Abstract
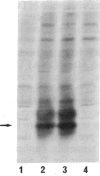
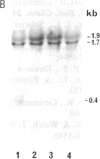
We have selected a metallothionein (MT) cDNA clone from a cadmium-resistant Drosophila melanogaster cell line. This clone includes an open reading frame coding for a 43-amino acid protein whose characteristics are a high cysteine content (12 cysteines, 28% of all residues) and a lack of aromatic amino acids. This protein differs markedly from the Drosophila MT (Mtn gene) previously reported [Lastowski-Perry, D., Otto, E. & Maroni, G. (1985) J. Biol. Chem. 260, 1527-1530). The MT system of Drosophila thus consists of at least two distantly related genes, in sharp contrast with vertebrate MT systems, in which the different members of MT gene families display high similarity. The gene corresponding to our MT cDNA (Mto) is inducible in Drosophila cell lines and in both larval and adult flies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couderc J. L., Sobrier M. L., Giraud G., Becker J. L., Dastugue B. Actin gene expression is modulated by ecdysterone in a Drosophila cell line. J Mol Biol. 1983 Mar 5;164(3):419–430. doi: 10.1016/0022-2836(83)90059-1. [DOI] [PubMed] [Google Scholar]
- Debec A., Mokdad R., Wegnez M. Metallothioneins and resistance to cadmium poisoning in Drosophila cells. Biochem Biophys Res Commun. 1985 Feb 28;127(1):143–152. doi: 10.1016/s0006-291x(85)80137-6. [DOI] [PubMed] [Google Scholar]
- Fogel S., Welch J. W. Tandem gene amplification mediates copper resistance in yeast. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5342–5346. doi: 10.1073/pnas.79.17.5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junakovic N. Variability in the molecular organization of the 5S RNA genes among strains of Drosophila melanogaster. Nucleic Acids Res. 1980 Aug 25;8(16):3611–3622. doi: 10.1093/nar/8.16.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Metallothioneins: proteins in search of function. Cell. 1985 May;41(1):9–10. doi: 10.1016/0092-8674(85)90051-0. [DOI] [PubMed] [Google Scholar]
- Kurtz D. T., Nicodemus C. F. Cloning of alpha 2u globulin cDNA using a high efficiency technique for the cloning of trace messenger RNAs. Gene. 1981 Mar;13(2):145–152. doi: 10.1016/0378-1119(81)90003-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lastowski-Perry D., Otto E., Maroni G. Nucleotide sequence and expression of a Drosophila metallothionein. J Biol Chem. 1985 Feb 10;260(3):1527–1530. [PubMed] [Google Scholar]
- Lerch K., Ammer D., Olafson R. W. Crab metallothionein. Primary structures of metallothioneins 1 and 2. J Biol Chem. 1982 Mar 10;257(5):2420–2426. [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Maroni G., Otto E., Lastowski-Perry D. Molecular and cytogenetic characterization of a metallothionein gene of Drosophila. Genetics. 1986 Mar;112(3):493–504. doi: 10.1093/genetics/112.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Michel F., Jacquier A., Dujon B. Comparison of fungal mitochondrial introns reveals extensive homologies in RNA secondary structure. Biochimie. 1982 Oct;64(10):867–881. doi: 10.1016/s0300-9084(82)80349-0. [DOI] [PubMed] [Google Scholar]
- Münger K., Germann U. A., Lerch K. Isolation and structural organization of the Neurospora crassa copper metallothionein gene. EMBO J. 1985 Oct;4(10):2665–2668. doi: 10.1002/j.1460-2075.1985.tb03985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemer M., Wilkinson D. G., Travaglini E. C., Sternberg E. J., Butt T. R. Sea urchin metallothionein sequence: key to an evolutionary diversity. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4992–4994. doi: 10.1073/pnas.82.15.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi S., Cardenosa G., Pine R., Huang P. C. Cadmium-induced accumulation of metallothionein messenger RNA in rat liver. J Biol Chem. 1981 Mar 10;256(5):2180–2184. [PubMed] [Google Scholar]
- Searle P. F., Davison B. L., Stuart G. W., Wilkie T. M., Norstedt G., Palmiter R. D. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984 Jul;4(7):1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]