Abstract
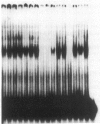
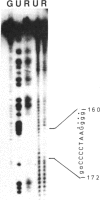
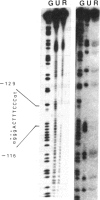
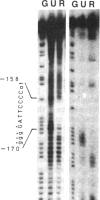
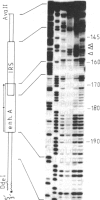
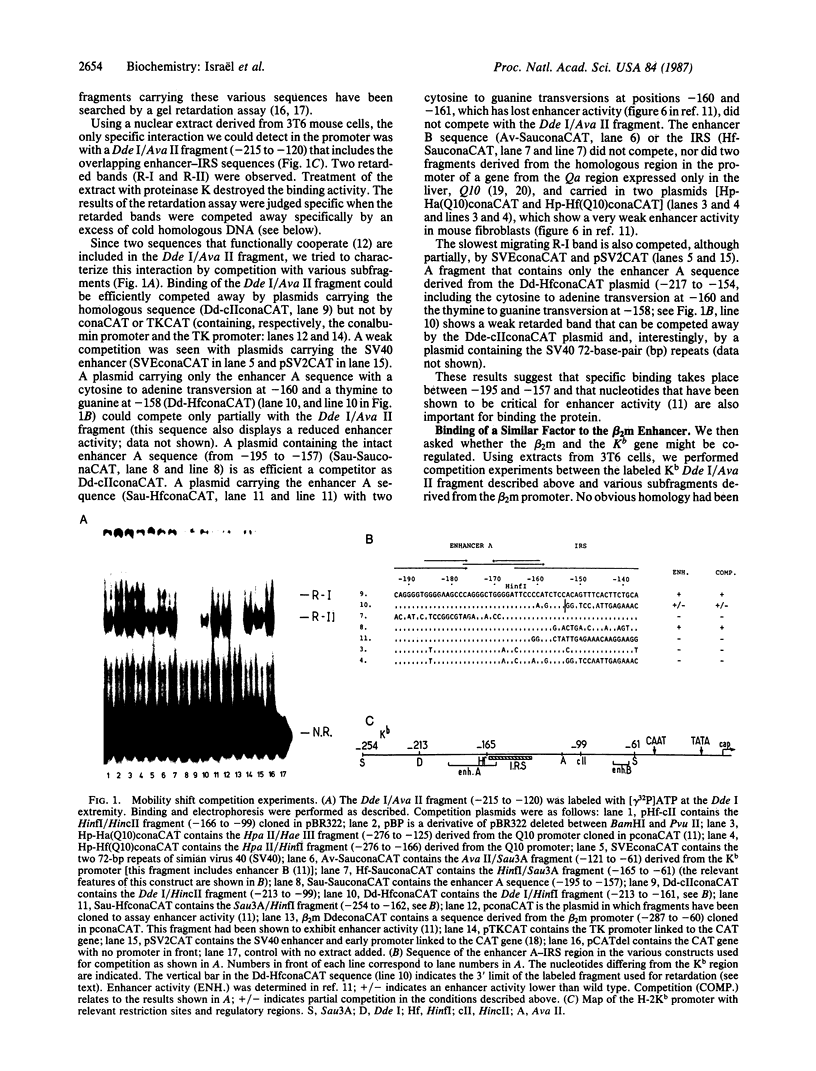
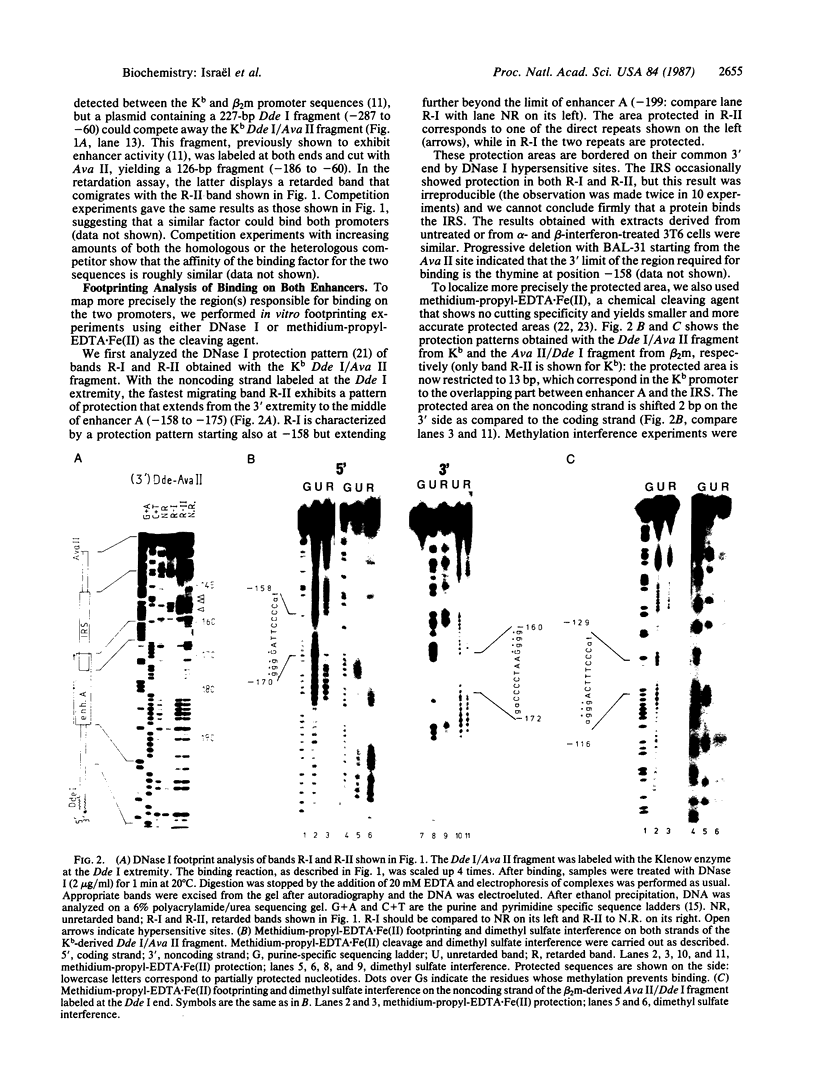
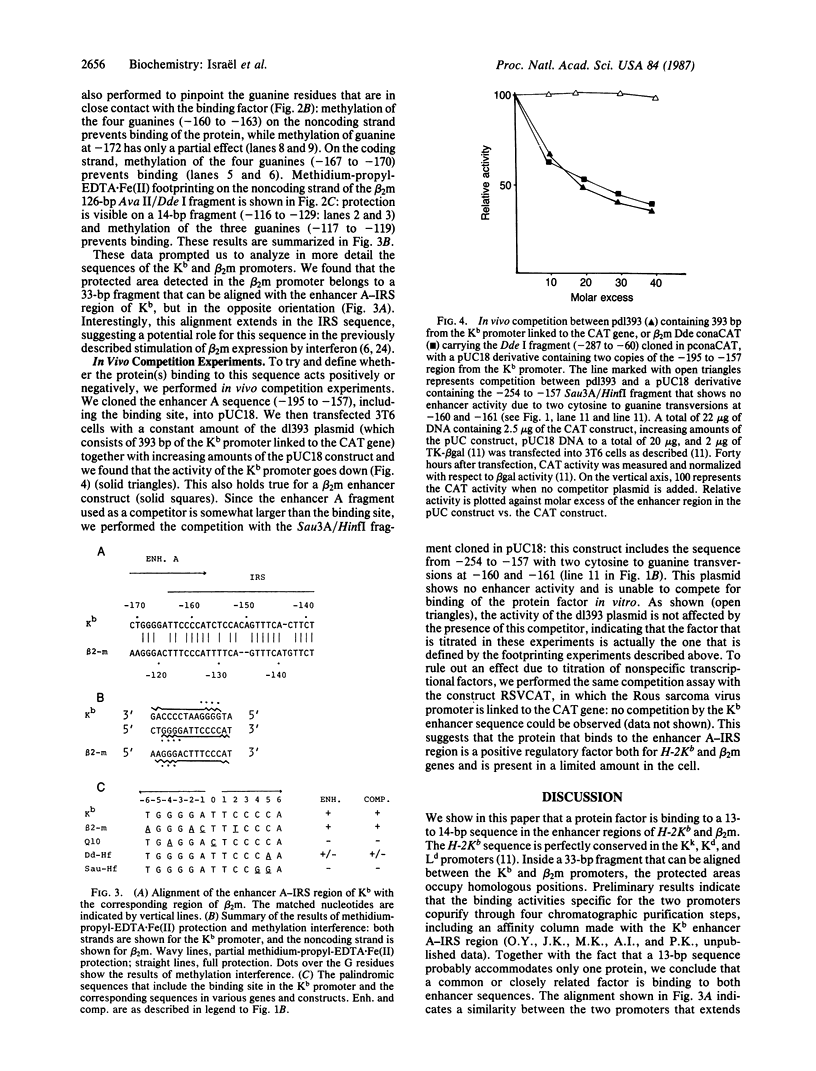
Using gel retardation and in vitro "footprinting", we have analyzed the interactions between nuclear proteins derived from various mouse cells and the enhancer and interferon response sequences of the H-2Kb gene. We have found that a protein factor binds a site in the enhancer sequence that partially overlaps the interferon response sequence. This factor also binds to a similar sequence lying in the opposite orientation in the promoter of the mouse beta 2-microglobulin gene, suggesting a common regulatory mechanism. Transfection competition experiments indicate that this factor acts as a positive element in the expression of H-2 and beta 2-microglobulin genes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcangioli B., Lescure B. Identification of proteins involved in the regulation of yeast iso- 1-cytochrome C expression by oxygen. EMBO J. 1985 Oct;4(10):2627–2633. doi: 10.1002/j.1460-2075.1985.tb03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone O. R., Milstein C. Control of HLA-A,B,C synthesis and expression in interferon-treated cells. EMBO J. 1982;1(3):345–349. doi: 10.1002/j.1460-2075.1982.tb01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverie J. M., Sauvaget I. Assessing the biological significance of primary structure consensus patterns using sequence databanks. I. Heat-shock and glucocorticoid control elements in eukaryotic promoters. Comput Appl Biosci. 1985;1(2):95–104. doi: 10.1093/bioinformatics/1.2.95. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Linnenbach A., Huebner K., Parnes J. R., Margulies D. H., Appella E., Seidman J. G. Control of expression of histocompatibility antigens (H-2) and beta 2-microglobulin in F9 teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5754–5758. doi: 10.1073/pnas.78.9.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin J. J., Lew A. M., Flavell R. A., Coligan J. E. Secretion of a soluble class I molecule encoded by the Q10 gene of the C57BL/10 mouse. EMBO J. 1985 Feb;4(2):369–374. doi: 10.1002/j.1460-2075.1985.tb03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous M., Nir U., Wallach D., Merlin G., Rubinstein M., Revel M. Interferon-dependent induction of mRNA for the major histocompatibility antigens in human fibroblasts and lymphoblastoid cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3082–3086. doi: 10.1073/pnas.79.10.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniger E., Varnum S. M., Ptashne M. Specific DNA binding of GAL4, a positive regulatory protein of yeast. Cell. 1985 Apr;40(4):767–774. doi: 10.1016/0092-8674(85)90336-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron I., Hokland M., Berg K. Enhanced expression of beta2-microglobulin and HLA antigens on human lymphoid cells by interferon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6215–6219. doi: 10.1073/pnas.75.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel A., Kimura A., Fournier A., Fellous M., Kourilsky P. Interferon response sequence potentiates activity of an enhancer in the promoter region of a mouse H-2 gene. Nature. 1986 Aug 21;322(6081):743–746. doi: 10.1038/322743a0. [DOI] [PubMed] [Google Scholar]
- Kimura A., Israël A., Le Bail O., Kourilsky P. Detailed analysis of the mouse H-2Kb promoter: enhancer-like sequences and their role in the regulation of class I gene expression. Cell. 1986 Jan 31;44(2):261–272. doi: 10.1016/0092-8674(86)90760-9. [DOI] [PubMed] [Google Scholar]
- Kress M., Cosman D., Khoury G., Jay G. Secretion of a transplantation-related antigen. Cell. 1983 Aug;34(1):189–196. doi: 10.1016/0092-8674(83)90149-6. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the expression of surface antigens on murine leukemia L 1210 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2785–2788. doi: 10.1073/pnas.70.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morello D., Daniel F., Baldacci P., Cayre Y., Gachelin G., Kourilsky P. Absence of significant H-2 and beta 2-microglobulin mRNA expression by mouse embryonal carcinoma cells. Nature. 1982 Mar 18;296(5854):260–262. doi: 10.1038/296260a0. [DOI] [PubMed] [Google Scholar]
- Morello D., Duprey P., Israel A., Babinet C. Asynchronous regulation of mouse H-2D and beta-2 microglobulin RNA transcripts. Immunogenetics. 1985;22(5):441–452. doi: 10.1007/BF00418090. [DOI] [PubMed] [Google Scholar]
- Morello D., Gachelin G., Dubois P., Tanigaki N., Pressman D., Jacob F. Absence of reaction of a xenogenic anti-H-2 serum with mouse embryonal carcinoma cells. Transplantation. 1978 Aug;26(2):119–125. doi: 10.1097/00007890-197808000-00012. [DOI] [PubMed] [Google Scholar]
- Ozato K., Wan Y. J., Orrison B. M. Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2427–2431. doi: 10.1073/pnas.82.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Piette J., Kryszke M. H., Yaniv M. Specific interaction of cellular factors with the B enhancer of polyoma virus. EMBO J. 1985 Oct;4(10):2675–2685. doi: 10.1002/j.1460-2075.1985.tb03987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa F., Berissi H., Weissenbach J., Maroteaux L., Fellous M., Revel M. The beta2-microglobulin mRNA in human Daudi cells has a mutated initiation codon but is still inducible by interferon. EMBO J. 1983;2(2):239–243. doi: 10.1002/j.1460-2075.1983.tb01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Dervan P. B. Methidiumpropyl-EDTA.Fe(II) and DNase I footprinting report different small molecule binding site sizes on DNA. Nucleic Acids Res. 1983 Aug 25;11(16):5555–5567. doi: 10.1093/nar/11.16.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Fellous M., Revel M. Preferential effect of gamma interferon on the synthesis of HLA antigens and their mRNAs in human cells. Nature. 1982 Oct 28;299(5886):833–836. doi: 10.1038/299833a0. [DOI] [PubMed] [Google Scholar]