Abstract
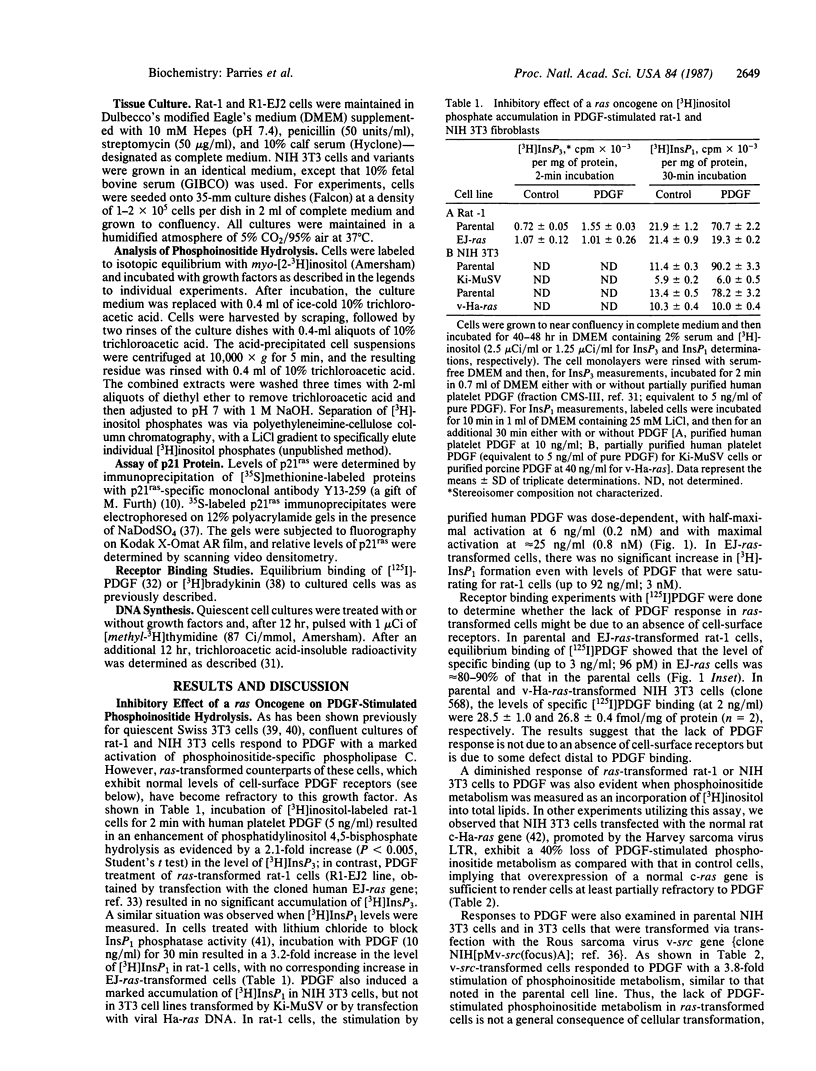
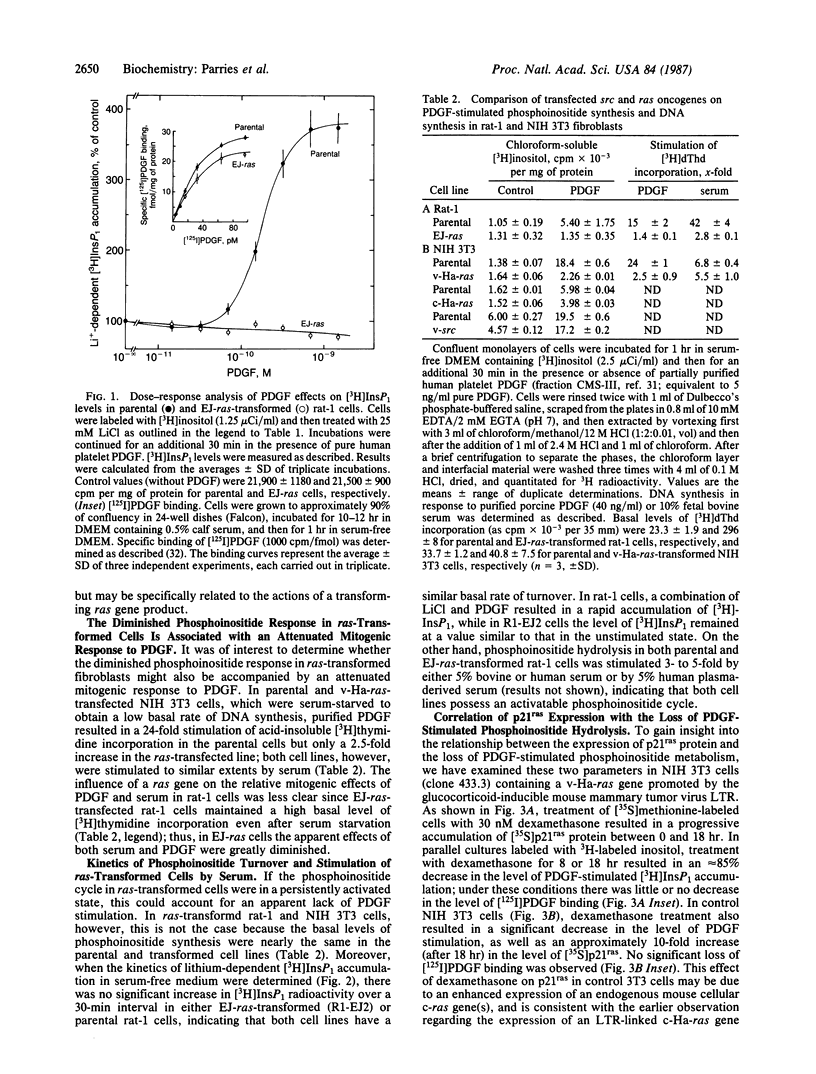
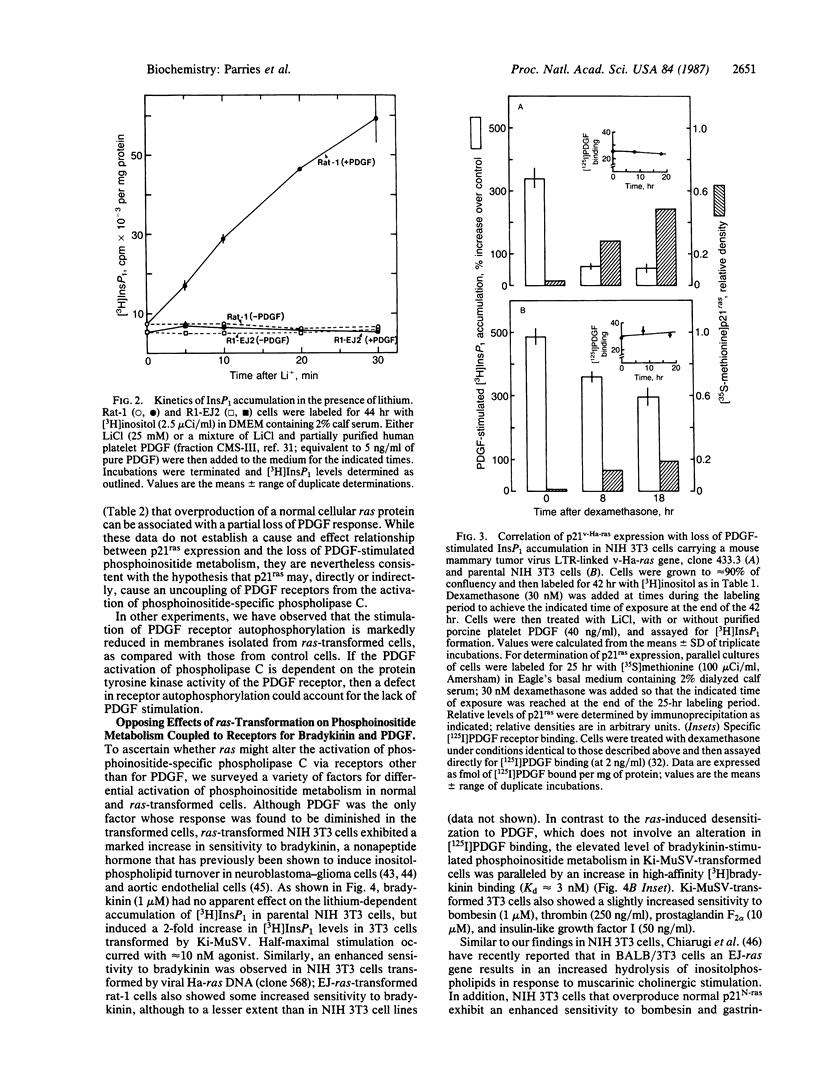
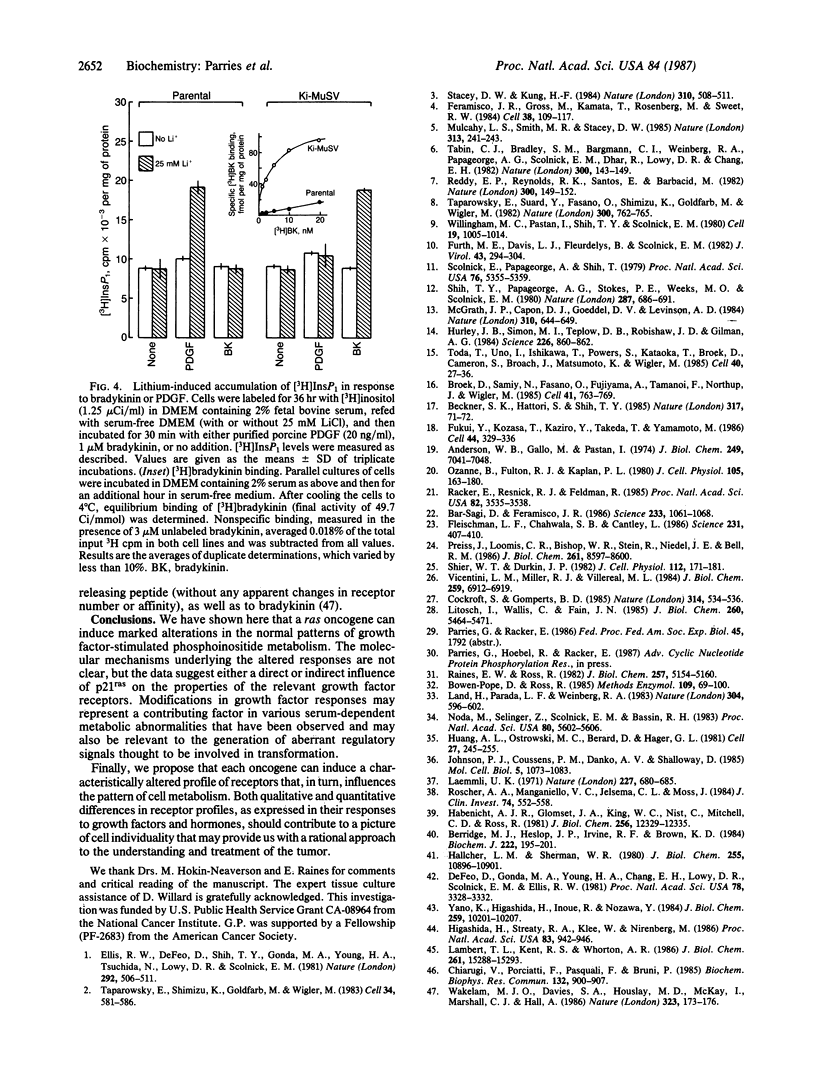
Expression of a transforming Harvey or Kirsten ras gene caused opposing effects in the ability of platelet-derived growth factor (PDGF) and bradykinin to activate phospholipase C-mediated phosphoinositide hydrolysis. In [3H]inositol-labeled rat-1 fibroblasts, PDGF (5 ng/ml) resulted in a 2-fold increase in the level of [3H]inositol trisphosphate (InsP3) after 2 min and, in the presence of LiCl, a 3- to 8-fold increase in the level of [3H]inositol monophosphate (InsP1) after 30 min. However, in EJ-ras-transfected rat-1 cells, which exhibit near normal levels of PDGF receptors, PDGF resulted in little or no accumulation of either [3H]InsP3 or [3H]InsP1. Similarly, marked stimulations by PDGF were observed in NIH 3T3 cells, as well as in v-src-transformed 3T3 cells, but not in 3T3 cells transformed by Kirsten sarcoma virus or by transfection with v-Ha-ras DNA. This diminished phosphoinositide response in ras-transformed cells was associated with a markedly attenuated mitogenic response to PDGF. On the other hand, both phosphoinositide metabolism and DNA synthesis in ras-transformed fibroblasts were stimulated several-fold by serum. In NIH 3T3 cells carrying a glucocorticoid-inducible v-Ha-ras gene, a close correlation was found between the expression of p21ras and the loss of PDGF-stimulated [3H]InsP1 accumulation. In contrast to this ras-induced desensitization to PDGF, ras-transformed NIH 3T3 cells exhibited an enhanced sensitivity to bradykinin; this effect was associated with an elevated level of high-affinity [3H]bradykinin binding. We propose that a ras gene product (p21) can, directly or indirectly, influence growth factor-stimulated phosphoinositide hydrolysis, as well as DNA synthesis, via alterations in the properties of specific growth factor receptors.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Gallo M., Pastan I. Adenylate cyclase activity in fibroblasts transformed by Kirsten or Moloney sarcoma viruses. Decreased activity and loss of response to prostaglandin E1. J Biol Chem. 1974 Nov 25;249(22):7041–7048. [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986 Sep 5;233(4768):1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Beckner S. K., Hattori S., Shih T. Y. The ras oncogene product p21 is not a regulatory component of adenylate cyclase. Nature. 1985 Sep 5;317(6032):71–72. doi: 10.1038/317071a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Methods for studying the platelet-derived growth factor receptor. Methods Enzymol. 1985;109:69–100. doi: 10.1016/0076-6879(85)09078-4. [DOI] [PubMed] [Google Scholar]
- Broek D., Samiy N., Fasano O., Fujiyama A., Tamanoi F., Northup J., Wigler M. Differential activation of yeast adenylate cyclase by wild-type and mutant RAS proteins. Cell. 1985 Jul;41(3):763–769. doi: 10.1016/s0092-8674(85)80057-x. [DOI] [PubMed] [Google Scholar]
- Chiarugi V., Porciatti F., Pasquali F., Bruni P. Transformation of BALB/3T3 cells with EJ/T24/H-ras oncogene inhibits adenylate cyclase response to beta-adrenergic agonist while increases muscarinic receptor dependent hydrolysis of inositol lipids. Biochem Biophys Res Commun. 1985 Nov 15;132(3):900–907. doi: 10.1016/0006-291x(85)91892-3. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- DeFeo D., Gonda M. A., Young H. A., Chang E. H., Lowy D. R., Scolnick E. M., Ellis R. W. Analysis of two divergent rat genomic clones homologous to the transforming gene of Harvey murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3328–3332. doi: 10.1073/pnas.78.6.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Fleischman L. F., Chahwala S. B., Cantley L. ras-transformed cells: altered levels of phosphatidylinositol-4,5-bisphosphate and catabolites. Science. 1986 Jan 24;231(4736):407–410. doi: 10.1126/science.3001936. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Kozasa T., Kaziro Y., Takeda T., Yamamoto M. Role of a ras homolog in the life cycle of Schizosaccharomyces pombe. Cell. 1986 Jan 31;44(2):329–336. doi: 10.1016/0092-8674(86)90767-1. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Higashida H., Streaty R. A., Klee W., Nirenberg M. Bradykinin-activated transmembrane signals are coupled via No or Ni to production of inositol 1,4,5-trisphosphate, a second messenger in NG108-15 neuroblastoma-glioma hybrid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):942–946. doi: 10.1073/pnas.83.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., Coussens P. M., Danko A. V., Shalloway D. Overexpressed pp60c-src can induce focus formation without complete transformation of NIH 3T3 cells. Mol Cell Biol. 1985 May;5(5):1073–1083. doi: 10.1128/mcb.5.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Litosch I., Wallis C., Fain J. N. 5-Hydroxytryptamine stimulates inositol phosphate production in a cell-free system from blowfly salivary glands. Evidence for a role of GTP in coupling receptor activation to phosphoinositide breakdown. J Biol Chem. 1985 May 10;260(9):5464–5471. [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B., Fulton R. J., Kaplan P. L. Kirsten murine sarcoma virus transformed cell lines and a spontaneously transformed rat cell-line produce transforming factors. J Cell Physiol. 1980 Oct;105(1):163–180. doi: 10.1002/jcp.1041050118. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Racker E., Resnick R. J., Feldman R. Glycolysis and methylaminoisobutyrate uptake in rat-1 cells transfected with ras or myc oncogenes. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3535–3538. doi: 10.1073/pnas.82.11.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Roscher A. A., Manganiello V. C., Jelsema C. L., Moss J. Autoregulation of bradykinin receptors and bradykinin-induced prostacyclin formation in human fibroblasts. J Clin Invest. 1984 Aug;74(2):552–558. doi: 10.1172/JCI111452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Papageorge A. G., Shih T. Y. Guanine nucleotide-binding activity as an assay for src protein of rat-derived murine sarcoma viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5355–5359. doi: 10.1073/pnas.76.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shier W. T., Durkin J. P. Role of stimulation of arachidonic acid release in the proliferative response of 3T3 mouse fibroblasts to platelet-derived growth factor. J Cell Physiol. 1982 Aug;112(2):171–181. doi: 10.1002/jcp.1041120204. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Papageorge A. G., Stokes P. E., Weeks M. O., Scolnick E. M. Guanine nucleotide-binding and autophosphorylating activities associated with the p21src protein of Harvey murine sarcoma virus. Nature. 1980 Oct 23;287(5784):686–691. doi: 10.1038/287686a0. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Taparowsky E., Shimizu K., Goldfarb M., Wigler M. Structure and activation of the human N-ras gene. Cell. 1983 Sep;34(2):581–586. doi: 10.1016/0092-8674(83)90390-2. [DOI] [PubMed] [Google Scholar]
- Taparowsky E., Suard Y., Fasano O., Shimizu K., Goldfarb M., Wigler M. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature. 1982 Dec 23;300(5894):762–765. doi: 10.1038/300762a0. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Vicentini L. M., Miller R. J., Villereal M. L. Evidence for a role of phospholipase activity in the serum stimulation of Na+ influx in human fibroblasts. J Biol Chem. 1984 Jun 10;259(11):6912–6919. [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]
- Yano K., Higashida H., Inoue R., Nozawa Y. Bradykinin-induced rapid breakdown of phosphatidylinositol 4,5-bisphosphate in neuroblastoma X glioma hybrid NG108-15 cells. J Biol Chem. 1984 Aug 25;259(16):10201–10207. [PubMed] [Google Scholar]


