intestinal mRNA showed enhanced synthesis of two protein bands at 57 and 63 kDa. The mRNA encoding the cotransporter is smaller (2.3 kb) than that (2.6-2.9 kb) encoding the 55-kDa facilitated glucose carrier in human hepatoma cells and rat brain.
Full text
PDF
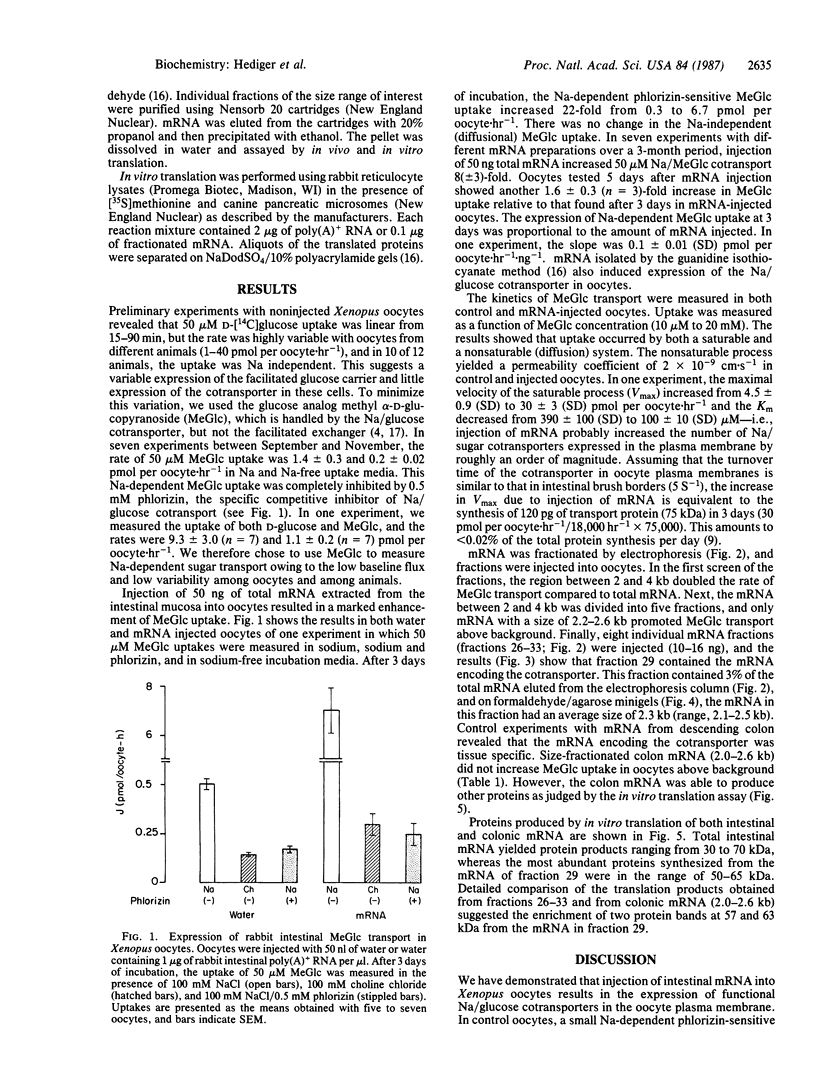
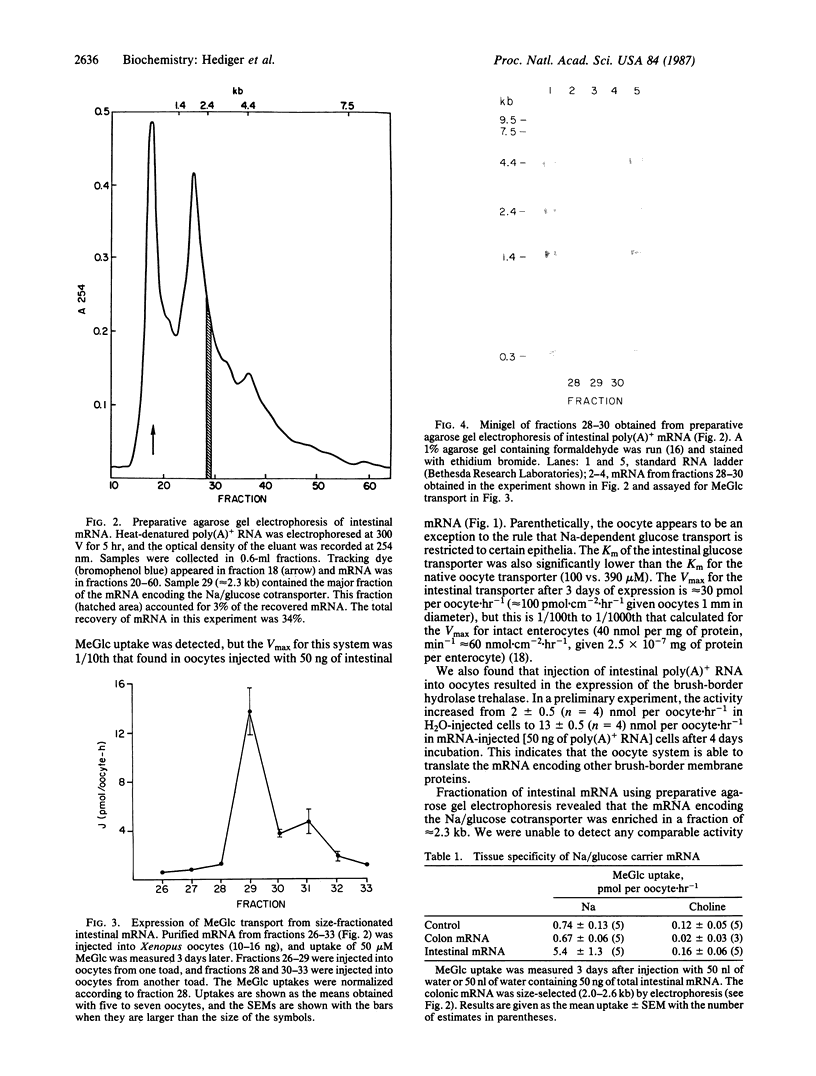

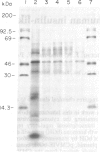
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard E. A., Miledi R., Sumikawa K. Translation of exogenous messenger RNA coding for nicotinic acetylcholine receptors produces functional receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1982 May 22;215(1199):241–246. doi: 10.1098/rspb.1982.0040. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprettere A. J., Van Acker K. J., Eggermont E., Carchon H., Evens M. Primary glucose-galactose malabsorption. Acta Paediatr Belg. 1980 Apr-Jun;33(2):121–123. [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Messenger RNA from human brain induces drug- and voltage-operated channels in Xenopus oocytes. 1984 Mar 29-Apr 4Nature. 308(5958):421–424. doi: 10.1038/308421a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Wickens M. P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- Hediger M. A. Apparatus and method for preparative gel electrophoresis. Anal Biochem. 1984 Nov 1;142(2):445–454. doi: 10.1016/0003-2697(84)90488-3. [DOI] [PubMed] [Google Scholar]
- Hediger M. A. High resolution preparative gel electrophoresis of DNA fragments and plasmid DNA using a continuous elution apparatus. Anal Biochem. 1986 Dec;159(2):280–286. doi: 10.1016/0003-2697(86)90344-1. [DOI] [PubMed] [Google Scholar]
- Karasov W. H., Diamond J. M. Adaptive regulation of sugar and amino acid transport by vertebrate intestine. Am J Physiol. 1983 Oct;245(4):G443–G462. doi: 10.1152/ajpgi.1983.245.4.G443. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Inui K., Takano M., Hori R. Distinction of three types of D-glucose transport systems in animal cells. Biochem Biophys Res Commun. 1985 Oct 30;132(2):490–496. doi: 10.1016/0006-291x(85)91160-x. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J. Sodium-sugar coupling stoichiometry in chick intestinal cells. Am J Physiol. 1984 Jul;247(1 Pt 1):C74–C82. doi: 10.1152/ajpcell.1984.247.1.C74. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J. alpha-Methylglucoside satisfies only Na+-dependent transport system of intestinal epithelium. Am J Physiol. 1981 Nov;241(5):C227–C232. doi: 10.1152/ajpcell.1981.241.5.C227. [DOI] [PubMed] [Google Scholar]
- Morgan M., Hanke P., Grygorczyk R., Tintschl A., Fasold H., Passow H. Mediation of anion transport in oocytes of Xenopus laevis by biosynthetically inserted band 3 protein from mouse spleen erythroid cells. EMBO J. 1985 Aug;4(8):1927–1931. doi: 10.1002/j.1460-2075.1985.tb03872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Peerce B. E., Wright E. M. Conformational changes in the intestinal brush border sodium-glucose cotransporter labeled with fluorescein isothiocyanate. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2223–2226. doi: 10.1073/pnas.81.7.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerce B. E., Wright E. M. Distance between substrate sites on the Na-glucose cotransporter by fluorescence energy transfer. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8092–8096. doi: 10.1073/pnas.83.21.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G., Kessler M., Hosang M., Weber J., Schmidt U. Biochemistry of the Na+, D-glucose cotransporter of the small-intestinal brush-border membrane. The state of the art in 1984. Biochim Biophys Acta. 1984 Sep 3;779(3):343–379. doi: 10.1016/0304-4157(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Wright E. M., van Os C. H., Mircheff A. K. Sugar uptake by intestinal basolateral membrane vesicles. Biochim Biophys Acta. 1980 Mar 27;597(1):112–124. doi: 10.1016/0005-2736(80)90155-8. [DOI] [PubMed] [Google Scholar]