Abstract
Inhalational anthrax is caused by spores of the bacterium Bacillus anthracis (B. anthracis), and is an extremely dangerous disease that can kill unvaccinated victims within 2 weeks. Modern antibiotic-based therapy can increase the survival rate to ∼50%, but only if administered presymptomatically (within 24–48 h of exposure). To discover host signaling responses to presymptomatic anthrax, label-free quantitative phosphoproteomics via liquid chromatography coupled to mass spectrometry was used to compare spleens from uninfected and spore-challenged mice over a 72 h time-course. Spleen proteins were denatured using urea, reduced using dithiothreitol, alkylated using iodoacetamide, and digested into peptides using trypsin, and the resulting phosphopeptides were enriched using titanium dioxide solid-phase extraction and analyzed by nano-liquid chromatography-Linear Trap Quadrupole-Orbitrap-MS(/MS). The fragment ion spectra were processed using DeconMSn and searched using both Mascot and SEQUEST resulting in 252,626 confident identifications of 6248 phosphopeptides (corresponding to 5782 phosphorylation sites). The precursor ion spectra were deisotoped using Decon2LS and aligned using MultiAlign resulting in the confident quantitation of 3265 of the identified phosphopeptides. ANOVAs were used to produce a q-value ranked list of host signaling responses. Late-stage (48–72 h postchallenge) Sterne strain (lethal) infections resulted in global alterations to the spleen phosphoproteome. In contrast, ΔSterne strain (asymptomatic; missing the anthrax toxin) infections resulted in 188 (5.8%) significantly altered (q<0.05) phosphopeptides. Twenty-six highly tentative phosphorylation responses to early-stage (24 h postchallenge) anthrax were discovered (q<0.5), and ten of these originated from eight proteins that have known roles in the host immune response. These tentative early-anthrax host response signaling events within mouse spleens may translate into presymptomatic diagnostic biomarkers of human anthrax detectable within circulating immune cells, and could aid in the identification of pathogenic mechanisms and therapeutic targets.
Anthrax is a life-threatening infectious disease that affects humans and many animal species, and Bacillus anthracis (B. anthracis), the causative agent of anthrax, is a Gram-positive, rod-shaped, nonmotile, endospore-forming bacterium (1, 2). During the 20th century, effective human and animal vaccines almost completely eradicated anthrax as a naturally occurring disease from the industrialized world. However, anthrax remains a major concern because several nations stockpiled weaponized B. anthracis spores, and because B. anthracis has been used as a bioterror weapon (e.g. the 2001 anthrax letters attack in the United States) (3). As a result, B. anthracis is classified as a Category A Select Agent by the Centers for Disease Control and Prevention (some attenuated strains are excluded).
The major routes of infection occur via inhalation, cutaneous abrasion, and ingestion of B. anthracis spores, which can survive for years in the environment. Inhalational anthrax is the most severe form of the disease, and in the past it was almost invariably lethal within ∼2 weeks. Fortunately, modern antibiotic-based therapies have significantly reduced anthrax mortality. As a result, 55% of the inhalational anthrax victims of the 2001 U. S. attack survived. However, antibiotics are effective only against proliferating B. anthracis, and therefore improve patient outcome only if administered presymptomatically (within 24–48 h of exposure), when the level of anthrax toxemia is low.
The major toxic factors of B. anthracis are the lethal and edema toxins encoded by the pXO1 plasmid, although other bacterial factors might additionally play important pathogenic roles. Lethal toxin is a proteolytic inhibitor of mitogen-activated protein kinase kinase, and edema toxin is a bacterial adenylate cyclase capable of increasing the levels of intracellular cyclic AMP. Both of these enzymatic activities have a profound effect on cell function and viability. The antiphagocytic capsule produced by proteins encoded by the pXO2 plasmid is also an important virulence factor, but it has no inherent toxicity. Elimination of these two plasmids greatly reduces the virulence of B. anthracis. For example, the Sterne strain (pXO1+, pXO2−) is an important vaccine strain that causes a mild infection in most animals, including humans. In DBA/2J mice, however, Sterne B. anthracis causes a highly lethal infection. Consequently, Sterne-challenged DBA/2J mice have become a valuable model of human anthrax. In contrast, the plasmidless ΔSterne strain (pXO1−, pXO2−) causes an asymptomatic, abortive infection in DBA/2J mice.
The molecular events that result in the high mortality of anthrax victims is still an active area of research, and there is no known cell model or biomarker that is predictive of patient outcome. After the 2001 attacks, police in the USA have investigated roughly 500 “white powder” hoaxes per year (4), and prophylactically prescribing powerful antibiotics can result in uninfected patients developing serious adverse effects. In a large-scale anthrax hoax or in an actual attack, first responders and hospitals would have great difficulty distinguishing uninfected and presymptomatic patients. Therefore, efforts in our laboratory are focused on identifying diagnostic host response biomarkers of presymptomatic anthrax, and we recently identified candidate anthrax biomarkers in the low-molecular-mass serum proteome of Sterne and ΔSterne spore-challenged DBA/2J mice using liquid chromatography coupled to (tandem) mass spectrometry (LC-MS[/MS])1 (5). Proteomics (6–12), including phosphoproteomics (13–16), has greatly benefited from recent advances in LC-MS(/MS), though tentative biomarkers still require validation by orthogonal experimentation.
In this investigation, host signaling responses to anthrax were discovered using label-free quantitative phosphoproteomics to compare spleens from uninfected and spore-challenged mice. The spleen was studied because of its role in the immune system, because it is among the first of the organs to display the presence of disseminating B. anthracis in the murine model, and because of the difficulty of reproducibly preparing sufficient masses of circulating immune cells directly from the blood of spore-challenged mice. Synergistically with its role as a hematopoietic organ (i.e. as a site of immune cell maturation, activation, and proliferation), the spleen also functions as a secondary lymphoid organ both by directly filtering the blood to capture pathogens and antigens, and by transiently concentrating phagocytes and antigen presenting cells (e.g. dendritic cells, granulocytes, macrophages, and monocytes) and antigen-specific B- and T-lymphocytes, resulting in an increase in the efficiency of the host immune response (17–20). The splenic marginated pool of immune cells is in dynamic equilibrium with the circulating immune cells, and includes 10–15% of the body's B lymphocytes (20, 21), 25% of the body's T lymphocytes (20, 21), 21–34% of the body's granulocytes (22, 23), and 50% of the body's monocytes (24) (this reserve of monocytes can exit the spleen and differentiate to become dendritic cells and macrophages). The strategy of using comparative proteomics to study altered tissues to generate lists of candidate biomarkers has been employed previously, primarily because of the difficulty of discovering biomarkers directly from the blood (25–32). Host response signaling events within mouse spleens may translate into presymptomatic diagnostic biomarkers of human anthrax detectable within circulating immune cells, and could aid in the identification of pathogenic mechanisms and therapeutic targets. In this study, thousands of mouse spleen phosphopeptides were confidently identified and quantitated, demonstrating that label-free quantitative phosphoproteomics is a viable alternative to stable isotope labeling (e.g. 18O, isobaric tag for relative and absolute quantification, Tandem Mass Tags, and stable isotope labeling with amino acids in cell culture).
EXPERIMENTAL PROCEDURES
Mouse Infection and Tissue Homogenization
The George Mason University Institutional Animal Care and Use Committee and the Biocon Animal Care and Use Committee/Institutional Review Board (Biocon Inc., Rockville, MD) approved all of the animal experimentation protocols. Using BSL-2 safety protocols at Biocon Inc., mice (Mus musculus strain DBA/2J, female, 8 weeks old, The Jackson Laboratory, Bar Harbor, ME) were either not injected at all (five mice) or were intraperitoneally injected with 100 μl of sterile H2O (15 mice), or 100 μl ΔSterne (pXO1−, pXO2−) B. anthracis spores in sterile H2O (5 × 106 spores/100 μl; 15 mice), or 100 μl 34F2 Sterne (pXO1+, pXO2−) B. anthracis spores in sterile H2O (5 × 106 spores/100 μl; 25 mice). The B. anthracis Sterne 34F2 and ΔSterne strains (33) and the spore preparation protocol (34) have been described previously. At t = 0 h, the five uninjected mice were prepared by terminal bleed from the orbital sinus using a glass Pasteur pipette, followed by cervical dislocation and dissection of the spleen, which was then placed into a 2-ml cryogenic vial and immediately snap frozen in liquid nitrogen. At 24, 48, and 72 h postchallenge, five mice from each of the remaining experimental conditions (H2O, ΔSterne, and Sterne) were prepared using the same dissection protocol. Fourteen of the Sterne-challenged mice were found dead in their cages prior to 72 h postchallenge (Sterne-infected mice have a high mortality rate at this time point) and were discarded, so only a single 72 h post-Sterne-challenge mouse spleen was isolated. The spleen samples were organized into five sample blocks by placing one biological replicate from each experimental condition into each block, and then randomizing the order of the samples within each block. The samples were processed in this (random) order within these blocks to reduce any possible systematic biases (35). Each sample was homogenized by manual disruption into 600 μl of freshly prepared Lysis Buffer (50 mm tris-HCl pH 8, 8 m urea), and BCA protein concentration assays (Thermo Fisher Scientific Inc., Waltham, MA) were performed.
Sample Digestion and Phosphopeptide Enrichment
Two milligrams (protein mass) of each spleen homogenate (∼30–50% of a mouse spleen) was diluted with Lysis Buffer to a final volume of 370 μl, and 200 ng of bovine β-casein was added to each sample to serve as a phosphoprotein standard. Dithiothreitol was added to a final concentration of 10 mm, and the samples were incubated at 60 °C for 30 min to reduce disulfide bridges. The sample proteins were alkylated by adding iodoacetamide to a final concentration of 50 mm and incubating the samples at room temperature for 20 min in darkness. The samples were diluted with H2O and 500 mm NH4HCO3 pH 9 such that the final urea concentration was 1 m and the final NH4HCO3 concentration was 100 mm. The sample proteins were digested into peptides by adding sequencing grade modified trypsin (1:100 [protein w:w] trypsin:sample; Promega Corp., Madison, WI) and incubating the samples at 37 °C for 21.5 h.
The samples were acidified in preparation for C-18 solid-phase extraction (SPE) by adding acetic acid to a final concentration of 2% v/v, and 8 pmol of phosphorylated angiotensin II was added to each sample to serve as a phosphopeptide standard. Each sample was centrifuged 40 min at 2000 × g at room temperature to pellet particulates (to avoid clogging the SPE column), and then the supernatants underwent C-18 solid phase extraction using a Sep-Pak C-18 SPE 1 cc 100 mg column (Waters Corp., Milford, MA). Centrifugation in a swinging-bucket rotor was used to equilibrate the Sep-Pak columns and to apply the samples and the wash buffer (0.1% v/v formic acid). The elution buffer (0.1% v/v formic acid, 80% v/v acetonitrile [ACN]) was applied slowly using a rubber bulb to produce positive air pressure.
The Sep-Pak eluates were concentrated in a SpeedVac (no heating; Thermo Fisher Scientific Inc.) to 150 μl. Next, 150 μl of freshly prepared TiO2 Loading Buffer (5% v/v trifluoroacetic acid [TFA], 80% v/v ACN, 225 mg/ml 2,5-dihydroxybenzoic acid [DHB]) was added to each sample, and the samples were microcentrifuged 10 min at 16,000 × g at room temperature to pellet any particulates that might clog the TiO2 SPE tip. Phosphopeptides from each supernatant were enriched using a Titansphere PHOS-TiO 200 μl centrifuge tip containing 3 mg of TiO2 media (GL Sciences Inc., Torrance, CA) using a protocol based on (36). Centrifugation was used to equilibrate the tips, apply the samples, apply the first wash buffer (freshly prepared 2% v/v TFA, 80% v/v ACN, 40 mg/ml DHB), the second wash buffer (2% v/v TFA, 80% v/v ACN), the first elution buffer (5% w/v [∼3M] NH4OH, to elute the phosphopeptides from the TiO2 resin), and the second elution buffer (2% v/v TFA, 80% v/v ACN, to elute the phosphopeptides from the Empore C-8 bonded silica membrane below the TiO2 resin inside the tips). The eluates were immediately concentrated in a SpeedVac (no heating) to 20 μl to evaporate the ACN and NH4OH, and the samples were acidified in preparation for C-18 SPE by adding 60 μl of 1% v/v acetic acid.
The samples underwent C-18 SPE using a ZipTip 10 μl pipette tip (Millipore Corp., Billerica, MA) with the Sep-Pak C-18 SPE wash and elution buffers described above. During the development of this protocol, it was found that skipping the ZipTip SPE resulted in clogging of the nano-LC-electrospray ionization (ESI) column/tip, and that performing this step solved this problem. Forty μl of H2O was added to each ZipTip eluate, and then the samples were concentrated in a SpeedVac (no heating) to 10 μl to evaporate the ACN. One pmol of angiotensin I was added to each sample to serve as a peptide standard, and 7 μl of 1% v/v acetic acid was added to each sample to acidify it in preparation for reversed-phase LC-MS(/MS). The samples were stored at −80 °C until LC-MS(/MS).
Mass Spectrometry
Nano-LC-ESI-MS(/MS) analyses were performed using a Surveyor LC system coupled to an LTQ-Orbitrap mass spectrometer (both Thermo Fisher Scientific Inc.; Buffer A: 0.1% v/v formic acid; Buffer B: 0.1% v/v formic acid, 80% v/v ACN) (37–38). Each nano-LC-ESI column/tip was prepared by laser-pulling a tip (Laser Based Micropipette Puller Model P-2000, Sutter Instrument Co., Novato, CA) onto a 30 cm length of 100 μm I.D. coated silica capillary tubing (Polymicro Technologies LLC, Phoenix, AZ), which was then packed with 15 cm of Magic C18AQ C-18 media (5 μm diameter, 200 A pores, Michrom Bioresources Inc., Auburn, CA) (note that this was just a single piece of capillary; there was no connector or frit between the column and tip). One nano-LC-ESI column/tip was used for sample blocks 1–2, and a second column/tip was used for sample blocks 3–5. Each sample was manually loaded using a pressure cell (Brechbuhler Inc., Houston, TX), the nano-LC-ESI column/tip was connected to the LC system, and the postsplit flow rate was manually calibrated to be 350 nL/min (∼70 bar, ∼70 μl/min presplit) using a calibrated micropipette. Note that because the sample was manually loaded in this manner, the phosphopeptides were never exposed to metal or other surfaces that might have caused sample loss (39). A 2 kV ESI voltage was applied and the ESI spray was observed. LC-MS(/MS) was performed using an instrument method that included a 120 min linear gradient (10–40% Buffer B) and a 40 min 100% Buffer B column regeneration step. The LTQ-Orbitrap was set to perform simultaneous Orbitrap-MS (400–1600 m/z, resolution = 100,000) and shotgun CID LTQ-MS2 (no Electron Transfer Dissociation, Higher Energy Collisional Dissociation, or Pulsed Q Collision Induced Dissociation was used) against the top eight most intense ions (top six for sample block 5). Dynamic exclusion was enabled to avoid repeatedly selecting intense ions for fragmentation (sample blocks 1 and 4 excluded from −0.1 to 1.6 m/z units about the precursor ion for 25 s; block 2 excluded from −0.1 to 1.6 m/z for 15 s; blocks 3 and 5 excluded from −1.1 to 1.6 m/z for 60 s). Multistage activation was performed during the analyses of sample blocks 4 and 5 (Neutral Loss Mass List = −97.98, −48.99, −32.66 m/z units). These spectra are available for download as .RAW files at the Proteome Commons Tranche data repository at https://proteomecommons.org/(Tranche Hash: j7rN4iodsyEQbWjTaWAqMthHEdlfsCqJc/WeLuA5xsrD0Tiu7NOEAwQWpWxhpAwl+hRPiBRzsc+K0cTFg2Kipfp+LDEAAAAAAAA9rw==).
Phosphopeptide Identification
MS2 spectra were processed using DeconMSn (v2.1.4.1, http://omics.pnl.gov/software/) (40), an implementation of the THRASH algorithm (41), which deisotoped the precursor ion isotopic profiles to determine accurate precursor ion monoisotopic masses, and then produced .dta text files of the fragment ion spectra. A short PERL script (dta_filt.pl) was used to delete tentative neutral-loss peaks (supplemental material), and then both Mascot (v2.2.06, Matrix Science Inc., Boston, MA) (42) and SEQUEST (v28 [rev. 12], Thermo Fisher Scientific Inc.) (43) were used to search for fully tryptic phosphopeptides (variable modifications: Ser, Thr, Tyr phosphorylation, Met oxidation; fixed modification: iodoacetamide Cys carboxyamidomethylation; ≤1 missed trypsin cleavage; ± 10 ppm precursor ion mass tolerance; ± 0.5 Da fragment ion mass tolerance [Mascot]; 1 Da fragment ion mass bins [SEQUEST]).
A FASTA text file consisting of five concatenated sets of protein sequences was used to search the spectra: (i) protein and peptide standards, (ii) common contaminants (e.g. porcine trypsin, human keratin), (iii) Bacillus anthracis Sterne proteins (this dataset did not include any plasmid proteins, retrieved July 24, 2009, Comprehensive Microbial Resource, J. Craig Venter Institute, http://www.jcvi.org/) (44), (iv) Bacillus anthracis Ames Ancestor plasmid proteins (only the pXO1 and pXO2 plasmid proteins, retrieved August 30, 2009, http://www.jcvi.org/) (44), and (v) mouse proteins (Mus musculus, v3.61, retrieved July 24, 2009, International Protein Index, http://www.ebi.ac.uk/IPI/IPIhelp.html) (45). Overall, there were 62,339 protein sequences total. Protein Digestion Simulator Basic (v2.1.2991.22796, http://omics.pnl.gov/software/) was used to validate the FASTA file, to produce reversed and scrambled FASTA decoy databases from the normal database, and to produce tables of tryptic peptides for Microsoft Access 2007 queries.
In addition to searching the normal protein sequence file, Mascot and SEQUEST were both used to search the two decoy databases. The decoy database searches were performed separately from the normal searches, and the estimated false discovery rate (FDR) was calculated using: FDR = 0.5 × (the number of reversed identifications + the number of scrambled identifications)/(the number of normal identifications). It has been argued that this FDR estimate overestimates the true FDR, and an alternative method using concatenated (normal + decoy) databases has been proposed (46). Empirically, a highly confident peptide identification will result from a highly confident first hit (the “top-ranked hit”; i.e. the highest-confidence peptide sequence that resulted from a FASTA database search against a single experimental fragment ion spectrum) and a much less confident second hit (the second-place hit). Although the concatenated database method may result in a more accurate FDR estimate, it unfortunately overestimates the confidence of the second hits (46). Therefore, the decoy database searches were performed separately, which resulted in relatively accurate second hit scores at the cost of possibly overestimating the FDR estimates.
The resulting Mascot (.dat) and SEQUEST (.out) output files were converted to tab-delimited text files using Mascot Output Parser (v2003–11-13, generously provided by Dr. Matthew Monroe, Pacific Northwest National Laboratory, Richland, WA) and Peptide File Extractor (console version, v1.1.3519.25650, http://omics.pnl.gov/software/). Microsoft Access 2003 was used to import each of the resulting text files and to perform queries in an automated fashion using macros. Systematic precursor ion mass measurement errors were determined and the search-space was reduced from 20 ppm (±10 ppm) to 7 ppm (±3.5 ppm about the systematic error) to reduce the FDR (supplemental Fig. 1) (a much more advanced algorithm to remove systematic precursor ion mass measurement errors has recently been published (47, 48)). Phosphopeptide FDR Estimator (v2009–10 − 26) (49) was used to calculate the Ambiguity Score (50) of each phosphorylation site identified by Mascot and SEQUEST, and also to perform a discriminant analysis using the SEQUEST data (supplemental Fig. 2; the resulting q-values are included as supplemental data but otherwise were not used to filter the data or to estimate FDRs of the filtered data). All of these steps were automated using Microsoft DOS command line scripting and Microsoft Access 2003 macros.
For each MS2 spectrum produced by DeconMSn, only the top-ranked hit was retained (one from each of the six searches: Mascot/SEQUEST, normal/reversed/scrambled). A two-step process was then used to filter the resulting data to reduce the FDR. First, if Mascot and SEQUEST agreed on the phosphopeptide identification (ignoring post-translational modification [PTM] localization), relatively mild data filtration criteria were employed (see supplemental Table 2 for a detailed description of all of the data filtration criteria). In the rare case that a single spectrum resulted in filter-passing Mascot and SEQUEST identifications of two different peptides, these identifications were discarded (only 515 spectra resulted in this situation). In the second step, strict data filtration criteria were employed to filter identifications made by only one of the two search engines (this resulted in a relatively small number of additional filter-passing identifications). FDRs of the filtered data were estimated using the decoy database method described above, and the data filtration criteria were designed so that ≤2.5% of the phosphopeptides were wrongly identified within each of 15 data categories (precursor ion charge states +1 – +5; identifications made by Mascot only, SEQUEST only, or both). Overall, this resulted in 252,626 filter-passing phosphopeptide identifications (FDR = 0.36%; an MS2 spectrum confidently identified by both Mascot and SEQUEST still counted as a single identification) of 6248 different phosphopeptides (FDR = 3.4%). This corresponded to 5782 phosphorylation sites. If phosphopeptides identified by only a single spectrum were excluded (“one-hit-wonders” are generally considered to be low-confidence), 5172 different phosphopeptides were identified (FDR = 1.9%), and if ≥3 identifications were required per phosphopeptide, 4652 different phosphopeptides were identified (FDR = 1.3%). Compared with using either Mascot or SEQUEST alone, using both search engines (i.e. “consensus scoring”) resulted in roughly triple the number of confidently identified phosphopeptides, in agreement with previous studies (51–55).
To manually validate the phosphopeptide identifications, ReAdW (v4.3.1, Institute for Systems Biology, Seattle, WA, http://www.proteomecenter.org/) was used to convert the LTQ-Orbitrap output .RAW files to .mzXML files, MS Data File Trimmer (v1.1.2991.28041, http://omics.pnl.gov/software/) was used to produce a single file containing the best MS2 spectra (i.e. the spectra that resulted in the highest-confidence identification of each phosphopeptide), and SpectrumLook (v1.5.37, http://omics.pnl.gov/software/) was used to browse the annotated spectra. These annotated spectra are available for download as a 212 MB .zip file at the Proteome Commons Tranche data repository at https://proteomecommons.org/(Tranche Hash: WsVcA7Y8lkPS/nO8sWIQTueMRiq6z6Vf4ZQRPSfLyH++tSbSXEAWCjHI+gxw2usbd+7ezHNKgetev8nSCyPBln/PDcoAAAAAAAAETg==).
Phosphopeptide Quantitation and ANOVAs
The LTQ-Orbitrap output files were deisotoped using Decon2LS (v1.0.2964.22547, http://omics.pnl.gov/software/) (56), an implementation of the THRASH algorithm (41). The resulting data were analyzed using MultiAlign (v1.1.2994.17669, http://omics.pnl.gov/software/), an implementation of the LCMSWARP (57) and AMT-tag (6–7) algorithms. The MultiAlign data were imported into a Microsoft Access 2007 database and “peak-matched” to the phosphopeptide identifications determined by the Mascot and SEQUEST analyses. A MultiAlign “feature” (a.k.a., a “unique mass class” or UMC) is a complete set of precursor ion m/z peaks originating from a single analyte and from a single LC-MS(/MS) analysis (i.e. a complete set of m/z peaks deconvolved of isotopic and charge state effects and then correlated by mass and elution time across multiple MS scans within a single LC-MS[/MS] file). For each individual LC-MS(/MS) analysis, one MultiAlign feature uniquely corresponds to one analyte. During peak-matching, a MultiAlign feature was matched to a Mascot/SEQUEST identification if and only if they were both from the same LC-MS(/MS) analysis, the MultiAlign feature apex was within ± 200 MS scans of the MS2 spectrum, and the MultiAlign feature mass was within ± 3.5 ppm of the precursor ion mass. A MultiAlign “cluster” (of features) is a complete set of MultiAlign features from a set of multiple, aligned LC-MS(/MS) analyses (i.e. for the complete set of aligned LC-MS[/MS] analyses, one MultiAlign cluster uniquely corresponds to one analyte). MultiAlign clusters that peak-matched to multiple phosphopeptides [ignoring PTM localization] were discarded unless the vast majority of the matching phosphopeptide identifications were of only one phosphopeptide (i.e. ≥fourfold the number of the second-most-numerous match). The vast majority of MultiAlign clusters only matched to a single phosphopeptide because of the enormous reduction in sample complexity that resulted from the TiO2 SPE. MultiAlign clusters with a mass range >16 ppm were discarded, “split” MultiAlign clusters (clusters confidently peak-matched to the same phosphopeptide [ignoring PTM localization]) were joined, and phosphopeptides that contained a missed trypsin cleavage site were discarded. MultiAlign was used to calculate the peak area (elution time versus intensity) of each feature, and each peak area was used as a relative measure of the abundance of the analyte. This resulted in 3265 confidently identified and quantitated phosphopeptides (FDR = 1.81% estimated by peak-matching the decoy database identifications). If ≥2 identifications were required per phosphopeptide, 3006 different phosphopeptides were confidently identified and quantitated (FDR = 1.3%), and if ≥3 identifications were required, 2837 different phosphopeptides were confidently identified and quantitated (FDR = 0.97%).
To eliminate systematic abundance value biases between the samples (due to, for example, slightly different sample phosphopeptide masses), a central tendency normalization (58) was performed (supplemental Fig. 3). Specifically, one LC-MS(/MS) dataset was selected to be the baseline, and for each “alignee” dataset, and for each confidently identified and quantitated phosphopeptide, the alignee to baseline abundance value ratio was calculated. The median of these ratios was calculated for each alignee and used as a normalization factor. Normalization was performed by dividing all of the alignee abundance values by this normalization factor.
The abundance values were log10-transformed because this increased the normality of the distribution of their z-scores (supplemental Fig. 4). Then, to determine which phosphopeptide abundance values were significantly different between the experimental groups, two different types of analysis of variance (ANOVA) tests were performed for each phosphopeptide using a MATLAB script (supplemental material) (35, 59). The first ANOVA was simply a parametric one-way ANOVA (missing values were excluded). The second ANOVA was a nonparametric Kruskal-Wallis one-way ANOVA by ranks (missing values were tied for the lowest rank). Each ANOVA resulted in a p value that is the probability of obtaining by random chance data at least as disproportionate as that observed. Both types of ANOVAs were employed because whereas the parametric ANOVA is a much more powerful test in general, it is unable to test for phosphopeptides that are present in some experimental conditions and absent from others. Other researchers have addressed the problem of missing values from proteomics datasets using data imputation (60), but this strategy is controversial so it was not used in this study. The resulting parametric p values were used to estimate parametric q-values using QVALUE (v1.1, http://genomics.princeton.edu/storeylab/qvalue/index.html) (61), and the nonparametric p values were used to estimate nonparametric q-values in the same way. A q-value is defined as the proportion of false positives incurred (i.e. the FDR) when a particular p value is considered significant. A single, robust q-value for each phosphopeptide could not be calculated because the parametric and nonparametric p values were not independent. Consequently, for each phosphopeptide the minimum of the parametric and nonparametric q-values was calculated and was used for data filtration and for ranking the confidence in the phosphorylation responses, and was considered an approximate estimate of the q-value of the combined ANOVAs. Unless specified otherwise, “q” or “q-value” refers to the minimum of the parametric and nonparametric q-values. To test the efficacy of the analysis strategy of the quantitative data, selected ion chromatograms of one of the phosphorylation responses were manually inspected (supplemental Fig. 5). Hierarchical cluster analyses of the significant data were performed using Genesis (v1.7.2, http://genome.tugraz.at/) (62) with average linkage correlations determined by Euclidean distance.
RESULTS
In this investigation, spleens from uninfected and spore-challenged mice were analyzed using label-free quantitative phosphoproteomics to discover host signaling responses to anthrax. A variety of organs and biofluids were retained from each mouse, and we ultimately focused on the spleen because of its role in the immune system, and because reproducibly isolating sufficient masses of circulating immune cells from whole blood using BSL2 protocols was found to be very challenging. Additionally, hemolysis was caused by the advanced Sterne infections, and the resulting highly variable volumes of blood recoverable from each mouse were often insufficient for our experiments.
Initially, method development experiments were performed to design and optimize sample preparation and mass spectrometry. Notably, several different phosphopeptide enrichment procedures were tested. Microcolumns prepared using a pressure cell, frit, and capillary tubing, and packed with Titansphere 5 μm TiO2 loose media (GL Sciences Inc.) (63) worked very well (∼5,000 phosphopeptide identifications per 2 h LC-LTQ-Orbitrap-MS[/MS] analysis) but suffered from low sample throughput (∼1 sample/5 h), and the reproducibility of the protocol was never carefully determined. TopTip 200 μl TiO2 centrifuge tips (Glygen Corp., Columbia, MD) worked poorly by comparison (∼1000 phosphopeptide identifications per 2 h LC-LTQ-Orbitrap-MS[/MS] analysis), and the TopTips retained more nonphosphopeptides than the Titansphere media. Fortunately, Titansphere PHOS-TiO 200 μl TiO2 centrifuge tips (GL Sciences Inc.) became available recently, and they performed just as well as the Titansphere microcolumns while enabling high sample throughput (∼20 samples/5 h), which in turn reduced sample processing time and eliminated the reproducibility concern. Three alterations to the protocol were investigated. First, using lactic acid instead of DHB (as in (64)) didn't significantly change the number of identified phosphopeptides per LC-LTQ-Orbitrap-MS(/MS) analysis. Second, including an insoluble component of the spleen homogenate in the tryptic digestion resulted in clogging of the Sep-Pak C-18 SPE, and therefore was excluded from consideration. Lastly, simplifying the TiO2 SPE procedure was found to have no adverse effect (described in detail in the Supplemental Protocol).
Similarly, a data analysis strategy needed to be developed to confidently discover the phosphoprotein signaling responses. To confidently identify the phosphopeptides, both Mascot and SEQUEST were used to perform database searching. This greatly mitigated the effect of the often poor fragmentation common to CID spectra of phosphopeptides, because both algorithms greatly complement one another. In fact, when the same FDR was required (of unique phosphopeptides), each algorithm alone resulted in roughly one-third of the identified phosphopeptides compared with using both search engines and requiring that both identified the same phosphopeptide (ignoring PTM localization). This is not surprising, as the use of multiple search engines (i.e. “consensus scoring”) has been shown to significantly improve peptide identification confidence (51–55). Additionally, a short Perl script was written (supplemental material) to remove tentative neutral-loss precursor ion peaks from the fragment ion spectra .dta files prior to the Mascot and SEQUEST analyses, and this significantly increased the number of identified phosphopeptides at the same FDR. Also, the use of DeconMSn instead of extract_msn enabled operation of the LTQ-Orbitrap with monoisotopic precursor ion selection turned off, and this also increased the number of identified phosphopeptides at the same FDR.
In a preliminary comparative phosphoproteomics experiment using six mice (two uninfected, two 24 h Sterne-infected, and two 48 h Sterne-infected), spectrum counting (simply counting confident phosphopeptide identifications as a rough measure of abundance) was found to be insufficient to identify tentative phosphoprotein signaling responses. However, reanalyzing the data using MultiAlign resulted in the discovery of 97 phosphopeptides that were altered significantly within the 48 h Sterne samples compared with the other four samples (44 were more abundant and 53 were less abundant). The two phosphoproteins affected most dramatically (both were ∼30-fold more abundant) have known roles in the immune system: interferon-γ-inducible p47 GTPase and Z-DNA binding protein (a cytosolic DNA sensor).
Based on the results of the preliminary experiments, a scaled-up discovery experiment using 60 mice was designed and performed (Fig. 1). At t = 0 h, five uninjected negative control (nc) mice were dissected, 15 nc mice were injected with H2O, 15 mice were injected with ΔSterne spores, and 25 mice were injected with Sterne spores. At t = 24, 48, and 72 h, five H2O, five ΔSterne, and five Sterne mice were dissected (14 of the Sterne mice died in their cages prior to t = 72 h and were discarded, so the 72 h Sterne experimental condition contained only one mouse spleen). Both the lethal Sterne strain and the asymptomatic ΔSterne strain were studied so that the virulence-specificity of the signaling responses could be determined. Signaling responses to Sterne but not ΔSterne might be useful markers of lethal anthrax or of virulent bacterial infection in general. The 46 resulting spleens were homogenized, and the resulting homogenate proteins were reduced using dithiothreitol, alkylated using iodoacetamide, and digested into peptides using trypsin. Phosphopeptides were enriched using TiO2 SPE and analyzed by nano-LC-LTQ-Orbitrap-MS(/MS). These spectra are available for download as .RAW files at the Proteome Commons Tranche data repository at https://proteomecommons.org/(Tranche Hash: j7rN4iodsyEQbWjTaWAqMthHEdlfsCqJc/WeLuA5xsrD0Tiu7NOEAwQWpWxhpAwl+hRPiBRzsc+K0cTFg2Kipfp+LDEAAAAAAAA9rw==).
Fig. 1.
Experimental design. Spleens from 46 mice (uninfected negative control, asymptomatic ΔSterne B. anthracis [missing the anthrax toxin], and lethal Sterne B. anthracis) were compared using label-free quantitative phosphoproteomics.
The resulting 46 nano-LC-LTQ-Orbitrap-MS(/MS) datasets plus the earlier 21 method development anthrax-mouse spleen nano-LC-LTQ-Orbitrap-MS(/MS) datasets were searched using both Mascot and SEQUEST against a combined B. anthracis + mouse FASTA text file of protein sequences. This resulted in 252,626 filter-passing phosphopeptide identifications (FDR = 0.36%) of 6248 different phosphopeptides (FDR = 3.4%). This corresponded to 5782 phosphorylation sites. If ≥2 identifications were required per phosphopeptide, 5172 different phosphopeptides were identified (FDR = 1.9%), and if ≥3 identifications were required, 4652 different phosphopeptides were identified (FDR = 1.3%). These data are included as supplemental Table 3, and the corresponding annotated spectra are available for download as a 212 MB .zip file at the Proteome Commons Tranche data repository at https://proteomecommons.org/(Tranche Hash: WsVcA7Y8lkPS/nO8sWIQTueMRiq6z6Vf4ZQRPSfLyH++tSbSXEAWCjHI+gxw2usbd+7ezHNKgetev8nSCyPBln/PDcoAAAAAAAAETg==).
The resulting 46 LTQ-Orbitrap datasets described above were also analyzed using Decon2LS and MultiAlign. Pairwise Pearson correlations of the log10-transformed MultiAlign abundance values displayed as a heatmap (Fig. 2) clearly show that the phosphoproteome profiles of the very sick (≥48 h Sterne) mice were globally different from the other profiles but not from each other. This was not surprising as these mice were moribund, and their spleens were pale pink (the normal spleens were blood red) and smaller than normal (roughly half the volume) based on visual observation.
Fig. 2.
Correlation analyses of the LC-MS(/MS) datasets. Spleen phosphoproteomics samples from 46 mice were analyzed by LC-MS(/MS), and the resulting data are displayed as pairwise Pearson correlations of the log10-transformed MultiAlign abundance values. Black (R2 ≤ 0.5) and yellow (R2 ≥ 0.9) indicate weak and strong correlations, respectively. The phosphoproteome profiles of the very sick (≥48 h Sterne) mice were globally different from the others, but not from each other.
The MultiAlign data were peak-matched to the filter-passing phosphopeptide identifications, and additional data filtration criteria were imposed. Of the 6248 confidently identified phosphopeptides, 3265 were confidently quantitated (FDR = 1.81% estimated by peak-matching the decoy database identifications). If ≥2 identifications were required per phosphopeptide, 3006 different phosphopeptides were confidently identified and quantitated (FDR = 1.3%), and if ≥3 identifications were required, 2837 different phosphopeptides were confidently identified and quantitated (FDR = 0.97%). These data are included as supplemental Table 4.
A global normalization of the abundance values was performed to eliminate systematic biases between the samples because of, for example, slightly different sample phosphopeptide masses (supplemental Fig. 3). Then log10-transformation was performed because it increased the normality of the z-score distribution of the abundance values (supplemental Fig. 4) indicating that the unadjusted values were approximately log-normal. A plot of the geometric mean (μG) versus geometric standard deviation (σG) of the globally normalized but otherwise unadjusted abundance values indicated that σG < 2 for most of the phosphopeptides (Fig. 3). This indicates that phosphorylation abundance changes of twofold upon infection might be statistically discernable. Also, it was noted that σG decreased as μG increased, likely because the Orbitrap intensity measurement is less accurate at lower precursor ion intensities.
Fig. 3.
Variance of the phosphopeptide abundance values. The MultiAlign phosphopeptide abundance values were globally normalized, and then the geometric mean (μG) and geometric standard deviation (σG) were calculated for each analyte across the 46 spleen samples. Confidence intervals of log-normal data take the form μGσG−n - μGσGn (e.g. the 95% confidence interval is from μGσG−2 to μGσG2, or from 0.5 × μG to 2 × μG if σG = 20.5). Phosphopeptide abundances altered twofold are potentially statistically discernable.
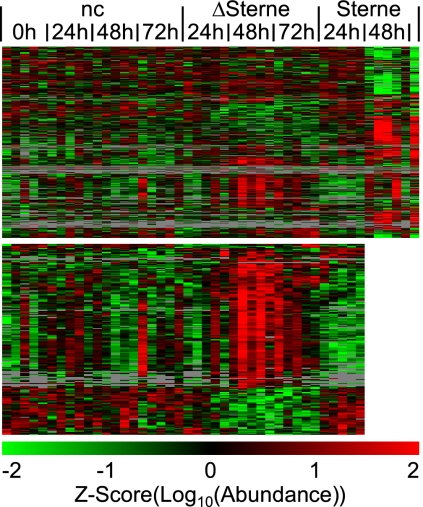
Following globally normalizing and log10-transforming the abundance values, ANOVAs were used to identify phosphopeptides that were disproportionately abundant in one or more of the experimental groups (35, 59). Of the 3265 confidently identified and quantitated phosphopeptides, 1173 (36%) were found to be significantly altered (q < 0.05). Note that by requiring q < 0.05, 5% of the data (59 phosphopeptides) are estimated to have been wrongly considered significant by the ANOVAs. The significantly altered phosphopeptide data were analyzed using a hierarchical cluster analysis and displayed as a heatmap (Fig. 4 Top; the corresponding data is included as supplemental Table 5). The ≥48 h Sterne samples were very different from the other samples, in agreement with Fig. 2. It should be noted that to justify performing the global normalization, it was assumed that any abundance differences between the samples would average out. This assumption was true even for the ≥48 h Sterne samples, as the global normalization successfully removed systematic biases from even these datasets (supplemental Fig. 3; note that the MultiAlign and global normalization baseline was the same sample, and that it was one of the 24 h ΔSterne samples).
Fig. 4.
Mouse spleen signaling responses to anthrax. Top Heatmap: The MultiAlign abundance values of the confidently identified and quantitated phosphopeptides (n = 3265) were globally normalized, log10-transformed, and analyzed by ANOVAs across the seven experimental conditions. The significant data (q<0.05, n = 1173) were z-score transformed across the 46 samples, and analyzed using a hierarchical cluster analysis. Each row represents a confidently identified phosphopeptide, each column represents a mouse spleen, and relative abundance values are indicated by color (green = relatively low, red = relatively high, gray = missing data). Most of the phosphopeptides were altered in the ≥48 h Sterne samples, correlating strongly with anthrax pathogenesis as the Sterne-infected mice began to die at 48 h postchallenge and the mice infected with the ΔSterne strain (missing the anthrax toxin) were asymptomatic. Bottom Heatmap: All of the data except for the ≥48 h Sterne data were reanalyzed by ANOVAs across the five remaining experimental conditions, and the significant data (q<0.05, n = 188) were analyzed using a hierarchical cluster analysis. The resulting heatmap depicts the mouse spleen signal transduction cascade responding to the asymptomatic ΔSterne infection.
Ideally, a post hoc analysis such as the Tukey-Kramer test would have been used to perform pairwise comparisons to determine which phosphopeptides were significantly different between specific pairs of experimental groups. Unfortunately, an algorithm to calculate the FDR of such analyses has yet to be discovered. Therefore, to discover which phosphopeptides were altered between the other experimental conditions (i.e. other than ≥48 h Sterne), the ANOVAs were recalculated excluding the ≥48 h Sterne data. This time, of the 3265 confidently identified and quantitated phosphopeptides, 188 (5.8%) were found to be significantly altered (q < 0.05). Note that by requiring q < 0.05, 5% of the data (nine phosphopeptides) are estimated to have been wrongly considered significant by the ANOVAs. The significantly altered phosphopeptide data were analyzed using a hierarchical cluster analysis and displayed as a heatmap (Fig. 4 Bottom; the corresponding data is included as supplemental Table 6). Almost all of these 188 phosphopeptides were disproportionately abundant within the ≥48 h ΔSterne samples. Clearly these phosphoproteins were a major component of the mouse spleen immune response to the asymptomatic ΔSterne challenge. Notably, this response was mostly absent at 24 h postchallenge, and it was decreased in intensity by 72 h postchallenge, in agreement with the abortive nature of the ΔSterne infection.
To discover phosphopeptides that were altered at 24 h postinfection, the ANOVAs were recalculated yet again, this time excluding the ≥48 h ΔSterne and Sterne data. This time, of the 3265 confidently identified and quantitated phosphopeptides, 26 (0.8%) were tentatively found to be altered (q<0.5). Note that by requiring q < 0.5, 50% of the data (13 phosphopeptides) are estimated to have been wrongly considered significant by the ANOVAs. The tentatively altered phosphopeptide data were analyzed using a hierarchical cluster analysis and displayed as a heatmap (Fig. 5; the corresponding data is included as supplemental Table 7). Almost all of the 26 phosphopeptides were affected the same way by the ΔSterne and Sterne strains, indicating similarity between the early host responses to the toxigenic and nontoxigenic infections. It's reasonable to question whether or not these early signaling events are specific to B. anthracis infection, or if they are not anthrax-specific but rather are common to bacterial infection in general.
Fig. 5.
Mouse spleen signaling responses to early-stage anthrax. To tentatively discover early-stage signaling responses, the analysis described in Fig. 4 was repeated except that the ≥48 h ΔSterne and Sterne data were excluded. Twenty-six phosphopeptides were tentatively found to be altered (q<0.5). Note that by requiring q<0.5, 50% of the data (13 phosphopeptides) were estimated to have been wrongly considered significant by the ANOVAs. Of these 26 phosphopeptides, ten were derived from eight proteins that have known roles in the immune system. Two of these eight proteins, Pram1 and Hemgn, each had two phosphopeptides that responded similarly (i.e. clustered together) at 24 h postchallenge, indicating that both proteins were probably genuinely affected during early-stage anthrax.
Ten of the 26 phosphopeptides were derived from eight proteins that have known roles in the immune system. Two of these phosphoproteins, Pram1 and Hemgn, each had two phosphorylation sites (on two separate phosphopeptides) that were affected similarly during early-anthrax (Fig. 5), indicating that these proteins were probably genuinely altered at 24 h postchallenge. Because the phosphopeptides from each of these two proteins clustered together, it's reasonable to speculate that these proteins were multiply phosphorylated/dephosphorylated by the same kinase/phosphatase. Of the 26 phosphopeptides, the one with the lowest q-value was derived from interferon-γ-inducible p47 GTPase, a regulator of immunity to intracellular pathogens (Gm12250; p[parametric] = 0.0000345; q[parametric] = 0.11). Overall, these 26 phosphopeptides constitute a q-value-ranked list of candidate biomarkers of early-stage anthrax available for targeted validation experiments using circulating immune cells. Ideally, a set of highly-confident (q < 0.001) early-anthrax candidate biomarkers would have been discovered. However, comparative proteome profiling of highly similar samples often has the problem of a small number of slightly altered proteins, making it challenging to confidently discover the earliest biomarkers of diseases in general.
In an attempt to further refine the list of 26 phosphopeptides, the results of the three sets of ANOVAs were compared, and it was found that 15 of the phosphopeptides were discovered by all three analyses (Fig. 6). Thus, these 15 phosphopeptides were mildly altered at 24 h postinfection, and then more significantly altered at ≥48 h postinfection. Six of the 15 phosphopeptides were derived from five proteins that have known roles in the host immune system. These include both Hemgn phosphopeptides, one of the two Pram1 phosphopeptides, and the interferon-γ-inducible p47 GTPase (Gm12250) phosphopeptide mentioned above. The other two phosphopeptides originated from Dok1 and Samsn1. Because these five phosphoproteins have known immune system functions, were mildly altered in the 24 h anthrax samples, and were significantly altered in the ≥48 h anthrax samples, these constitute the highest-confidence candidate phosphoprotein biomarkers of early-stage anthrax discovered by this study.
Fig. 6.
Venn diagram of the ANOVA results. ANOVAs were performed using all of the data (upper left circle; same data as in Fig. 4 Top), all but the ≥48 h Sterne data (upper right circle; same data as in Fig. 4 Bottom), and all but the ≥48 h ΔSterne and Sterne data (bottom circle; same data as in Fig. 5). Fifteen phosphopeptides were found to be slightly altered at 24 h postchallenge, and then significantly altered at ≥48 h.
DISCUSSION
Recently developed sample preparation, mass spectrometry, and data analysis technologies have greatly advanced quantitative phosphoproteomics. In this study, the spleens of B. anthracis spore-challenged mice were analyzed using label-free quantitative phosphoproteomics, thousands of phosphopeptides were confidently identified and quantitated, and many candidate biomarkers of anthrax were discovered. Sterne infections resulted in global alterations to the spleen phosphoproteome starting at 48 h postchallenge, correlating well with the morphological changes to the spleens and the severity of the symptoms at this stage of the disease. Asymptomatic ΔSterne infections resulted in 188 significantly altered phosphopeptides, and the corresponding phosphoproteins likely have important host response functions in the spleen.
Twenty-six phosphopeptides were tentatively discovered to be altered at 24 h postchallenge. Eight of the corresponding proteins have known roles in the immune system: (i) Dido1 (death-inducer obliterator), an interleukin-3 activated apoptotic factor, (ii) Dok1 (docking protein), a negative regulator downstream of Toll-like receptors, (iii) Gm12250 (interferon-γ-inducible p47 GTPase), a regulator of immunity to intracellular pathogens, (iv) Hemgn (hemogen), a hematopoietic cell-specific protein that regulates proliferation and differentiation and resists cell death through activation of NFκB, (v) Map3k5 (mitogen-activated protein kinase kinase kinase), which is activated by the T-cell costimulatory receptor and regulates apoptosis upon infection and B-cell activation and proliferation, (vi) Mllt4 (afadin), a regulator of hematopoietic cell-cell adhesion, (vii) Pram1 (PML-RARA-regulated adapter), a regulator of T-cell receptor mediated activation of, for example, NFκB, and (viii) Samsn1 (SH3 SAM containing hematopoietic adaptor), a B-cell receptor immunoinhibitor. These represent excellent candidate phosphoprotein biomarkers for validation studies of circulating immune cells using, for example, triple quadrupole mass spectrometry or reverse phase protein microarrays. A validated mouse spleen signaling response of early-stage anthrax might translate into a presymptomatic biomarker of human anthrax detectable within circulating immune cells. Alternatively, additional discovery experiments would likely result in a set of higher-confidence candidate biomarkers. For example, label-free quantitative phosphoproteomics could be used to study circulating immune cells isolated from subhuman primates given an intratracheal administration of a B. anthracis spore aerosol of a highly pathogenic BSL-3 strain such as the Ames strain. Additionally, phosphoproteome profiling of human circulating immune cells incubated with B. anthracis bacteria or from recipients of anthrax vaccination might be informative. Used as diagnostic markers, phosphoprotein biomarkers of presymptomatic anthrax could potentially provide physicians with a key tool to fight anthrax in the event of a bioterror attack. More broadly, they may be useful biomarkers of immune response in general.
The recent development of phosphopeptide enrichment strategies, high-resolution nano-LC-MS(/MS), software designed to analyze mass spectrometry data, and countless other technologies have enabled confident, high-throughput, shotgun phosphopeptide identification and simultaneous comparative quantitation. Besides innumerable applications to life science research, future clinical applications of quantitative phosphoproteomics might include rapid phosphoproteome profiling of tumors to differentially diagnose cancer and of circulating immune cells to test for infection, inflammation, and immune disorders. Combined with other omics technologies, quantitative phosphoproteomics might help foster the emerging science of systems biology, and together these synergistic analytical methods will hopefully lead to more comprehensive models of biological processes and the development of personalized medicine.
Acknowledgments
We gratefully acknowledge the contributions of Myung-Chul Chung, Jianghong Deng, Jennifer Guernsey, Matthew E. Monroe, Aarthi Narayanan, and Mark M. Ross.
Footnotes
* This work was supported by the US Department of Energy grant DE-FC52-FC04NA25455.
 This article contains supplemental material S1–S7, supplemental Figs. S1–S5, supplemental Protocol, and supplemental Tables S1–S7.
This article contains supplemental material S1–S7, supplemental Figs. S1–S5, supplemental Protocol, and supplemental Tables S1–S7.
1 The abbreviations used are:
- LC-MS(/MS)
- liquid chromatography-tandem MS
- ACN
- acetonitrile
- ANOVA
- analysis of variance
- DHB
- 2,5-dihydroxybenzoic acid
- FDR
- false discovery rate
- nc
- negative control
- PTM
- post-translational modification
- SPE
- solid-phase extraction
- TFA
- trifluoroacetic acid.
REFERENCES
- 1. Mock M., Fouet A. (2001) Anthrax. Ann. Rev. Microbiol. 55, 647–671 [DOI] [PubMed] [Google Scholar]
- 2. Inglesby T. V., O'Toole T., Henderson D. A., Bartlett J. G., Ascher M. S., Eitzen E., Friedlander A. M., Gerberding J., Hauer J., Hughes J., McDade J., Osterholm M. T., Parker G., Perl T. M., Russell P. K., Tonat K. (2002) Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287, 2236–2252 [DOI] [PubMed] [Google Scholar]
- 3. Graham B., Talent J., Allison G., Cleveland R., Rademaker S., Roemer T., Sherman W., Sokolski H., Verma R. (2008) World at risk: The report of the commission on the prevention of WMD proliferation and terrorism, Random House, New York [Google Scholar]
- 4. Drogin B. (March 8, 2009) Anthrax hoaxes pile up, as does their cost. Los Angeles Times [Google Scholar]
- 5. Narayanan A., Zhou W., Ross M., Tang J., Liotta L., Petricoin E., Kashanchi F., Bailey C., Popov S. (2009) Discovery of Infectious Disease Biomarkers in Murine Anthrax Model Using Mass Spectrometry of the Low-Molecular-Mass Serum Proteome. J. Proteomics Bioinform. 2, 408–415 [Google Scholar]
- 6. Zimmer J. S., Monroe M. E., Qian W. J., Smith R. D. (2006) Advances in proteomics data analysis and display using an accurate mass and time tag approach. Mass Spectrometry Rev. 25, 450–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu T., Belov M. E., Jaitly N., Qian W. J., Smith R. D. (2007) Accurate mass measurements in proteomics. Chem. Rev. 107, 3621–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yates J. R., Ruse C. I., Nakorchevsky A. (2009) Proteomics by mass spectrometry: approaches, advances, and applications. Ann. Rev. Biomed. Eng. 11, 49–79 [DOI] [PubMed] [Google Scholar]
- 9. Feng X., Liu X., Luo Q., Liu B. F. (2008) Mass spectrometry in systems biology: an overview. Mass Spectrometry Rev. 27, 635–660 [DOI] [PubMed] [Google Scholar]
- 10. Gstaiger M., Aebersold R. (2009) Applying mass spectrometry-based proteomics to genetics, genomics and network biology. Nat. Rev. 10, 617–627 [DOI] [PubMed] [Google Scholar]
- 11. Nesvizhskii A. I., Vitek O., Aebersold R. (2007) Analysis and validation of proteomic data generated by tandem mass spectrometry. Nat. Meth. 4, 787–797 [DOI] [PubMed] [Google Scholar]
- 12. Matthiesen R. (2007) Methods, algorithms and tools in computational proteomics: a practical point of view. Proteomics 7, 2815–2832 [DOI] [PubMed] [Google Scholar]
- 13. Macek B., Mann M., Olsen J. V. (2009) Global and site-specific quantitative phosphoproteomics: principles and applications. Ann. Rev. Pharmacol.Toxicol. 49, 199–221 [DOI] [PubMed] [Google Scholar]
- 14. Thingholm T. E., Jensen O. N., Larsen M. R. (2009) Analytical strategies for phosphoproteomics. Proteomics 9, 1451–1468 [DOI] [PubMed] [Google Scholar]
- 15. Grimsrud P. A., Swaney D. L., Wenger C. D., Beauchene N. A., Coon J. J. (2010) Phosphoproteomics for the masses. ACS Chem. Biol. 5, 105–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nita-Lazar A., Saito-Benz H., White F. M. (2008) Quantitative phosphoproteomics by mass spectrometry: past, present, and future. Proteomics 8, 4433–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brendolan A., Rosado M. M., Carsetti R., Selleri L., Dear T. N. (2007) Development and function of the mammalian spleen. Bioessays 29, 166–177 [DOI] [PubMed] [Google Scholar]
- 18. Mebius R. E., Kraal G. (2005) Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616 [DOI] [PubMed] [Google Scholar]
- 19. Paul W. E. (2008) Fundamental immunology, 6th Ed., pp 38–42, Lippincott, Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 20. Cesta M. F. (2006) Normal structure, function, and histology of the spleen. Toxicol. Pathol. 34, 455–465 [DOI] [PubMed] [Google Scholar]
- 21. Hoffbrand A. V., Catovsky D., Tuddenham E. (2005) Postgraduate Haematology, 5th Ed., pp 359–60, Blackwell Publishing, Oxford, UK [Google Scholar]
- 22. Peters A. M., Saverymuttu S. H., Bell R. N., Lavender J. P. (1985) Quantification of the distribution of the marginating granulocyte pool in man. Scand. J. Haematol. 34, 111–120 [DOI] [PubMed] [Google Scholar]
- 23. Peters A. M., Saverymuttu S. H., Keshavarzian A., Bell R. N., Lavender J. P. (1985) Splenic pooling of granulocytes. Clin. Sci. 68, 283–289 [DOI] [PubMed] [Google Scholar]
- 24. Swirski F. K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J. L., Kohler R. H., Chudnovskiy A., Waterman P., Aikawa E., Mempel T. R., Libby P., Weissleder R., Pittet M. J. (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beretta L. (2009) Comparative analysis of the liver and plasma proteomes as a novel and powerful strategy for hepatocellular carcinoma biomarker discovery. Cancer Lett. 286, 134–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen R., Pan S., Brentnall T. A., Aebersold R. (2005) Proteomic profiling of pancreatic cancer for biomarker discovery. Mol. Cell Proteomics 4, 523–533 [DOI] [PubMed] [Google Scholar]
- 27. Johann D. J., Jr., Wei B. R., Prieto D. A., Chan K. C., Ye X., Valera V. A., Simpson R. M., Rudnick P. A., Xiao Z., Issaq H. J., Linehan W. M., Stein S. E., Veenstra T. D., Blonder J. (2010) Combined blood/tissue analysis for cancer biomarker discovery: application to renal cell carcinoma. Anal. Chem. 82, 1584–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKinney K. Q., Lee Y. Y., Choi H. S., Groseclose G., Iannitti D. A., Martinie J. B., Russo M. W., Lundgren D. H., Han D. K., Bonkovsky H. L., Hwang S. I. (2010) Discovery of putative pancreatic cancer biomarkers using subcellular proteomics. J. Proteomics. 2011 Jan 1; 74(1): 79–88 [DOI] [PubMed] [Google Scholar]
- 29. Poschmann G., Sitek B., Sipos B., Hamacher M., Vonend O., Meyer H. E., Stühler K. (2009) Cell-based proteome analysis: the first stage in the pipeline for biomarker discovery. Biochim. Biophys. Acta 1794, 1309–1316 [DOI] [PubMed] [Google Scholar]
- 30. Schiess R., Wollscheid B., Aebersold R. (2009) Targeted proteomic strategy for clinical biomarker discovery. Mol. Oncol. 3, 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whiteaker J. R., Zhang H., Zhao L., Wang P., Kelly-Spratt K. S., Ivey R. G., Piening B. D., Feng L. C., Kasarda E., Gurley K. E., Eng J. K., Chodosh L. A., Kemp C. J., McIntosh M. W., Paulovich A. G. (2007) Integrated pipeline for mass spectrometry-based discovery and confirmation of biomarkers demonstrated in a mouse model of breast cancer. J. Proteome Res. 6, 3962–3975 [DOI] [PubMed] [Google Scholar]
- 32. Zhou L., Cai M., Ling X. B., Wang Q., Lau K., Zhao J. J., Schilling J., Chen L. (2009) Cancer biomarker discovery via targeted profiling of multiclass tumor tissue-derived proteomes. Clin. Proteomics 5, 163–169 [Google Scholar]
- 33. Bradburne C., Chung M. C., Zong Q., Schlauch K., Liu D., Popova T., Popova A., Bailey C., Soppet D., Popov S. (2008) Transcriptional and apoptotic responses of THP-1 cells to challenge with toxigenic, and non-toxigenic Bacillus anthracis. BMC Immunol. 9, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Popov S. G., Villasmil R., Bernardi J., Grene E., Cardwell J., Wu A., Alibek D., Bailey C., Alibek K. (2002) Lethal toxin of Bacillus anthracis causes apoptosis of macrophages. Biochem. Biophys. Res. Commun. 293, 349–355 [DOI] [PubMed] [Google Scholar]
- 35. Oberg A. L., Vitek O. (2009) Statistical design of quantitative mass spectrometry-based proteomic experiments. J. Proteome Res. 8, 2144–2156 [DOI] [PubMed] [Google Scholar]
- 36. Thingholm T. E., Jørgensen T. J., Jensen O. N., Larsen M. R. (2006) Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nat. Protoc. 1, 1929–1935 [DOI] [PubMed] [Google Scholar]
- 37. Scigelova M., Makarov A. (2006) Orbitrap mass analyzer–overview and applications in proteomics. Proteomics 6 Suppl 2, 16–21 [DOI] [PubMed] [Google Scholar]
- 38. Perry R. H., Cooks R. G., Noll R. J. (2008) Orbitrap mass spectrometry: instrumentation, ion motion and applications. Mass Spectrometry Rev. 27, 661–699 [DOI] [PubMed] [Google Scholar]
- 39. Zhao R., Ding S. J., Shen Y., Camp D. G., 2nd, Livesay E. A., Udseth H., Smith R. D. (2009) Automated metal-free multiple-column nanoLC for improved phosphopeptide analysis sensitivity and throughput. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877, 663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mayampurath A. M., Jaitly N., Purvine S. O., Monroe M. E., Auberry K. J., Adkins J. N., Smith R. D. (2008) DeconMSn: a software tool for accurate parent ion monoisotopic mass determination for tandem mass spectra. Bioinformatics 24, 1021–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Horn D. M., Zubarev R. A., McLafferty F. W. (2000) Automated reduction and interpretation of high resolution electrospray mass spectra of large molecules. J. Am. Soc. Mass Spectrometry 11, 320–332 [DOI] [PubMed] [Google Scholar]
- 42. Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
- 43. Eng J. K., McCormack A. L., Yates J. R., 3rd (1994) An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrometry 5, 976–989 [DOI] [PubMed] [Google Scholar]
- 44. Peterson J. D., Umayam L. A., Dickinson T., Hickey E. K., White O. (2001) The Comprehensive Microbial Resource. Nucleic Acids Res. 29, 123–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kersey P. J., Duarte J., Williams A., Karavidopoulou Y., Birney E., Apweiler R. (2004) The International Protein Index: an integrated database for proteomics experiments. Proteomics 4, 1985–1988 [DOI] [PubMed] [Google Scholar]
- 46. Elias J. E., Gygi S. P. (2007) Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Meth. 4, 207–214 [DOI] [PubMed] [Google Scholar]
- 47. Petyuk V. A., Jaitly N., Moore R. J., Ding J., Metz T. O., Tang K., Monroe M. E., Tolmachev A. V., Adkins J. N., Belov M. E., Dabney A. R., Qian W. J., Camp D. G., 2nd, Smith R. D. (2008) Elimination of systematic mass measurement errors in liquid chromatography-mass spectrometry based proteomics using regression models and a priori partial knowledge of the sample content. Analyt. Chem. 80, 693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Petyuk V. A., Mayampurath A. M., Monroe M. E., Polpitiya A. D., Purvine S. O., Anderson G. A., Camp D. G., 2nd, Smith R. D. (2010) DtaRefinery, a software tool for elimination of systematic errors from parent ion mass measurements in tandem mass spectra data sets. Mol. Cell Proteomics 9, 486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Du X., Yang F., Manes N. P., Stenoien D. L., Monroe M. E., Adkins J. N., States D. J., Purvine S. O., Camp D. G., 2nd, Smith R. D. (2008) Linear discriminant analysis-based estimation of the false discovery rate for phosphopeptide identifications. J. Proteome Res. 7, 2195–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beausoleil S. A., Villén J., Gerber S. A., Rush J., Gygi S. P. (2006) A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 24, 1285–1292 [DOI] [PubMed] [Google Scholar]
- 51. Searle B. C. (2010) Scaffold: a bioinformatic tool for validating MS/MS-based proteomic studies. Proteomics 10, 1265–1269 [DOI] [PubMed] [Google Scholar]
- 52. McHugh L., Arthur J. W. (2008) Computational methods for protein identification from mass spectrometry data. PLoS Comput. Biol. 4, e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Price T. S., Lucitt M. B., Wu W., Austin D. J., Pizarro A., Yocum A. K., Blair I. A., FitzGerald G. A., Grosser T. (2007) EBP, a program for protein identification using multiple tandem mass spectrometry datasets. Mol. Cell Proteomics 6, 527–536 [DOI] [PubMed] [Google Scholar]
- 54. Sultana T., Jordan R., Lyons-Weiler J. (2009) Optimization of the use of consensus methods for the detection and putative identification of peptides via mass spectrometry using protein standard mixtures. J. Proteomics Bioinform. 2, 262–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Manes N. P., Estep R. D., Mottaz H. M., Moore R. J., Clauss T. R., Monroe M. E., Du X., Adkins J. N., Wong S. W., Smith R. D. (2008) Comparative proteomics of human monkeypox and vaccinia intracellular mature and extracellular enveloped virions. J. Proteome Res. 7, 960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jaitly N., Mayampurath A., Littlefield K., Adkins J. N., Anderson G. A., Smith R. D. (2009) Decon2LS: An open-source software package for automated processing and visualization of high resolution mass spectrometry data. BMC Bioinformatics 10, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jaitly N., Monroe M. E., Petyuk V. A., Clauss T. R., Adkins J. N., Smith R. D. (2006) Robust algorithm for alignment of liquid chromatography-mass spectrometry analyses in an accurate mass and time tag data analysis pipeline. Analyt. Chem. 78, 7397–7409 [DOI] [PubMed] [Google Scholar]
- 58. Callister S. J., Barry R. C., Adkins J. N., Johnson E. T., Qian W. J., Webb-Robertson B. J., Smith R. D., Lipton M. S. (2006) Normalization approaches for removing systematic biases associated with mass spectrometry and label-free proteomics. J. Proteome Res. 5, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de la Fuente van Bentem S., Mentzen W. I., de la Fuente A., Hirt H. (2008) Towards functional phosphoproteomics by mapping differential phosphorylation events in signaling networks. Proteomics 8, 4453–4465 [DOI] [PubMed] [Google Scholar]
- 60. Polpitiya A. D., Qian W. J., Jaitly N., Petyuk V. A., Adkins J. N., Camp D. G., 2nd, Anderson G. A., Smith R. D. (2008) DAnTE: a statistical tool for quantitative analysis of -omics data. Bioinformatics 24, 1556–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Storey J. D., Tibshirani R. (2003) Statistical significance for genomewide studies. Proc. Natl. Acad. Sci.U.S.A. 100, 9440–9445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sturn A., Quackenbush J., Trajanoski Z. (2002) Genesis: cluster analysis of microarray data. Bioinformatics 18, 207–208 [DOI] [PubMed] [Google Scholar]
- 63. Zhou W., Ross M. M., Tessitore A., Ornstein D., Vanmeter A., Liotta L. A., Petricoin E. F., 3rd (2009) An initial characterization of the serum phosphoproteome. J. Proteome Res. 8, 5523–5531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sugiyama N., Masuda T., Shinoda K., Nakamura A., Tomita M., Ishihama Y. (2007) Phosphopeptide enrichment by aliphatic hydroxy acid-modified metal oxide chromatography for nano-LC-MS/MS in proteomics applications. Mol. Cell Proteomics 6, 1103–1109 [DOI] [PubMed] [Google Scholar]