Abstract
Bioorthogonal chemical reporters are useful tools for visualizing and identifying post-translational modifications on proteins. Here we report the proteomic analysis of mammalian proteins targeted by a series of fatty acid chemical reporters ranging from myristic to stearic acid. The large-scale analysis of total cell lysates from fully solubilized Jurkat T cells identified known fatty-acylated proteins and many new candidates, including nuclear proteins and in particular histone H3 variants. We demonstrate that histones H3.1, H3.2, and H3.3 are modified with fatty acid chemical reporters and identify the conserved cysteine 110 as a new site of S-acylation on histone H3.2. This newly discovered modification of histone H3 could have implications for nuclear organization and chromatin regulation. The unbiased proteomic analysis of fatty-acylated proteins using chemical reporters has revealed a greater diversity of lipid-modified proteins in mammalian cells and identified a novel post-translational modification of histones.
Protein fatty-acylation encompasses several covalent modification of proteins with lipids that plays important roles in eukaryotic physiology and human disease (1). In particular, N-myristoylation and S-palmitoylation comprise the two major classes of protein fatty-acylation that provide crucial mechanisms to spatially and temporally control the activities of proteins in eukaryotic cells (see Fig. 1A) (1). N-Myristoylation is a constitutive modification that is characterized by the attachment of myristic acid (14:0) to N-terminal glycine residues through the action of N-myristoyltransferases (2). In contrast, S-palmitoylation or S-acylation describes the reversible addition of palmitic (16:0) and other fatty acids onto the thiol side chain of cysteine residues (3), which can be installed by a conserved family of Asp-His-His-Cys-containing protein acyltransferases (DHHC-PATs)1 (4). The terms “palmitoylation” and S-acylation are commonly used interchangeably and represent a heterogeneous modification with fatty acids both longer and shorter than palmitate which also varying in their degree of saturation. Stearate (18:0), oleate (18:1), arachidonate (20:4), and eicosapentaenoate (20:5) have all been shown to be incorporated onto palmitoylated proteins (5, 6). Fatty-acylation of proteins typically increases their affinity to membranes and subsequent interaction with other proteins for cell signaling (1). A survey of some known fatty-acylated proteins (i.e. Ras, Lck, and PSD-95) highlights essential roles for protein fatty-acylation in cellular proliferation, lymphocyte activation, and synaptic transmission respectively (1).
Fig. 1.
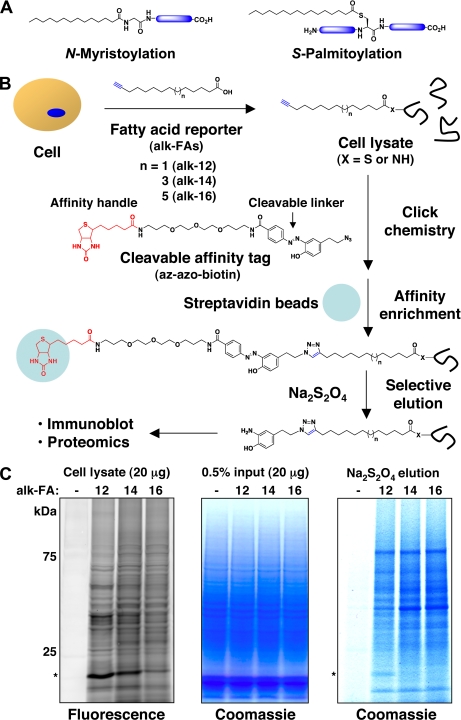
Protein fatty-acylation and its analysis with bioorthogonal chemical reporters. A, Chemical modification of fatty-acylated proteins. Palmitoylation is heterogeneous in length and saturation; only palmitic acid is shown. B, Schematic for proteomic analysis of fatty-acylated proteins with chemical reporters using cleavable affinity tags. Jurkat T cells were metabolically labeled with DMSO control (-) or alk-FAs (20 μm myristic analogs, 100 μm all other analogs; 6–8 h). Total cell lysates were then subjected to click chemistry reaction with az-azo-biotin (100 μm), small molecule removal, affinity-enrichment with streptavidin-beads, selective elution from beads with sodium dithionite (Na2S2O4, 25 mm, 1 h), and separation by SDS-PAGE. Selectively recovered proteins were analyzed by immunoblot using specific antibodies or stained with Coomassie blue and processed for mass spectrometry-based protein identification. X = NH (N-myristoylation) or S (S-palmitoylation). C, Profile of alkynyl-fatty acid-labeled proteins in total cell lysates visualized by click chemistry reaction with az-rhodamine/in-gel fluorescence scanning (left panel) or with az-azo-biotin, streptavidin enrichment, Na2S2O4 elution and Coomassie blue staining (right panel). Coomassie staining of total cell lysates from each sample (-, alk-12, alk-14, alk-16) demonstrates comparable amounts of protein loading for both fluorescence imaging and affinity enrichment of labeled proteins (middle panel).
The diversity of fatty-acylated proteins and their mechanisms of regulations are not completely understood (1). Prediction algorithms such as Myrbase (7) and CSS-Palm (8) provide useful bioinformatic tools for the analysis of candidate fatty-acylated proteins based on previously observed modification motifs, but require experimental validation and offer no insight into the post-transcriptional mechanisms of fatty-acylation. For example, alternative splicing of mRNA transcripts may yield substrates for N-myristoylation as in the case of the p35 isoform of OCA-B, a transcription factor required for late-stage B-cell development (9, 10). Alternatively, protein fatty-acylation may be altered during cellular regulation. During programmed cell death, the apoptosis effector tBid is post-translationally N-myristoylated following proteolytic cleavage by the cysteine protease caspase-3, which targets tBid to mitochondrial membranes during apoptosis (11, 12). Dissecting the regulatory mechanisms of S-palmitoylation in mammals also presents a significant challenge as ∼23 DHHC-PATs have been identified in human and mouse genomes with overlapping substrate specificities (13). Moreover, the functions of fatty-acylated proteins can be regulated by other post-translational modifications (PTMs) such as phosphorylation, as exemplified by studies on MARCKS (myristoylated alanine-rich C-kinase substrate) (14), c-Abl kinase (15, 16), and potassium channels (17). Robust experimental methods are therefore needed to fully appreciate the diversity and functional roles of protein fatty-acylation.
Two chemical methods have been developed to overcome the limitations of radiolabeled fatty acids for the detection and identification of fatty-acylated proteins (18). The acyl-biotin exchange (ABE) protocol developed by Drisdel and Green exploits the differential sensitivity of thioesters to hydroxylamine (NH2OH) for selective biotinylation of S-acylated proteins and nonradioactive visualization by streptavidin blot (19) as well as enrichment of S-acylated peptides or proteins for proteomic analysis by mass spectrometry (20–23). The global profiling of S-palmitoylation in budding yeast with ABE identified several new S-palmitoylated proteins and highlighted the differential and overlapping substrate specificities of the DHHC-PATs (20, 21). The application of ABE to neurons revealed isoform-specific S-palmitoylation of the GTPase Cdc42 in the brain as well as many new candidate S-palmitoylated proteins (22). Enrichment of S-acylated peptides from mammalian cells by ABE has also expanded the list of S-palmitoylated proteins and sites of S-acylation (23, 24). Alternatively, we (25–27) and others (28–34) have developed fatty acid chemical reporters of protein fatty-acylation that enable rapid nonradioactive detection of fatty-acylated proteins from mammalian cells using bioorthogonal ligation methods (35) such as the Staudinger ligation or CuI-catalyzed [3 + 2] azide-alkyne cycloaddition (CuAAC) (36), often termed “click chemistry” (see Fig. 1B) (18). Metabolic labeling of mammalian cells with azido/alkynyl-fatty acid (az/alk-FA) chemical reporters followed by reaction of azide/alkyne-modified proteins with biotinylated or fluorescent detection tags allows the visualization of fatty-acylated proteins by streptavidin blot (25, 26, 28–31, 33) or in-gel fluorescence (27, 32, 34, 37), respectively. The direct comparison of various detection modes has demonstrated that metabolic labeling of cells with alkynyl-fatty acids (alk-FAs) in combination with CuAAC and in-gel fluorescence scanning affords the optimal method for the visualization of protein fatty-acylation (27). Indeed, the analysis of various mammalian cell lines with fatty acid chemical reporters and CuAAC/in-gel fluorescence scanning revealed unique and diverse profiles of fatty-acylated proteins (27). Large-scale profiling of S-acylated proteins in Jurkat T cell membrane fractions with an alkynyl-palmitate reporter (17-octadecynoic acid abbreviated 17-ODYA or alk-16) by Martin and Cravatt using CuAAC labeling/enrichment methods, on-bead proteolysis and multidimensional protein identification technology (MudPIT) recovered many new candidate S-palmitoylated proteins and identified a family of serine hydrolases targeted to membranes by palmitoylation (32). Herein, we describe the proteomic analysis of fatty-acylated proteins in mammalian cells in which we employ a series of fatty acid chemical reporters representing myristic to stearic acids (see Fig. 1B and supplemental Fig. S2). This survey is unbiased in that all cellular proteins were solubilized and analyzed; no fractionation such as membrane preparations was used. Our studies of total cell lysates from Jurkat T cells have revealed known and novel candidate fatty-acylated proteins, including nuclear components such as S-acylated histone H3 variants.
EXPERIMENTAL PROCEDURES
High-performance Liquid Chromatography-MS (HPLC-MS) Analysis of Biotinylated Detection Tags
Biotinylated detection tags were diluted to 100 μm in 50 mm triethanolamine (TEA), 150 mm NaCl and treated for 1 h with the reductants specified in supplemental Fig. S1. The reactions were terminated by reverse phase extraction on a C18 column, washed, eluted, and vacuum concentrated. Samples were analyzed on a Waters Acquity HPLC and separated on a C18 column. The gradient of solvent A (water with 0.1% TFA) and solvent B (CH3CN with 0.1% TFA) was increased from 1% B to 40% B over 9 min, held at 60% B for 1 min, and 100% B for 2 min. Mass to charge ratios were measured on a Thermo Scientific TSQ Vantage triple stage quadrupole.
Cell Culture and Metabolic Labeling
Jurkat T cells (human T lymphoma) were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin (P/S). Cells were maintained in a humidified 37 °C incubator with 5% CO2. Trypan blue exclusion was used to determine cell viability. Cells were labeled at 5 × 106 cells/ml for 8 h in 25 μm myristic acid analogs (alk-12 and az-12) or 50 μm palmitic (alk-14) and stearic acid analogs (az-15 and alk-16) in RPMI 1640 containing 2% charcoal dextran-treated FBS (CD-FBS) and P/S. Cells were spun down, washed once with phosphate-buffered saline (PBS), snap frozen in liquid N2 and stored at −80 °C.
Preparation of Total Cell Lysates
Cells were lysed first in a hypotonic buffer (5 mm TEA, pH 7.4, 5 mm MgCl2, 10 mm PMSF, Roche Complete EDTA-free protease inhibitors) to which benzonase at 1000 U/ml had been added. Cells were intermittently vortexed and sonicated for 30 min at 4 °C after which an equal volume of 8% SDS, 300 mm NaCl, 100 mm TEA pH 7.4 was added. Cells were again sonicated and incubated for 10 min at room temperature (RT); at this point the solution was clear. The lysate was centrifuged at 10,000 × g, 0 °C for 30 min and the supernatant removed and stored at −80 °C until future use. Protein concentration of cell lysates was determined by a BCA assay using BSA as a standard and equal amounts of protein from 2–20 mg were precipitated overnight with eight volumes of methanol at −80 °C. The precipitated proteins were resolubilized to 5–15 mg/ml in 4% SDS, 150 mm NaCl and 50 mm TEA pH 7.4 containing 0.5 mm PMSF and 1× Roche Complete protease inhibitors with sonication.
CuAAC Labeling of Cell Lysates and In-Gel Fluorescence Scanning
Cell lysates were labeled in 4% SDS, 150 mm NaCl, and 50 mm TEA with protease inhibitors at 1 mg/ml. Protein was reacted for 2 h at RT with 100 μm azido- or alkynyl-rhodamine (see supplemental Fig. S2) or biotinylated detection tags (supplemental Figs. S1 and S2) from a 10 mm dimethyl sulfoxide (DMSO) stock solution, 1 mm tris(2-carboxyethyl)phosphine hydrochloride (TCEP) from a 50 mm freshly prepared aqueous stock solution, 100 μm tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA) from a 25 mm DMSO stock, and 1 mm CuSO4 from a 50 mm freshly prepared aqueous stock solution. The reactions were terminated by the addition of 8 volumes of −20 °C methanol and placed at −20 °C overnight. Precipitated proteins were pelleted by centrifugation (18,000 × g, 10 min, at 0 °C) and the supernatant discarded. Protein pellets were air dried for 10 min, resuspended in 35 μl of resuspension buffer (4% SDS, 50 mm triethanolamine pH 7.4, 150 mm NaCl), diluted with 12.5 μl 4× reducing SDS-loading buffer (40% glycerol, 200 mm Tris-HCl pH 6.8, 8% SDS, 0.4% bromphenol blue), and 2.5 μl 2-mercaptoethanol, heated for 3 min at 95 °C and ∼20 μg of protein was loaded per gel lane for separation by SDS-PAGE (4–20% Bio-Rad Criterion Tris-HCl gel). Protein gels were then visualized by in-gel fluorescence scanning with an Amersham Biosciences Typhoon 9400 variable mode imager (excitation 532 nm, 580 nm filter, 30 nm band-pass).
Immunoprecipitations and CuAAC Labeling/In-Gel Fluorescence Scanning
Jurkat cells were labeled and lysed as described above with the exception that 8% SDS was replaced with 2% Brij-97 for a final lysate in 1% Brij-97, 50 mm TEA pH 7.4, and 150 mm NaCl. 500 μg of Brij cell lysate was mixed with 10 μl protein G beads and 2 μg anti-HSP90 and incubated for 1 h with agitation at RT. Histone IPs were performed as described before (38). Beads were washed 3× with Brij-97 buffer and resuspended in 14 μl of 4% SDS buffer containing all CuAAC reagents. Beads were reacted for 1 h at RT after which 5 μl of 4× LDS sample buffer and 1 μl BME were added. 20 μl of supernatant were taken and analyzed by SDS-PAGE. Gels were scanned as described above.
Transfection and Metabolic Labeling of Constructs Bearing Exogenous Tags
293T cells were grown to 95% confluency in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% and 100 U/ml penicillin and 100 mg/ml streptomycin. Cells were transfected with various plasmids using lipofectamine-2000 according to the manufacturer's instructions. After 10 h, media was supplemented either with 100 μm palmitic acid or 100 μm alk-14. Eight hours later cells were harvested using a cell scraper, washed with PBS and frozen at −80 °C until use. Protein pellets were handled identically as for Jurkat cells. Plasmids were as follows: PP1γ C-terminally tagged with EGFP pEGFP-C1 (Lei Tan and Tarun Kapoor), HSP90α N-terminally His- and C-terminally 3× FLAG tagged in pcDNA3.1+ (Laura Donlin and Alexander Tarakhovsky), and H3 variants C-terminally Myc-His tagged in pcDNA4 (Aaron Goldberg and David Allis (39)).
Western Blot Analysis
Proteins separated by SDS-PAGE were transferred (50 mm tris base, 40 mm glycine, 0.0375% SDS, 20% MeOH in deionized water, Bio-Rad Trans-Blot Semi-Dry Cell, 20 V, 1 h) onto a polyvinylidene fluoride (PVDF) membrane. PVDF membrane was incubated with PBST containing blocking buffer (5% nonfat dried milk, 1% BSA, and 0.1% Tween-20 in PBS) for 1 h at 25 °C. The membrane was incubated with primary antibodies (manufacture's recommendations), washed three times with PBST and incubated with horseradish peroxidase-conjugated secondary-antibodies (∼1:20,000 in blackening buffer, Jackson Immunoresearch, West Grove, PA). Blots were visualized with ECL Western blotting detection reagents (Amersham Biosciences).
Streptavidin Enrichment of Azide/Alkyne-modified Proteins
Total cell lysates were reacted with azido- or alkynyl-biotinylated detection tags (for synthesis see supplemental Fig. S2) as described above and protein pellets washed with −80 °C methanol three times. The protein pellet was resuspended in 4% SDS buffer to which 10 mm EDTA was added to solubilized protein-chelated copper. The labeled cell lysate was diluted to ∼0.5% SDS with 1% Brij 97 in 150 mm NaCl, 50 mm TEA at pH 7.4 and incubated with streptavidin agarose at 0.2 ml slurry/1 mg of protein for 1 h at RT. The streptavidin beads were washed in batch format six times with 20 column volumes of 2% SDS in PBS, 8 m urea in 250 mm ammonium bicarbonate (ABC), 2.5 m NaCl in PBS, 0.5 m ABC, 0.25 m ABC, and finally 0.05 m ABC. Proteins were eluted from the streptavidin beads with 150 μl of 25 mm sodium dithionite in 0.25% SDS twice and subsequently with 150 μl 25 mm sodium dithionite in 8 m urea twice for 15 min each at RT. The sodium dithionite eluants were combined, exchanged to 50 mm ABC, and concentrated to 14 μl on a 10 kDa MW Centricon. The recovered protein were then diluted with reducing SDS-loading buffer, heated for 5 min at 95 °C, separated by SDS-PAGE on 4–20% Tris-Gly gels and stained with Coomassie blue. Each lane from the gel were cut into 10–24 pieces and digested by previously described protocols (40). Samples were analyzed on an LTQ-Orbitrap and data analyzed as described below. A total of 18 runs were analyzed, composed of six negative controls (DMSO), five myristic acid analog (az-12/alk-12), three palmitic acid analog (alk-14), and four stearic acid analog (az-15/alk-16).
Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis
LC-MS analysis was performed with a Dionex 3000 nano-HPLC coupled to an LTQ-Orbitrap ion trap mass spectrometer (ThermoFisher). Peptides were pressure loaded onto a homemade 75 μm diameter, 15 cm C18 reverse phase column and separated with a gradient running from 95% buffer A (HPLC water with 0.1% formic acid) and 5% buffer B (HPLC grade CH3CN with 0.1% formic acid) to 55% B over 30 min, next ramping to 95% B over 10 min and holding 95% B for 10 min. One full MS scan (300–2000 MW) was followed by three data-dependent scans of the nth most intense ions with dynamic exclusion enabled. The spray voltage was set to 1.94 kV and the flow rate through the column was set to 0.25 μl/min.
Analysis of Protein Hits From Mass Spectrometry Data
Peptides were identified using SEQUEST version 28 (revision 13) and X!Tandem searched against the human International Protein Index (IPI) database version 3.50 (74,016 entries); searches were also run against the same database containing a randomized version of all sequences (148,032 entries). Mass tolerances were set at 10 ppm precursor mass and 1 Da fragment mass. Cysteines were considered constantly modified by carboxyamidomethylation. Allowable variable modifications in SEQUEST included oxidation on methionine and tryptophan, deamidation of asparagine and glutamine, and acetylation on the N terminus and lysine; lysine acetylation was rarely observed except for histones. Allowable variable modifications in X!Tandem were the same with the addition of amino acid cyclization with an N-terminal glutamine or iodoacetamide-capped cysteine (-NH3), or glutamic acid (-OH) resulting in N-terminal pyrrolidone carboxylic acids.
SEQUEST and X!Tandem search results were imported into Scaffold for PeptideProphet filtering. To generate a large space for PeptideProphet to model possible incorrect identifications, peptides were initially searched against a database generated with KRNLH trypsin specificity allowing five missed cleavages. Only peptides judged by PeptideProphet to have a ≥95% likelihood of correct assignment were accepted. PeptideProphet probabilities are a combined function of both Xcorr and Δcn and, in particular with high mass accuracy data, some spurious peptide identifications received a high probability due to a very high Δcn despite a low Xcorr (i.e. < 1). These spurious hits represent, for example, environmental contaminants that match very well in mass to one possible peptide, but which exhibit minimal fragmentation. Thus, in addition to the primary PeptideProphet probability filter, all peptide identifications with an Xcorr ≤1, Δcn ≤ 0.02 or E ≤ 1 were deemed unacceptable. With these filter criteria, SEQUEST Xcorr averaged ∼3.1 and X!Tandem E ∼2.9.
Following this peptide-level filter, proteins were deemed identified if they matched two or more tryptic peptides with a maximum of three missed cleavages. Thereafter, high probability semi- and nontryptic peptides from the initial broad search were accepted as additional sequence coverage. Only proteins identified in more than two runs are listed. This analysis was also performed with a randomized database appended to the real sequences. No randomized proteins were present after these filter criteria. Proteins were allowed to share homologous peptides and redundant proteins were identified similar to the procedure described by Kearney et al. (41). Protein accession numbers matching a subset of peptides were determined and are not counted in the number of identified proteins. For example, if protein P1 is matched by peptides A, B, and C and protein P2 is matched by A and B, P2 is interpreted as a subhit and not counted. Likewise, in the case that two accession numbers match exactly the same peptides, the first (lowest numerical) IPI accession number is reported and the others are listed and not counted. Note that all accession numbers matching a distinct set of peptides are listed. For example, if protein P3 is matched by peptides D, E, and F; protein P4 is matched by peptides D, E, and G, and protein P5 is matched by peptides D, F, and G, then P3, P4, and P5 will all be listed. In these cases, accession numbers are highlighted with an asterisk. Supplemental Table S7 lists the peptides associated with each IPI accession number with shared peptides underlined and supplemental Table S8 lists all IPI numbers that match to a particular peptide.
Independent MS runs were normalized by determining the analog with the most accepted and identified peptides (“counts”) in each run and normalizing those maximum counts to a constant value. This normalization constant was then applied to the counts for all proteins of all other analogs; averaged normalized counts were calculated for each protein across all runs. The first two myristic acid analog experiments were handled slightly differently because these experiments lacked other analogs. In this case, the averages of the normalized myristic acid signals from all experiments except these first two were first calculated. A constant was determined for the first two experiments to bring the total maximum signal of myristic analog counts to this normalized average. To allow presentation across different abundances, these averaged, normalized spectral counts were presented as percentage of maximum, with full red representing 100% of maximal signal strength and full white representing 0%. Fig. 2 and supplemental Fig. S4 also show the total positive abundance and total abundance normalized for protein molecular weight. For the normalized abundance, spectral counts were scaled such that: (1) the total number of counts remains the same; and (2) counts are linearly scaled by the molecular weight of the proteins. Thus, if two proteins, P1 at 50 kDa and P2 at 100 kDa, were both observed to have 10 spectral counts, the adjusted abundance of P1 would be 13.3 and P2 would be 6.7 (total = 20 and P1 abundance twice that of P2).
Fig. 2.
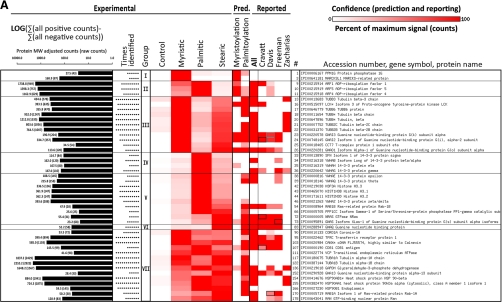
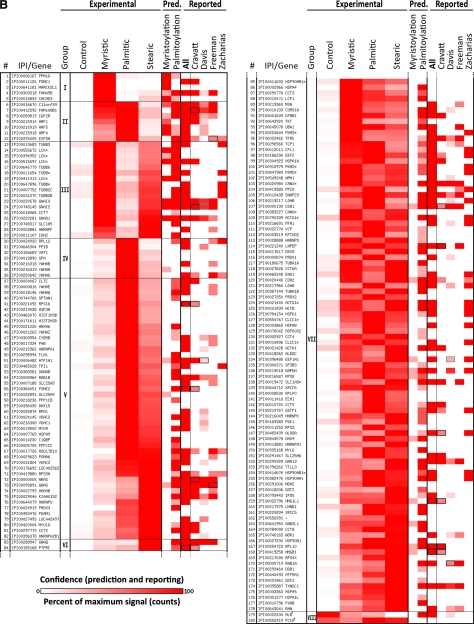
Proteins identified from Jurkat T cells metabolically labeled with click-chemistry compatible fatty acids of different chain lengths (myristic acid = az- and alk-12), (palmitic = alk-14), (stearic = az-15 and alk-16) or DMSO carrier control (-); high confidence only (≥ 5 identifications with fivefold enrichment over DMSO control). A, Selected examples; B, full list (next page). Proteins with the same gene symbol but different IPI numbers are marked with an asterisks (*). Under experimental, intensity of heat map coloration represents normalized peptide counts for each protein hit averaged over 6 independent proteomic experiments. Proteins are clustered by their propensity to be modified from short to long chain fatty acids and assigned to seven groups: I: modified mainly by myristic acid; II: modified mainly by myristic and palmitic acids; III: myristic and stearic acids; IV: palmitic acid; V: palmitic and stearic acids; VI: palmitic acid; VII: myristic, palmitic and stearic acids. Group VIII (next page) are proteins enriched in the negative control (marked with a dagger (†); counts are negatives in excess of positive). Proteins are sorted by the correlation between their averaged normalized signal strengths and the physical length of the fatty acid chains. Intensity of red in prediction and in comparative analysis to other reported experimental datasets represents confidence of prediction or identification, respectively, given criteria set by each work. N-terminal myristoylation was predicted by Myrbase. S-palmitoylated was predicted by CSS-Palm. In comparative analysis, a black box means a protein was identifiable by our data that matched an accession number published in other data sets, but which differs from the reported accession number of this figure because of additional sequence coverage or uncertainty in peptide-level identification. A gray box represents the same situation, except that (1) both an unlisted, interpretable accession number and the exact accession number listed match published reports; and (2) the confidence of the unlisted number is higher than the accession number listed here. In that case, the higher confidence of the unlisted accession number is shown. In A, at left, the total number of peptide counts in excess of any negative (log scale) and the number of times the protein was identified. Nearly four orders of magnitude are spanned (11–8075 raw counts). See also supplemental Fig. S4.
Bioinformatic Analysis of Protein Identifications
For prediction of N-myristoylation and S-palmtoylation, MYRbase and CSS-Palm were used respectively. In the case of N-myristoylation, three levels of confidence in order of decreasing certainty were respectively given the numerical values (red shading) of 100%, 67 and 33%. Confidence levels of 100 and 67% correspond respectively to the “RELIABLE” values and “TWILIGHT ZONE” predictions of MYRbase. The lowest confidence level reflects no prediction of myristoylation by MYRbase, but the presence of an N-terminal glycine or methionine-glycine. Likewise, protein sequences predicted to be palmitoylated by the CSS-Palm set at the “high,” “medium,” and “low” cutoffs were assigned S-palmitoylation prediction confidence values of 100%, 67%, and 33% respectively. For comparison of our data with published data sets, three separate approaches were required for each published set. For comparative analysis of Martin and Cravatt data, both IPI numbers and gene symbols were compared. High confidence proteins were assigned 100% confidence and medium confidence proteins 50% coloration. In all comparisons, caution must be exercised as each IPI number may have more than one gene symbol and likewise one gene symbol may exist for multiple IPI numbers. Because of the difference in species, all comparisons involving the Davis data were performed solely at the level of gene symbol (protein homology). Gene symbols for all accession numbers from both datasets were taken from current database entries; in the cases where gene symbols had not yet been entered, they were determined by BLAST. Gene symbols were compared and the confidence for Davis data calculated as follows: for both the cultured neuron and rat synaptosomal fractions, confidences of 50%, 33% and 16.7% were assigned respectively to high, medium and low confidence protein identifications as classified by Davis and coworkers. The confidences for the cultured neuron and rat synaptosomal fractions were then summed, so that a protein found in both and characterized at a high confidence received a total of 100% confidence, whereas a protein found once in a high confidence and once in a medium confidence would receive 83% red shading. For Zacharias data, all reported peptides were searched against the human IPI database; these IPI numbers were directly compared with the data here. Those peptides observed more than once were assigned 100% confidence, whereas those observed once were assigned 50% confidence. Freeman data was treated like Cravatt with high confidence proteins assigned 100% coloration and medium confidence identifications assigned 50%.
RESULTS
Characterization of Cleavable Affinity Reagents for CuAAC-based Proteomics
To facilitate the identification of azide- and alkyne-modified proteins targeted by bioorthogonal chemical reporters, we utilized clickable and cleavable affinity reagents for CuAAC, streptavidin enrichment, and selective elution of recovered polypeptides for proteomic analysis (Fig. 1B). Although the biotin-avidin interaction provides an excellent system for selective detection and retrieval of biomolecules, the high affinity binding (∼KD 10−15 m) of this interaction makes quantitative elution of biotinylated proteins from streptavidin beads challenging. Indeed, selective elution strategies using protease- (42–44), pH- (45–47), redox- (48–50), or photo-cleavable (51–53) linkers have been described to recover peptides or proteins following streptavidin enrichment. We employed the azobenzene linker as this functional group can be efficiently cleaved by mild reduction with sodium dithionite (Na2S2O4) (48–50, 54) and is stable to click chemistry conditions (38, 55). Exposure of azido-azo-biotin previously generated by our laboratory (Fig. 1B) (38) to various reducing agents demonstrated the azobenzene linkage was stable to reducing agents such as TCEP (tris(2-carboxyethyl)phosphine), but readily cleaved with 25 mm Na2S2O4 (supplemental Fig. S1). These experiments demonstrated that the cleavable affinity tags, alk-azo-biotin and az-azo-biotin, are compatible with click chemistry (1 mm TCEP) and reductive alkylation (10 mm TCEP) reaction conditions used for proteomic studies. We have previously utilized az-azo-biotin for CuAAC-based proteomic analysis of acetylated proteins in mammalian cells (38) and lipoproteins in bacteria (56). For this study, alk-azo-biotin was also synthesized to explore CuAAC, enrichment and identification of proteins targeted by azido-fatty acids (supplemental Fig. S2).
Proteomic Analysis of Fatty-Acylated Proteins in Mammalian Cells
To identify proteins targeted by fatty acid reporters, az-FA- and alk-FA-labeled Jurkat T cell lysates were reacted with the alk-azo-biotin and az-azo-biotin, respectively, incubated with streptavidin beads and extensively washed with denaturing buffers to remove nonspecifically bound proteins (Fig. 1B). Proteins that remained bound to streptavidin beads were selective eluted with 25 mm Na2S2O4, separated by SDS-PAGE and analyzed by Coomassie blue staining or immunoblotted for specific proteins (Fig. 1B). Coomassie blue staining of the polypeptides selectively eluted from streptavidin beads revealed significantly greater amounts of proteins recovered from alk-FA and az-FA-labeled cell lysates compared with control (-) (Fig. 1C and supplemental Fig. S3). A fraction of the cell lysates analyzed in parallel demonstrated equal levels of input material (Fig. 1C and supplemental Fig. S3). The profile of selectively recovered polypeptides roughly mirrors the fatty-acylated proteins visualized by CuAAC/in-gel fluorescence scanning, although the modes of protein detection are different (Fig. 1C). For example, the ∼20-kDa polypeptide that is preferentially labeled in az-12/alk-12 cell lysates by in-gel fluorescence is also apparent by the Coomassie blue-stained gel of selectively recovered proteins from streptavidin enrichment and Na2S2O4 elution (Fig. 1C, marked with an asterisk, and supplemental Fig. S3A, B). Western blot analysis of az-FA-labeled samples for known fatty-acylated proteins in Jurkat T cells such as Lck (25) and Tfr (57, 58) demonstrated the specificity of the CuAAC, streptavidin enrichment and Na2S2O4 elution protocol (supplemental Fig. S3C).
Having confirmed the specificity and low background of CuAAC and affinity enrichment methods, we proceeded with the large-scale identification of proteins targeted by the az/alk-FAs in Jurkat T cells. Az/alk-FA-labeled and control total cell lysates were reacted with clickable and cleavable affinity reagents (alk-azo-biotin, az-azo-biotin, respectively), purified by streptavidin enrichment and sodium dithionite elution protocol described above (Fig. 1B). The Coomassie blue-stained gels of the recovered proteins were then processed using standard protocols for gel-based proteomics and analyzed by nano-reverse-phase liquid chromatography and tandem mass spectrometry for protein identification on a LTQ-Orbitrap. Two initial proteomic runs were performed with az-12-labeled cell lysates (supplemental Fig. S3D), followed by four additional experiments using az/alk-FAs of different chain length (Fig. 1C, supplemental Fig. S3B and E). Tandem MS/MS analysis of tryptic peptides and subsequent database searches using Sequest and X-tandem! with a 95% peptide probability acceptance criteria and a two-unique peptide cutoff for protein identification revealed many known fatty-acylated proteins as well as new candidate fatty-acylated proteins not present in, or significantly enriched compared with, negative controls (supplemental Tables S1 and S2). Preliminary analysis of proteins selectively recovered from az-12 and alk-12-labeled cell lysates yielded a similar composition of protein hits (supplemental Tables S1, S2, and S6), which is consistent with previous experiments that demonstrated az-FA and alk-FA both function as efficient chemical reporters of protein fatty-acylation on known proteins such as Lck, LAT, and Ras (27). Proteomic data based on peptide spectral counts from negative control lanes (-, n = 6), myristic (az-12, n = 3, and alk-12, n = 3), palmitic (alk-14, n = 3) and stearic (az-15, n = 1, alk-16, n = 3) acid analogs were therefore combined and collated to evaluate proteins that were selectively labeled with fatty acid chemical reporters and separated into high (“rank A”) and medium (“rank B”) confidence groups (supplemental Tables S1 and S2). The normalized peptide spectral counts from the protein hits were represented in a heat map for comparative analysis (Fig. 2 and supplemental Fig. S4). In the high confidence group, a total of 178 proteins were identified ≥5 times in az/alk-FA labeled cells with ≥fivefold enrichment of normalized peptide counts over negative; only two proteins were inversely enriched in the negative controls (Fig. 2B and supplemental Fig. S4). Another 183 proteins in the medium confidence list were selectively identified at least twice in az/alk-FA labeled cells and with enrichment at least threefold above negative control (supplemental Table S2). Direct comparison of selectively identified protein hits from parallel proteomic analyses suggested that the high confidence protein hits are reproducibly recovered from independent experiments. Many known candidate fatty-acylated proteins were recovered from our proteomic studies. For example, N-myristoylated proteins such as ADP-ribosylation factors (ARF1, ARF4, and ARF5), Src-family kinase Lck, protein phosphatase 1G (PPM1G), G-protein subunits (Gi, Gk, and Go) and myristoylated, alanine-rich protein kinase C substrate (MARCKS), as well as S-palmitoylated proteins including the transferrin receptor (TfR), calnexin (CANX), N-Ras, and tetraspanins (CD81 and CD82) were identified from az/alk-FA labeled Jurkat T cells in the high confidence group (Fig. 2 and supplemental Fig. S4).
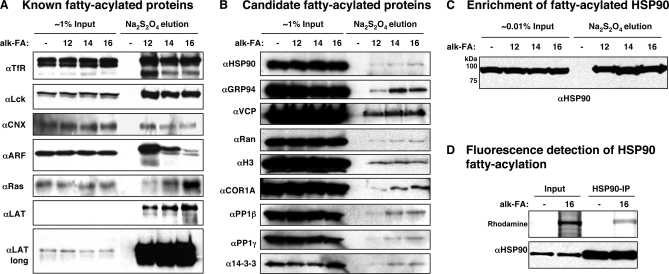
To evaluate the specific recovery of protein hits from az/alk-FA-labeled cell lysates in our proteomic data set, total cell lysates and Na2S2O4 eluants were analyzed by Western blot. Immunoblot analysis for known fatty-acylated proteins such as transferrin receptor (TfR), Lck, calnexin (CANX), linker-associated with T cell activation (LAT), N-Ras, and ADF-ribosylation factors (ARFs) confirmed mass spectrometric identification and specific retrieval from az/alk-FA-labeled cell lysates (Fig. 3A and supplemental Fig. 3C). We also evaluated other proteins from our proteomic dataset that have not been previously confirmed to be fatty-acylated. For example, Western blot analysis for heat shock proteins (HSP90 and GRP94), phosphatase subunits (PP1β and PP1γ) and other proteins (Ran, histone H3s, coronin-1A (COR1A), 14–3-3 proteins, transitional endoplasmic reticulum ATPase (p97/VCP)) confirmed their selective retrieval and identification by mass spectrometry (Fig. 3B). The analysis of fatty-acylated protein levels in total cell lysates compared with the amount of protein recovered from affinity enrichment affords a qualitative measure of how much protein is metabolically labeled with fatty acid reporters. N-myristoylated proteins such as Lck and ARFs appeared to be recovered in significant amounts compared with total cell lysates (Fig. 3A). Interestingly, the S-palmitoylated protein LAT was robustly enriched in Na2S2O4 elutions compared with cell lysates (Fig. 3A). In contrast, relatively low amounts of HSP90, GRP94, PP1β, PP1γ, and Ran are recovered from fatty acid reporter labeling and affinity enrichment compared with cell lysates (Fig. 3B), indicating a large dynamic range of fatty-acylation stoichiometry. The robust signal for known fatty-acylated proteins is likely a factor in their previous discovery. As expected, increasing the amount of alk-FA/CuAAC enriched proteins relative to total cell lysate demonstrated more robust relative signal intensity of alk-FA labeled endogenous HSP90 (Fig. 3C). Fatty-acylation of endogenous HSP90 was also confirmed following alk-16 labeling of Jurkat cells, immunoprecipitation, CuAAC reaction with az-rhodamine and in-gel fluorescence scanning (Fig. 3D).
Fig. 3.
Immunoblot analysis of selectively recovered proteins. A, Known fatty-acylated proteins. B, New candidate proteins. C, Robust enrichment of endogenously fatty-acylated HSP90. D, In-gel fluorescence detection of immunoprecipitated HSP90 metabolically labeled with alkynyl-fatty acid following CuAAC reaction with az-rhodamine.
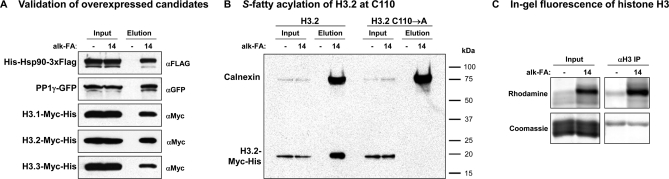
To further validate mass spectrometric and antibody detection of low level fatty-acylation of proteins, as well as clarify highly homologous histone H3 variants, we analyzed the fatty acid reporter labeling and enrichment of overexpressed constructs bearing epitope-tags (Fig. 4A). These experiments employed alk-14, as this analog yielded the broadest labeling among the candidate proteins analyzed. The results for HSP90α, PP1γ, and the histone H3 variants recapitulated the data obtained for endogenously expressed proteins and revealed similar labeling and enrichment of histones H3.1, H3.2, and H3.3. Because all histone H3 variants share a single conserved cysteine at position 110, we analyzed alk-14 labeling and enrichment of epitope-tagged H3.2 and C110A mutant. Endogenous calnexin was examined simultaneously as a positive control (Fig. 4B). Although calnexin signal was similar for both alk-14 labeled samples, H3.2 labeling was completely abrogated in the C110A construct, indicating that Cys110 is the site of H3.2 fatty-acylation. These results highlight the utility of bioorthogonal chemical reporters for large-scale profiling of fatty-acylated and identification of novel fatty-acylated proteins from fully solubilized cell lysates.
Fig. 4.
Further validation of candidate proteins. A, Validation of Hsp90, PP1γ, and histone H3 variants by transfection with tagged constructs. B, Identification of Cys110 in histone H3.2 as the site of S-palmitoylation. Although endogenous calnexin is similarly enriched and detected, all recovery of H3.2 is lost with a C110A mutation. C, Isolation of histone H3 by immunoprecipitation and in-gel fluorescence detects fatty-acylation directly.
Comparative Analysis of Fatty-acylation Proteomic Studies
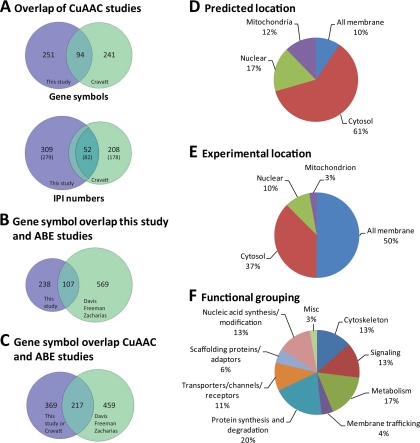
A comparative analysis of our proteomics dataset with other recently described large-scale studies of fatty-acylated proteins (22–24, 32) yields a significant degree of overlap with reported fatty-acylated proteins as well as new candidate fatty-acylated proteins (Fig. 5 and supplemental Fig. S5). The proteomic analysis of alk-16 (17-octadecynoic acid or 17-ODYA) labeled Jurkat T cells by Martin and Cravatt using CuAAC ligation with biotin-azide, streptavidin enrichment and on-bead digestion followed by multidimensional protein identification technology (MudPIT)-based protein identification yielded ∼125 high confidence fatty-acylated protein hits and another ∼200 proteins with medium confidence (32). Of the total list of fatty-acylated proteins identified by Martin and Cravatt, 52 IPI numbers (or 82 considering possible alternate identifications) and 94 gene symbols were shared with our dataset, representing a 17–28% overlap (Fig. 5A). In addition to the variability intrinsic to MS identification studies, two key features likely account for differences among our Jurtkat T cell datasets. Our studies were performed with total cell lysates that included nuclear and cytosolic proteins, compared with enriched membrane fractions from the Martin and Cravatt studies (32). Moreover, the clickable and cleavable reagents (alk-azo-biotin and az-azo-biotin) enabled the use of gel-based proteomics strategies, whereas Martin and Cravatt utilized on-bead protease digestion and MudPIT-based protein identification methods (32). Our analysis also employed multiple fatty acid reporters in particular myristic acid analogs, and recovered N-myristoylated proteins (e.g. PPM1G, MARCKS, ARF4, and ARF5) not present in the Martin and Cravatt dataset (Fig. 2 and supplemental Fig. S4), although alk-16 (17-ODYA) was also reported to target some N-myristoylated proteins (32). While many known and new candidate fatty-acylated proteins from total cell lysates were identified in our high and medium confidence datasets (Figs. 2–4), selective recovery and enrichment of some known S-palmitoylated proteins such as LAT not reaching these confidence thresholds could be clearly observed by Western blot analysis of sodium dithionite elutions (Fig. 3A). Time of incubation with fatty acid analogs, differential recovery of peptides by gel-based versus MudPIT proteomic strategies and subtleties in MS analysis could all play a role in the differences observed between this study and that of Martin and Cravatt.
Fig. 5.
Overlap of fatty-acylated protein studies and analysis of selectively recovered proteins from this study. A, Comparative analysis of high and medium confidence protein hits recovered CuAAC studies by gene symbol and IPI numbers. For IPI numbers, parentheses represent values including possible interpretations of peptide identification not reported in Fig. 2 or supplemental Fig. S4. B, Overlap of this study with acyl-biotin exchange studies. C, Overlap of all CuAAC studies and ABE studies by gene symbol. D, Subcellular distribution of high confidence protein hits based upon WoLF PSORT prediction algorithm. E, Subcellular distribution of high confidence protein hits based upon biochemical fraction studies of Jurkat T cells. F, Biological pathway analysis of high confidence protein hits.
ABE yields complementary datasets in comparison to CuAAC-based systems, as metabolic incorporation of analogs is not required. The ABE analysis of membrane fractions from rat neural sources by the Davis laboratory yielded ∼113 high confidence and ∼318 medium confidence S-acylated proteins (22), 49 of these protein hits were also identified in our proteomic dataset when compared by gene symbol (supplemental Tables S1, S2, and S3). ABE can also be performed with isotopically-encoded thiol-alkylating reagents (iCAT) for comparative analysis of S-acylated peptides, which identified ∼46 S-palmitoylated proteins as well as their potential sites of modification from HeLa cells (23). Our study shares 29 gene symbols with this data and if the peptides of HeLa cells are searched against our dataset, 61 IPI numbers are common (supplemental Tables S1, S2, and S3). ABE enrichment of S-acylated peptides from lipid-raft and nonlipid raft associated proteins from a human prostate cancer cell line has also yielded ∼67 known and ∼331 candidate S-acylated proteins as well as many potential sites of Cys modification (24); of these, 69 were also identified by our study by gene symbol or 68 by IPI number (supplemental Tables S1, S2, and S3). Although the ABE and fatty acid reporter proteomic studies were performed in different cell types and protein samples, the proteomic studies using fatty acid chemical reporters in combination with CuAAC suggests that bioorthogonal ligation methods can have higher signal-to-noise (Fig. 2, supplemental Fig. S4) (32). For example, ∼one fourth of proteins recovered from alk-FA labeling were also found in negative controls, whereas ∼two thirds of proteins recovered from ABE experiments were recovered from samples not treated with hydroxylamine. With respect to the chemical linkage of fatty acid protein modification, fatty chemical reporters enable the analysis of N-myristoylated proteins and other potential sites of amino acid modification (32) (supplemental Tables S1 and S2), whereas ABE selectively targets S-acylated proteins (20, 22–24) and has enabled identification of site modifications (23, 24). Although the total number of proteins identified by ABE studies from three different protein sources was higher, the overlap with this study was not substantially greater (107 gene symbols versus 94 gene symbols; Fig. 5B and 5C). Overall, both methods provide important insight into the diversity and biochemical properties of fatty-acylated proteins.
Within all five studies, ∼70–80% of proteins were observed in only one study (supplemental Fig. S5D and supplemental Table S3), ∼15–20% were observed in two studies, ∼5–7% were observed in three and only 2% were observed in four studies. No proteins were observed in all studies, reflective of the different methods of analysis, sample preparation and protein sources. Of the high confidence lists only, C11orf59, Canx, FAM108B1, Gna13, Gnai2, Gnai3, Gnaq, Gnas, KIAA0152, Snap23, Tfrc, and Txndc1 were shared among four studies; expanding this to include medium confidence hits, Cd81, Dnajc5, GNAO1, Igf2r, Rab1b, Rap2b, RPL12, Rpl5, RPS3A, Scamp3, and SLC25A5 are also observed. The greatest overlap between studies both in number of shared gene symbols and percentage overlap was observed between the Cravatt and Freeman studies (35%, 127 symbols), followed by Cravatt and this study (28%, 94 symbols), and Davis and Freeman (27%, 100 symbols). The high degree of overlap between this study and Cravatt's is likely because of the use of the same cell type (Jurkat T cells) and fatty acid chemical reporters. The Cravatt, Davis and Freeman datasets all analyzed membrane fractions of mammalian cells, which likely favored identification of similar protein compositions.
Functional Analysis of Fatty-acylated Proteins Targeted by Chemical Reporters
The large-scale analysis of proteins labeled with az/alk-FA chemical reporters of different chain lengths provides the opportunity to evaluate the specificity and diversity of protein fatty-acylation in mammalian cells. To determine the specificity of protein labeling with az/alk-FA reporters, the protein hits identified from our proteomics experiments were sorted into groups based on the frequency of their recovery with different chain length of az/alk-FA reporters using normalized peptide spectral counts. A survey of the protein hits revealed many proteins were labeled with multiple fatty acid chemical reporters (group VII) (Fig. 2, supplemental Fig. S4). In contrast, some proteins were labeled in a chain length-dependent fashion (group I and III) (Fig. 2, supplemental Fig. S4). For example, 44 proteins were only labeled by myristic analogs (az-12, alk-12) (group I) and 56 proteins were uniquely labeled by stearic acid analogs (az-15, alk-16) (group III). Many proteins labeled by alk-14 were targeted by shorter and longer fatty acid reporters (Fig. 2, supplemental Fig. S4), consistent with our previous observations that this fatty acid analog labels both N-myristoylated and S-palmitoylated proteins (27). The fatty acid chain length labeling preferences were also evident by Western blot analysis of selectively recovered proteins (Fig. 3A and 3B). N-myristoylated proteins such as ARFs were preferentially labeled by alk-12, whereas S-palmitoylated Ras was most efficiently recovered with alk-16 (Fig. 3A). Alternatively, N-myristoylated and S-palmitoylated proteins such as Lck were recovered with multiple fatty acid reporters (Fig. 3A). Other known S-palmitoylated proteins such as the transferrin receptor did not reveal a significant preference for length of fatty acid reporters (Fig. 3A). Differences in relative signal intensity of isoforms of the same protein between input and elution, such as with the transferrin receptor (Fig. 3A), likely represent preferential fatty-acylation of particular isoforms.
A comparative analysis of our proteomic dataset with the N-myristoylation prediction program, Myrbase (7), demonstrates that proteins containing N-terminal glycines were preferentially labeled by myristic analogs (az-12/alk-14) (Fig. 2, supplemental Fig. S4). In contrast, CSS-Palm prediction program based on previously reported S-palmitoylated proteins (8) identified protein hits throughout our dataset that did not correlate with fatty acid chemical reporters of specific chain length (Fig. 2, supplemental Fig. S4). These observations are consistent with the reported specificity of protein fatty-acylation, which demonstrated that NMTs preferentially utilize myristoyl-CoA through a relatively defined enzyme active site for N-myristoylation of proteins (59), whereas S-acylated proteins typically contain a heterogeneous composition of fatty acids of different chain lengths and unsaturation (5, 6, 60, 61).
We also evaluated the subcellular localization (supplemental Table S4, Fig. 5D and 5E) and biological pathways (supplemental Table 5, Fig. 5F) associated with the proteins labeled with fatty acid chemical reporters. It should be noted that protein samples from our studies represent an unbiased total cell lysates from lysis and solubilization using strong detergent (4% SDS) and nuclease treatment (benzonase), which eliminated the presence of any insoluble pellet. Our lysate thus includes nuclear, cytoplasmic, organelle, and membrane proteins. The analysis of protein localization for our dataset using the WoLF PSORT subcellular location prediction program (62) reveals the majority of fatty acid reporter-labeled proteins are expected to be cytosolic (61%), with the remainder distributed to the nuclei (17%), mitochondria (12%), and membranes (10%) (Fig. 5D). This prediction contrasts with recent experimental data from Jurkat T cells generated by biochemical subcellular fractionation and proteomic analysis (63). Az/alk-FA-labeled proteins partition primarily in membranes (50%), followed by cytosol (37%), nucleus (10%), and mitochondria (3%) (Fig. 5E). The difference between the predicted and experimental subcellular localization of our dataset suggested that many proteins assigned to be cytosolic may be membrane-associated through fatty-acylation. Interestingly, our analysis from total cell lysates also recovered several nuclear proteins, suggesting protein fatty-acylation could contribute to dynamic targeting of these proteins in the nucleus. A survey of biological pathways that are associated with the proteins targeted by our fatty acid reporters suggested that protein fatty-acylation is involved in many cellular processes ranging from signal transduction to protein synthesis and degradation (Fig. 5F).
DISCUSSION
Unraveling the diversity of fatty-acylated proteins and the underlying regulatory mechanisms of protein fatty-acylation in eukaryotes is a challenging task. Here we report the large-scale identification of fatty-acylated proteins in mammalian cells using azide/alkyne-functionalized fatty acids from an unbiased, fully solubilized protein preparation. The enrichment and proteomic analysis of proteins targeted by fatty acid reporters has revealed 57 known fatty-acylated proteins as well as 109 new candidate proteins (by gene symbol) with high confidence that may be regulated by protein fatty-acylation; extending the list to include high and medium confidence, this study identified 191 new candidate proteins and shared 154 identifications (supplemental Fig. S5A, S5B, and S5C). The comparison of azide- and alkynyl-fatty acid chemical reporters of various chain lengths highlights the utility and selectivity of both classes of lipid analogs for detecting fatty-acylated proteins in cells.
The discovery of S-fatty acylation on histone H3 variants represents a new class of histone modification whose implications will require further study. In particular, mechanism of fatty-acylation (supplemental Fig. S6), dynamics, stability, and overall function remain open questions. Cys110 of H3 variants lies buried deep within the nucleosome at the end of the H3 α-2 helix, occupying the center of a four-helix bundle at the H3-H3′ interface between the two H3-H4 dimers of the (H3-H4)2 tetramer. Through hydrophobic interactions and hydrogen bonds, this region alone causes tetramer formation (64–67). Disrupting the hydrophobic surfaces with a C110E mutation results in obligatory H3-H4 dimers (68). Without denaturation, C110 is chemically inaccessible to a wide variety of sulfhydryl reactive reagents conditions (69–72). The involvement of this residue in chromatin structure has been long discussed in the context of an H3-H3 disulfide (73, 74). However, four separate crystallographic studies, including one under oxidative conditions, have shown intersulfur distances in assembled nucleosomes to be incompatible with a disulfide linkage (64–67). Indeed, this residue has been shown to become chemically accessible only during active gene transcription (75–77). Additionally, in HPLC histone purifications, small quantities of hydrophobic (late-eluting) species are observed following the main H3 peak (78), possibly representing the presence of fatty-acylated H3 species.
As with the other fatty-acylated proteins identified in our survey, the mechanism of histone H3 S-fatty acylation could be because of chemical S-acylation (background reactivity, supplemental Fig. S6A), substrate-mediated S-acylation (auto- or self-acylation, supplemental Fig. S6B), enzyme-catalyzed S-acylation (i.e. DHHC-PATs, supplemental Fig. S6C), or some combination of these factors (supplemental Fig. S6). At sufficient concentrations, coenzyme A activated fatty acids are capable of spontaneous chemical S-acylation on cysteine residues of proteins (GAPDH (79); SNAP-25 (80); tubulin (81, 82); rhodopsin (83); G protein α subunits (84); myelin proteolipid protein (MPP) (85); actin, lactate dehydrogenase, fetuin (86); Bet3 (87)), and also short peptides containing the natural site of protein palmitoylation (86, 88). Many of the proteins demonstrated to undergo chemical acylation are also substrates for PATs (SNAP-25, (89); tubulin and G protein subunits, (82); rhodopsin, (83)), and many also undergo catalytic self-palmitoylation (GAPDH (79), SNAP-25 (80), MPP (85), and Bet3 (87)). Likewise, DHHC-PATs load themselves prior to palmitoyl transfer through an auto-acylation event (90). Auto-S-palmitoylation can occur in a regulated fashion such as in response to fatty acid levels (GAPDH (79)) or protein-protein interactions (SNAP-25 palmitoylation is 100-fold greater when bound in the SNARE complex (80); likewise, the presence of Gβγ enhances Gα palmitoylation (84)). This combination of mechanisms has complicated analysis of S-palmitoylation regulation.
S-acylation depends on two factors: the concentration of activated CoA ester and the relative reactivity of the cysteine. Reactivity depends on chemical accessibility and the concentration of thiolate anion capable of a nucleophillic attack, which is enhanced by physical proximity to basic residues (87, 91–93) (supplemental Fig. S6B and S6C). For DHHC-PATs, mutation of the cysteine-proximal basic residues (aspartic acid and histidine) sharply reduces or abrogates transferase activity (90, 94). Local concentration of fatty acid-CoA, and thus acylation, is favored by hydrophobic residues near a site of modification (91, 95); similarly, DHHC-PATs can place a bound fatty acid at a specific residue. Within mammalian cells, the global concentration of activated free fatty acids is kept to low nanomolar concentrations by the presence of acyl-CoA binding protein (ACBP) (96, 97). Because most studies of chemical acylation have not included ACBP and have extended the concentration of activated fatty acids even into the millimolar range, their results must be interpreted with great caution. Upon the inclusion of ACBP, chemical acylation of peptides and proteins has been shown to be eliminated; this buffering does not affect DHHC-PAT activity (86, 96, 98) and the effect on self-palmitoylating proteins has generally not been studied. Cytosolic H3 modification including acetylation or methylation by early acting enzymes prior to chromatin incorporation has been demonstrated (99, 100) and given the ER and perinuclear localization of multiple DHHC-PATs (101), it is possible H3 variants are modified before nuclear incorporation, during nuclear breakdown or possibly at the periphery of the nucleus during specific regulatory events (supplemental Fig. S7). The lack of chain length dependence (Figs. 2 and 3, supplemental Fig. S4) could be reflective of the broad substrate specificity of DHHC-PATs.
Functionally, the S-fatty-acylation of histone H3 variants might, like methylation and acetylation (102), alter nuclear events by establishing a binding platform for effector proteins like histone chaperones (supplemental Fig. S7). Alternatively, S-palmitoylation could affect the properties of chromatin itself, perhaps resulting in new, stabilizing hydrophobic interactions within one or between several nucleosomes, or nucleosomes and other chromatin proteins. Palmitoylation might also play a role in the suborganellular structure of the nucleus including spatial and functional compartmentalization of proteinaceous nuclear bodies, and chromatin domains and territories (103, 104). PTMs including methylation and acetylation show distinct nuclear localization, which can change with tissue type, cellular state and cell cycle (105). Such PTMs are well established to modulate gene activity by changing higher-order nuclear organization. For example, the addition of active chromatin marks like H3K9 acetylation or H3K4me2 results in chromatin decondensation and the formation of chromatin loops (106). Whether S-palmitoylation is involved in the regulatory protein-protein interactions of subnuclear organization remains to be seen. Particularly tempting is the potential involvement of S-palmitoylation in perinuclear tethering of chromatin to the nuclear envelope (supplemental Fig. S7), a localization generally associated with inactive heterochromatin and gene silencing (103, 104). Indeed, H3 variants are enriched in the nuclear shell fraction of HeLa cells (107). Although such localization might involve direct nuclear membrane tethering, it also could reflect a specific protein interaction. H3 has been shown to interact with membrane-bound lamin B-receptor (LBR) (108) and BAF (barrier-to-autointegration factor) (109), which in turn bind multiple inner nuclear membrane proteins (110, 111). The carboxyl-terminal tails of nuclear lamins have also been shown to bind H3 (112). Given the dynamic nature of S-palmitoylation (4), it will be interesting to determine the status of C110 palmitoylation (and potentially H3.1 C96) in conjunction with other PTMs, through the cell cycle and under various states, as well as the relative levels of modification between the H3 variants and their localizations.
Deciphering the function, regulation, and meaning of protein fatty-acylation will be an exciting field for years to come. This survey and others demonstrate that many more cellular proteins are modified with fatty acids than previously appreciated. Using fully solubilized mammalian cells, we discovered S-acylation of histone H3 variants and proteins from all cell compartments. The highly conserved Cys110 was determined by site-directed mutagenesis to be the site of modification for H3.2; the function of this PTM remains to be elucidated and may be involved in perinuclear chromatin silencing. The large-scale identification of fatty-acylation sites on proteins remains a significant challenge and will be essential for dissecting the functional roles of protein fatty-acylation in various cellular pathways. The characterization of specific acyltransferases and lipases responsible for individual fatty-acylated proteins will be key for determining their functions.
Acknowledgments
We thank Prof. David Allis for helpful discussions and reagents as well as Aaron Goldberg, Lei Tan, Prof. Tarun Kapoor, Laura Donlin and Prof. Alexander Tarakhovsky for plasmids. We also thank the Rockefeller Proteomics Resource Center and in particular Dr. Haiteng Deng for mass spectrometry analysis. H. C. H. acknowledges support from The Rockefeller University, Irma T. Hirschl/Monique Weill-Coulier Trust, Ellison Medical Foundation, NIH/NIDA (1R21DA025751-01) and NIH/NIGMS (1R01GM087544-01A2). Y.-Y. Y. thanks Anderson Cancer Foundation for a post-doctoral fellowship. A. S. R. thanks the New York Community Trust-Heiser Grant for a post-doctoral fellowship. G. C. thanks the Rockefeller/Sloan-Kettering/Cornell Tri-Institutional Program in Chemical Biology.
Footnotes
 This article contains supplemental Figs. S1 to S7 and supplemental Tables S1 to S8.
This article contains supplemental Figs. S1 to S7 and supplemental Tables S1 to S8.
1 The abbreviations used are:
- 17-ODYA
- 17-octadecynoic acid
- ABC
- ammonium bicarbonate
- ABE
- acyl-biotin exchange
- ACBP
- Acyl-CoA binding protein
- ARFs
- ADP-ribosylation factors
- BAF
- barrier-to-autointegration factor
- BSA
- bovine serum albumin
- CuAAC
- CuI-catalyzed [3+2] azide-alkyne cycloaddition
- DMSO
- dimethyl sulfoxide
- DHHC-PATs
- Asp-His-His-Cys-containing protein acyltransferases
- EDTA
- ethylenediaminetetraacetic acid
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- H3
- histone H3 and variants
- HPLC
- high performance liquid chromatography
- HSP90
- heat shock protein 90
- IPI
- International Protein Index
- LAT
- linker-associated with T-cell activation
- LC-MS
- liquid chromatography-mass spectrometry
- Lck
- lymphocyte-specific protein tyrosine kinase
- MARCKS
- myristoylated alanine-rich C-kinase substrate
- MudPIT
- multidimensional protein identification technology
- PBS
- phosphate buffered saline
- PBST
- PBS with 0.1% tween-20
- PP1β
- Serine/threonine-protein phosphatase PP1β catalytic subunit
- PP1γ
- Serine/threonine-protein phosphatase PP1γ catalytic subunit
- PTM
- post-translational modification
- Ras
- rat sarcoma small GTPase
- RPMI
- Roswell Park Memorial Institute (medium)
- RT
- room temperature (∼22 °C)
- TEA
- triethanolamine
- TFA
- trifluoroacetic acid.
REFERENCES
- 1. Resh M. D. (2006) Trafficking and signaling by fatty-acylated and prenylated proteins. Nat. Chem. Biol. 2, 584–590 [DOI] [PubMed] [Google Scholar]
- 2. Farazi T. A., Waksman G., Gordon J. I. (2001) The biology and enzymology of protein N-myristoylation. J. Biol. Chem. 276, 39501–39504 [DOI] [PubMed] [Google Scholar]
- 3. Resh M. D. (2006) Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE 2006, re14. [DOI] [PubMed] [Google Scholar]
- 4. Linder M. E., Deschenes R. J. (2007) Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 8, 74–84 [DOI] [PubMed] [Google Scholar]
- 5. DeMar J. C., Jr., Anderson R. E. (1997) Identification and quantitation of the fatty acids composing the CoA ester pool of bovine retina, heart, and liver. J. Biol. Chem. 272, 31362–31368 [DOI] [PubMed] [Google Scholar]
- 6. Liang X., Nazarian A., Erdjument-Bromage H., Bornmann W., Tempst P., Resh M. D. (2001) Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction. J. Biol. Chem. 276, 30987–30994 [DOI] [PubMed] [Google Scholar]
- 7. Maurer-Stroh S., Gouda M., Novatchkova M., Schleiffer A., Schneider G., Sirota F. L., Wildpaner M., Hayashi N., Eisenhaber F. (2004) MYRbase: analysis of genome-wide glycine myristoylation enlarges the functional spectrum of eukaryotic myristoylated proteins. Genome Biol. 5, R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou F., Xue Y., Yao X., Xu Y. (2006) CSS-Palm: palmitoylation site prediction with a clustering and scoring strategy (CSS). Bioinformatics 22, 894–896 [DOI] [PubMed] [Google Scholar]
- 9. Yu X., Wang L., Luo Y., Roeder R. G. (2001) Identification and characterization of a novel OCA-B isoform. implications for a role in B cell signaling pathways. Immunity 14, 157–167 [PubMed] [Google Scholar]
- 10. Siegel R., Kim U., Patke A., Yu X., Ren X., Tarakhovsky A., Roeder R. G. (2006) Nontranscriptional regulation of SYK by the coactivator OCA-B is required at multiple stages of B cell development. Cell 125, 761–774 [DOI] [PubMed] [Google Scholar]
- 11. Zha J., Weiler S., Oh K. J., Wei M. C., Korsmeyer S. J. (2000) Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science 290, 1761–1765 [DOI] [PubMed] [Google Scholar]
- 12. de Jonge H. R., Hogema B., Tilly B. C. (2000) Protein N-myristoylation: critical role in apoptosis and salt tolerance. Sci STKE 2000, PE1. [DOI] [PubMed] [Google Scholar]
- 13. Tsutsumi R., Fukata Y., Fukata M. (2008) Discovery of protein-palmitoylating enzymes. Pflugers Arch. 456, 1199–1206 [DOI] [PubMed] [Google Scholar]
- 14. Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. (1989) All ras proteins are polyisoprenylated but only some are palmitoylated. Cell 57, 1167–1177 [DOI] [PubMed] [Google Scholar]
- 15. Nagar B., Hantschel O., Young M. A., Scheffzek K., Veach D., Bornmann W., Clarkson B., Superti-Furga G., Kuriyan J. (2003) Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell 112, 859–871 [DOI] [PubMed] [Google Scholar]
- 16. Hantschel O., Nagar B., Guettler S., Kretzschmar J., Dorey K., Kuriyan J., Superti-Furga G. (2003) A myristoyl/phosphotyrosine switch regulates c-Abl. Cell 112, 845–857 [DOI] [PubMed] [Google Scholar]
- 17. Tian L., Jeffries O., McClafferty H., Molyvdas A., Rowe I. C., Saleem F., Chen L., Greaves J., Chamberlain L. H., Knaus H. G., Ruth P., Shipston M. J. (2008) Palmitoylation gates phosphorylation-dependent regulation of BK potassium channels. Proc. Natl. Acad. Sci. U.S.A. 105, 21006–21011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charron G., Wilson J., Hang H. C. (2009) Chemical tools for understanding protein lipidation in eukaryotes. Curr. Opin. Chem. Biol. 13, 382–391 [DOI] [PubMed] [Google Scholar]
- 19. Drisdel R. C., Green W. N. (2004) Labeling and quantifying sites of protein palmitoylation. BioTechniques 36, 276–285 [DOI] [PubMed] [Google Scholar]
- 20. Roth A. F., Wan J., Bailey A. O., Sun B., Kuchar J. A., Green W. N., Phinney B. S., Yates J. R., 3rd, Davis N. G. (2006) Global analysis of protein palmitoylation in yeast. Cell 125, 1003–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roth A. F., Wan J., Green W. N., Yates J. R., Davis N. G. (2006) Proteomic identification of palmitoylated proteins. Methods 40, 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang R., Wan J., Arstikaitis P., Takahashi H., Huang K., Bailey A. O., Thompson J. X., Roth A. F., Drisdel R. C., Mastro R., Green W. N., Yates J. R., 3rd, Davis N. G., El-Husseini A. (2008) Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature 456, 904–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang J., Planey S. L., Ceballos C., Stevens S. M., Jr., Keay S. K., Zacharias D. A. (2008) Identification of CKAP4/p63 as a major substrate of the palmitoyl acyltransferase DHHC2, a putative tumor suppressor, using a novel proteomics method. Mol. Cell Proteomics 7, 1378–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang W., Di Vizio D., Kirchner M., Steen H., Freeman M. R. (2010) Proteome-scale characterization of human s-acylated proteins in lipid raft-enriched and non-raft membranes. Mol. Cell Proteomics. 9, 54–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hang H. C., Geutjes E. J., Grotenbreg G., Pollington A. M., Bijlmakers M. J., Ploegh H. L. (2007) Chemical probes for the rapid detection of Fatty-acylated proteins in Mammalian cells. J. Am. Chem. Soc. 129, 2744–2745 [DOI] [PubMed] [Google Scholar]
- 26. Ching W., Hang H. C., Nusse R. (2008) Lipid-independent secretion of a Drosophila Wnt protein. J. Biol. Chem. 283, 17092–17098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charron G., Zhang M. M., Yount J. S., Wilson J., Raghavan A. S., Shamir E., Hang H. C. (2009) Robust fluorescent detection of protein fatty-acylation with chemical reporters. J. Am. Chem. Soc. 131, 4967–4975 [DOI] [PubMed] [Google Scholar]
- 28. Kostiuk M. A., Corvi M. M., Keller B. O., Plummer G., Prescher J. A., Hangauer M. J., Bertozzi C. R., Rajaiah G., Falck J. R., Berthiaume L. G. (2008) Identification of palmitoylated mitochondrial proteins using a bio-orthogonal azido-palmitate analogue. FASEB J. 22, 721–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin D. D., Vilas G. L., Prescher J. A., Rajaiah G., Falck J. R., Bertozzi C. R., Berthiaume L. G. (2008) Rapid detection, discovery, and identification of post-translationally myristoylated proteins during apoptosis using a bio-orthogonal azidomyristate analog. FASEB J. 22, 797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heal W. P., Wickramasinghe S. R., Bowyer P. W., Holder A. A., Smith D. F., Leatherbarrow R. J., Tate E. W. (2008) Site-specific N-terminal labelling of proteins in vitro and in vivo using N-myristoyl transferase and bioorthogonal ligation chemistry. Chem. Commun. (Camb), 4, 480–482 [DOI] [PubMed] [Google Scholar]
- 31. Heal W. P., Wickramasinghe S. R., Leatherbarrow R. J., Tate E. W. (2008) N-Myristoyl transferase-mediated protein labelling in vivo. Org. Biomol. Chem. 6, 2308–2315 [DOI] [PubMed] [Google Scholar]
- 32. Martin B. R., Cravatt B. F. (2009) Large-scale profiling of protein palmitoylation in mammalian cells. Nat. Methods 6, 135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hannoush R. N., Arenas-Ramirez N. (2009) Imaging the lipidome: omega-alkynyl fatty acids for detection and cellular visualization of lipid-modified proteins. ACS Chem. Biol. 4, 581–587 [DOI] [PubMed] [Google Scholar]
- 34. Yap M. C., Kostiuk M. A., Martin D. D., Perinpanayagam M. A., Hak P. C., Siddam A., Majjigapu J. R., Rajaiah G., Keller B. O., Prescher J. A., Wu P., Bertozzi C. R., Falck J. R., Berthiaume L. G. (2010) Rapid and selective detection of fatty acylated proteins using {omega}-alkynyl-fatty acids and click chemistry. J. Lipid Res. 51, 1566–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sletten E. M., Bertozzi C. R. (2009) Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. Engl. 48, 6974–6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meldal M., Tornøe C. W. (2008) Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 108, 2952–3015 [DOI] [PubMed] [Google Scholar]
- 37. Tsou L. K., Zhang M. M., Hang H. C. (2009) Clickable fluorescent dyes for multimodal bioorthogonal imaging. Org. Biomol. Chem. 7, 5055–5058 [DOI] [PubMed] [Google Scholar]
- 38. Yang Y. Y., Ascano J. M., Hang H. C. (2010) Bioorthogonal chemical reporters for monitoring protein acetylation. J. Am. Chem. Soc. 132, 3640–3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goldberg A. D., Banaszynski L. A., Noh K. M., Lewis P. W., Elsaesser S. J., Stadler S., Dewell S., Law M., Guo X., Li X., Wen D., Chapgier A., DeKelver R. C., Miller J. C., Lee Y. L., Boydston E. A., Holmes M. C., Gregory P. D., Greally J. M., Rafii S., Yang C., Scambler P. J., Garrick D., Gibbons R. J., Higgs D. R., Cristea I. M., Urnov F. D., Zheng D., Allis C. D. (2010) Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 140, 678–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hellman U., Wernstedt C., Góñez J., Heldin C. H. (1995) Improvement of an “In-Gel” digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal. Biochem. 224, 451–455 [DOI] [PubMed] [Google Scholar]
- 41. Kearney R. E., Blondeau F., McPherson P. S., Bell A. W., Servant F., Drapeau M., de Grandpre S., Bergeron J. J. M. (2005) Elimination of redundant protein identifications in high throughput proteomics. 2005 27th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vols 1–7, 4803–4806 7776 [DOI] [PubMed] [Google Scholar]
- 42. Speers A. E., Cravatt B. F. (2005) A tandem orthogonal proteolysis strategy for high-content chemical proteomics. J. Am. Chem. Soc. 127, 10018–10019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weerapana E., Speers A. E., Cravatt B. F. (2007) Tandem orthogonal proteolysis-activity-based protein profiling (TOP-ABPP)–a general method for mapping sites of probe modification in proteomes. Nat. Protoc. 2, 1414–1425 [DOI] [PubMed] [Google Scholar]
- 44. Dieterich D. C., Link A. J., Graumann J., Tirrell D. A., Schuman E. M. (2006) Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT). Proc. Natl. Acad. Sci. U.S.A. 103, 9482–9487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fauq A. H., Kache R., Khan M. A., Vega I. E. (2006) Synthesis of acid-cleavable light isotope-coded affinity tags (ICAT-L) for potential use in proteomic expression profiling analysis. Bioconjug. Chem. 17, 248–254 [DOI] [PubMed] [Google Scholar]
- 46. Park K. D., Liu R., Kohn H. (2009) Useful tools for biomolecule isolation, detection, and identification: acylhydrazone-based cleavable linkers. Chem. Biol. 16, 763–772 [DOI] [PubMed] [Google Scholar]
- 47. Dirksen A., Yegneswaran S., Dawson P. E. (2010) Bisaryl hydrazones as exchangeable biocompatible linkers. Angew. Chem. Int. Ed. Engl. 49, 2023–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jaffe C. L., Lis H., Sharon N. (1980) New clevable photoreactive heterobifunctional cross-linking reagents for studying membrane organization. Biochemistry 19, 4423–4429 [DOI] [PubMed] [Google Scholar]
- 49. Fonovic M., Verhelst S. H., Sorum M. T., Bogyo M. (2007) Proteomic evaluation of chemically cleavable activity based probes. Mol. Cell Proteomics. 6, 1761–1770 [DOI] [PubMed] [Google Scholar]
- 50. Verhelst S. H., Fonović M., Bogyo M. (2007) A mild chemically cleavable linker system for functional proteomic applications. Angew. Chem. Int. Ed. Engl. 46, 1284–1286 [DOI] [PubMed] [Google Scholar]
- 51. Zhou H., Ranish J. A., Watts J. D., Aebersold R. (2002) Quantitative proteome analysis by solid-phase isotope tagging and mass spectrometry. Nat. Biotechnol. 20, 512–515 [DOI] [PubMed] [Google Scholar]
- 52. Kim H. Y., Tallman K. A., Liebler D. C., Porter N. A. (2009) An azido-biotin reagent for use in the isolation of protein adducts of lipid-derived electrophiles by streptavidin catch and photo-release. Mol. Cell Proteomics 8, 2080–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Orth R., Sieber S. A. (2009) A photolabile linker for the mild and selective cleavage of enriched biomolecules from solid support. J. Org. Chem. 74, 8476–8479 [DOI] [PubMed] [Google Scholar]
- 54. Denny J. B., Blobel G. (1984) I-125-Labeled Crosslinking Reagent That Is Hydrophilic, Photoactivatable, and Cleavable through an Azo Linkage. Proc. Natl. Acad. Sci. U.S.A. 81, 5286–5290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Landi F., Johansson C. M., Campopiano D. J., Hulme A. N. Synthesis and application of a new cleavable linker for “click”-based affinity chromatography. Org. Biomol. Chem. 8, 56–59 [DOI] [PubMed] [Google Scholar]
- 56. Rangan K. J., Yang Y.-Y., Charron G., Hang H. C. (2010) Rapid visualization and large-scale profiling of bacterial lipoproteins with chemical reporters. J. Am. Chem. Soc. 132, 10628–10629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alvarez E., Girones N., Davis R. J. (1990) Inhibition of the receptor-mediated endocytosis of diferric transferrin is associated with the covalent modification of the transferrin receptor with palmitic acid. J. Biol. Chem. 265, 16644–16655 [PubMed] [Google Scholar]
- 58. Omary M. B., Trowbridge I. S. (1981) Covalent binding of fatty acid to the transferrin receptor in cultured human cells. J. Biol. Chem. 256, 4715–4718 [PubMed] [Google Scholar]
- 59. Gordon J. I., Duronio R. J., Rudnick D. A., Adams S. P., Gokel G. W. (1991) Protein N-myristoylation. J. Biol. Chem. 266, 8647–8650 [PubMed] [Google Scholar]
- 60. Liang X., Lu Y., Neubert T. A., Resh M. D. (2002) Mass spectrometric analysis of GAP-43/neuromodulin reveals the presence of a variety of fatty acylated species. J. Biol. Chem. 277, 33032–33040 [DOI] [PubMed] [Google Scholar]
- 61. Liang X., Lu Y., Wilkes M., Neubert T. A., Resh M. D. (2004) The N-terminal SH4 region of the Src family kinase Fyn is modified by methylation and heterogeneous fatty acylation: role in membrane targeting, cell adhesion, and spreading. J. Biol. Chem. 279, 8133–8139 [DOI] [PubMed] [Google Scholar]
- 62. Horton P., Park K. J., Obayashi T., Fujita N., Harada H., Adams-Collier C. J., Nakai K. (2007) WoLF PSORT: protein localization predictor. Nucleic Acids Res. 35, W585–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu L., Hwang S. I., Rezaul K., Lu L. J., Mayya V., Gerstein M., Eng J. K., Lundgren D. H., Han D. K. (2007) Global survey of human T leukemic cells by integrating proteomics and transcriptomics profiling. Mol. Cell Proteomics 6, 1343–1353 [DOI] [PubMed] [Google Scholar]
- 64. Wood C. M., Sodngam S., Nicholson J. M., Lambert S. J., Reynolds C. D., Baldwin J. P. (2006) The oxidised histone octamer does not form a H3 disulphide bond. Biochim. Biophys. Acta 1764, 1356–1362 [DOI] [PubMed] [Google Scholar]
- 65. Luger K., Mäder A. W., Richmond R. K., Sargent D. F., Richmond T. J. (1997) Crystal structure of the nucleosome core particle at 2.8 angstrom resolution. Nature 389, 251–260 [DOI] [PubMed] [Google Scholar]
- 66. Chantalat L., Nicholson J. M., Lambert S. J., Reid A. J., Donovan M. J., Reynolds C. D., Wood C. M., Baldwin J. P. (2003) Structure of the histone-core octamer in KCl/phosphate crystals at 2.15 angstrom resolution. Acta Crystallographica 59, 1395–1407 [DOI] [PubMed] [Google Scholar]
- 67. Lambert S. J., Nicholson J. M., Chantalat L., Reid A. J., Donovan M. J., Baldwin J. P. (1999) Purification of histone core octamers and 2.15 angstrom X-ray analysis of crystals in KCl phosphate. Acta Crystallographica 55, 1048–1051 [DOI] [PubMed] [Google Scholar]
- 68. Banks D. D., Gloss L. M. (2004) Folding mechanism of the (H3-H4)(2) histone tetramer of the core nucleosome. Protein Sci. 13, 1304–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bode J., Standt U. D. (1978) Structural Aspects of Histone Complexes and Nucleosomes Revealed by the Accessibility of Cysteine Side-Chains. Zeitschrift Fur Naturforschung C-a Journal of Biosciences 33, 884–890 [DOI] [PubMed] [Google Scholar]
- 70. Wong N. T., Candido E. P. (1978) Histone H-3 Thiol Reactivity as a Probe of Nucleosome Structure. Journal of Biological Chemistry 253, 8263–8268 [PubMed] [Google Scholar]
- 71. Lewis P. N., Chiu S. S. (1980) Effect of Histone H-3 Sulfhydryl Modifications on Histone-Histone Interactions and Nucleosome Formation and Structure. Eur. J. Biochem. 109, 369–376 [DOI] [PubMed] [Google Scholar]
- 72. Ferrari N., Pfeffer U., Vidali G. (1987) Nucleosomal structure as probed by H3 histone thiol reactivity. Conformation of H3 histone variants is differently affected by thiol group reagents. Cell Biophys. 10, 1–13 [DOI] [PubMed] [Google Scholar]
- 73. Garrard W. T., Nobis P., Hancock R. (1977) Histone H-3 Disulfide Reactions in Interphase, Mitotic, and Native Chromatin. J. Biol. Chem. 252, 4962–4967 [PubMed] [Google Scholar]
- 74. Hake S. B., Allis C. D. (2006) Histone H3 variants and their potential role in indexing mammalian genomes: The “H3 barcode hypothesis”. Proc. Natl. Acad. Sci. U.S.A.103, 6428–6435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen T. A., Allfrey V. G. (1987) Rapid and Reversible Changes in Nucleosome Structure Accompany the Activation, Repression, and Superinduction of Murine Fibroblast Protooncogenes C-Fos and C-Myc. Proc. Natl. Acad. Sci. U.S.A. 84, 5252–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen T. A., Smith M. M., Le S. Y., Sternglanz R., Allfrey V. G. (1991) Nucleosome Fractionation by Mercury Affinity-Chromatography - Contrasting Distribution of Transcriptionally Active DNA-Sequences and Acetylated Histones in Nucleosome Fractions of Wild-Type Yeast-Cells and Cells Expressing a Histone-H3 Gene Altered to Encode a Cysteine-110 Residue. J. Biol. Chem. 266, 6489–6498 [PubMed] [Google Scholar]
- 77. Bazett-Jones D. P., Mendez E., Czarnota G. J., Ottensmeyer F. P., Allfrey V. G. (1996) Visualization and analysis of unfolded nucleosomes associated with transcribing chromatin. Nucl. Acids Res. 24, 321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shechter D., Dormann H. L., Allis C. D., Hake S. B. (2007) Extraction, purification and analysis of histones. Nat. Protocols 2, 1445–1457 [DOI] [PubMed] [Google Scholar]
- 79. Yang J., Gibson B., Snider J., Jenkins C. M., Han X., Gross R. W. (2005) Submicromolar concentrations of palmitoyl-CoA specifically thioesterify cysteine 244 in glyceraldehyde-3-phosphate dehydrogenase inhibiting enzyme activity: A novel mechanism potentially underlying fatty acid induced insulin resistance. Biochemistry 44, 11903–11912 [DOI] [PubMed] [Google Scholar]
- 80. Veit M. (2000) Palmitoylation of the 25-kDa synaptosomal protein (SNAP-25) in vitro occurs in the absence of an enzyme, but is stimulated by binding to syntaxin. Biochem. J. 345 Pt 1, 145–151 [PMC free article] [PubMed] [Google Scholar]
- 81. Wolff J., Zambito A. M., Britto P. J., Knipling L. (2000) Autopalmitoylation of tubulin. Protein Sci. 9, 1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hiol A., Caron J. M., Smith C. D., Jones T. L. (2003) Characterization and partial purification of protein fatty acyltransferase activity from rat liver. Biochim. Biophys. Acta 1635, 10–19 [DOI] [PubMed] [Google Scholar]
- 83. Veit M., Sachs K., Heckelmann M., Maretzki D., Hofmann K. P., Schmidt M. F. (1998) Palmitoylation of rhodopsin with S-protein acyltransferase: enzyme catalyzed reaction versus autocatalytic acylation. Biochim. Biophys. Acta 1394, 90–98 [DOI] [PubMed] [Google Scholar]
- 84. Duncan J. A., Gilman A. G. (1996) Autoacylation of G protein alpha subunits. J. Biol. Chem. 271, 23594–23600 [DOI] [PubMed] [Google Scholar]
- 85. Bizzozero O. A., McGarry J. F., Lees M. B. (1987) Autoacylation of Myelin Proteolipid Protein with Acyl Coenzyme-A. J. Biol. Chem. 262, 13550–13557 [PubMed] [Google Scholar]
- 86. Bano M. C., Jackson C. S., Magee A. I. (1998) Pseudo-enzymatic S-acylation of a myristoylated yes protein tyrosine kinase peptide in vitro may reflect non-enzymatic S-acylation in vivo. Biochem. J. 330 (Pt 2), 723–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kümmel D., Heinemann U., Veit M. (2006) Unique self-palmitoylation activity of the transport protein particle component Bet3: a mechanism required for protein stability. Proc. Natl. Acad. Sci. U.S.A. 103, 12701–12706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Quesnel S., Silvius J. R. (1994) Cysteine-Containing Peptide Sequences Exhibit Facile Uncatalyzed Transacylation and Acyl-Coa-Dependent Acylation at the Lipid Bilayer Interface. Biochemistry 33, 13340–13348 [DOI] [PubMed] [Google Scholar]
- 89. Washbourne P. (2004) Greasing transmission: palmitoylation at the synapse. Neuron 44, 901–902 [DOI] [PubMed] [Google Scholar]
- 90. Mitchell D. A., Mitchell G., Ling Y., Budde C., Deschenes R. J. Mutational analysis of Saccharomyces cerevisiae Erf2 reveals a two-step reaction mechanism for protein palmitoylation by DHHC enzymes (2010) J. Biol. Chem. 285, 38104–38114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bélanger C., Ansanay H., Qanbar R., Bouvier M. (2001) Primary sequence requirements for S-acylation of beta(2)-adrenergic receptor peptides. FEBS Lett. 499, 59–64 [DOI] [PubMed] [Google Scholar]
- 92. Bizzozero O. A., Bixler H., Parkhani J., Pastuszyn A. (2001) Nitric oxide reduces the palmitoylation of rat myelin proteolipid protein by an indirect mechanism. Neurochem. Res. 26, 1127–1137 [DOI] [PubMed] [Google Scholar]
- 93. Britto P. J., Knipling L., Wolff J. (2002) The local electrostatic environment determines cysteine reactivity of tubulin. J. Biol. Chem. 277, 29018–29027 [DOI] [PubMed] [Google Scholar]
- 94. Mitchell D. A., Vasudevan A., Linder M. E., Deschenes R. J. (2006) Protein palmitoylation by a family of DHHC protein S-acyltransferases. J. Lipid Res. 47, 1118–1127 [DOI] [PubMed] [Google Scholar]
- 95. Bizzozero O. A., Bixler H. A., Pastuszyn A. (2001) Structural determinants influencing the reaction of cysteine-containing peptides with palmitoyl-coenzyme A and other thioesters. Biochim. Biophys. Acta 1545, 278–288 [DOI] [PubMed] [Google Scholar]
- 96. Leventis R., Juel G., Knudsen J. K., Silvius J. R. (1997) Acyl-CoA binding proteins inhibit the nonenzymic S-acylation cysteinyl-containing peptide sequences by long-chain acyl-CoAs. Biochemistry 36, 5546–5553 [DOI] [PubMed] [Google Scholar]
- 97. Faergeman N. J., Knudsen J. (1997) Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem. J. 323, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dunphy J. T., Schroeder H., Leventis R., Greentree W. K., Knudsen J. K., Silvius J. R., Linder M. E. (2000) Differential effects of acyl-CoA binding protein on enzymatic and non-enzymatic thioacylation of protein and peptide substrates. Biochim. Biophys. Acta 1485, 185–198 [DOI] [PubMed] [Google Scholar]
- 99. Loyola A., Almouzni G. (2007) Marking histone H3 variants: How, when and why? Trends Biochem. Sci. 32, 425–433 [DOI] [PubMed] [Google Scholar]
- 100. Loyola A., Bonaldi T., Roche D., Imhof A., Almouzni G. (2006) PTMs on H3 variants before chromatin assembly potentiate their final epigenetic state. Mol. Cell 24, 309–316 [DOI] [PubMed] [Google Scholar]
- 101. Ohno Y., Kihara A., Sano T., Igarashi Y. (2006) Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim. Biophys. Acta 1761, 474–483 [DOI] [PubMed] [Google Scholar]
- 102. Garske A. L., Oliver S. S., Wagner E. K., Musselman C. A., LeRoy G., Garcia B. A., Kutateladze T. G., Denu J. M. (2010) Combinatorial profiling of chromatin binding modules reveals multisite discrimination. Nat. Chem. Biol. 6, 283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Towbin B. D., Meister P., Gasser S. M. (2009) The nuclear envelope - a scaffold for silencing? Current Opinion Genetics Develop. 19, 180–186 [DOI] [PubMed] [Google Scholar]
- 104. Misteli T. (2005) Concepts in nuclear architecture. Bioessays 27, 477–487 [DOI] [PubMed] [Google Scholar]
- 105. Bártová E., Krejcí J., Harnicarová A., Galiová G., Kozubek S. (2008) Histone modifications and nuclear architecture: A review. J. Histochem. Cytochem. 56, 711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chambeyron S., Bickmore W. A. (2004) Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Develop. 18, 1119–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bouvier D., Hubert J., Seve A. P., Bouteille M. (1985) Characterization of Lamina-Bound Chromatin in the Nuclear Shell Isolated from Hela-Cells. Exp. Cell Res. 156, 500–512 [DOI] [PubMed] [Google Scholar]
- 108. Polioudaki H., Kourmouli N., Drosou V., Bakou A., Theodoropoulos P. A., Singh P. B., Giannakouros T., Georgatos S. D. (2001) Histones H3/H4 form a tight complex with the inner nuclear membrane protein LBR and heterochromatin protein 1. Embo. Reports 2, 920–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. De, Oca R. O. M., Lee K. K., Wilson K. L. (2005) Binding of barrier to autointegration factor (BAF) to histone H3 and selected linker histones including H1.1. J. Biol. Chem. 280, 42252–42262 [DOI] [PubMed] [Google Scholar]
- 110. Furukawa K. (1999) LAP2 binding protein 1 (L2BP1/BAF) is a candidate mediator of LAP2-chromatin interaction. J. Cell Sci. 112, 2485–2492 [DOI] [PubMed] [Google Scholar]
- 111. Lee K. K., Haraguchi T., Lee R. S., Koujin T., Hiraoka Y., Wilson K. L. (2001) Distinct functional domains in emerin bind lamin A and DNA-bridging protein BAF. J. Cell Sci. 114, 4567–4573 [DOI] [PubMed] [Google Scholar]
- 112. Taniura H., Glass C., Gerace L. (1995) A Chromatin Binding-Site in the Tail Domain of Nuclear Lamins That Interacts with Core Histones. J. Cell Biol. 131, 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]