Abstract
There is increasing evidence of a biochemical link between lipid oxidation and bone metabolism. Paraoxonase 1 (PON1) prevents the oxidation of low-density lipoprotein (LDL) and metabolizes biologically active phospholipids in oxidized LDLs. Here, we performed association analyses of genetic variation in PON1 to ascertain its contribution to osteoporotic fractures (OFs) and bone mineral density (BMD). We directly sequenced the PON1 gene in 24 Korean individuals and identified 26 sequence variants. A large population of Korean postmenopausal women (n = 1,329) was then genotyped for eight selected PON1 polymorphisms. BMD at the lumbar spine and femoral neck was measured using dual-energy X-ray absorptiometry. Lateral thoracolumbar (T4-L4) radiographs were obtained for vertebral fracture assessment, and the occurrence of non-vertebral fractures (i.e., wrist, hip, forearm, humerus, rib, and pelvis) was examined using self-reported data. Multivariate analyses showed that none of the polymorphisms was associated with BMD at either site. However, +5989A>G and +26080T>C polymorphisms were significantly associated with non-vertebral and vertebral fractures, respectively, after adjustment for covariates. Specifically, the minor allele of +5989A>G exerted a highly protective effect against non-vertebral fractures (OR = 0.59, P = 0.036), whereas the minor allele of +26080T>C was associated with increased susceptibility to vertebral fractures (OR = 1.73, P = 0.020). When the risk for any OFs (i.e., vertebral or non-vertebral) was considered, the statistical significance of both polymorphisms persisted (P = 0.002-0.010). These results suggest that PON1 polymorphisms could be one of useful genetic markers for OF risk in postmenopausal women.
Keywords: aryldialkylphosphatase; bone density; fractures, bone; polymorphism, single nucleotide; postmenopause
Introduction
Osteoporotic fractures (OFs) among elderly people are effectively a worldwide epidemic, and the predicted aging of populations will further increase the burden of these minimal trauma fractures on healthcare systems (Melton, 1993). In addition to high costs, OFs are associated with high morbidity and disability, high risk for long term institutionalization, and increased risk of death (Cooper et al., 1993; Melton, 1993). One central objective of bone biology research is to identify all important factors that underlie OFs, with the ultimate goal of intervening effectively and reducing the risk and incidence of OFs. Most previous studies have concentrated on extrinsic and non-genetic environmental factors, and studies on the genetic determinants of OFs are relatively rare, although recent study has reported that the age-adjusted heritability of OFs is about 27% (Michaelsson et al., 2005). Furthermore, genetic studies of bone have largely been confined to a consideration of bone mineral density (BMD) because this parameter is relatively easy to measure (Kanis, 1997). However, these results may not accurately reflect the heritability of OFs, because BMD is not the only important risk factor for OF; many other identified and/or unidentified intrinsic factors are also important (Kleerekoper et al., 1985; Faulkner et al., 1993; Harris et al., 1998). Therefore, studies of the genetic influence on OFs per se are essential.
There is increasing evidence of a biochemical link between lipid oxidation and bone biology. Increased lipid oxidation causes oxidative stress and reduces Wnt signaling, thereby decreasing the differentiation and survival of osteoblasts (Almeida et al., 2009). In addition, oxidized lipids have direct adverse effects on cellular components by increasing adipogenesis of marrow stromal cells at the expense of their osteogenic differentiation (Parhami et al., 1999) and inducing osteoclastic differentiation via a cAMP-mediated pathway (Tintut et al., 2002). Therefore, genes related to lipid oxidation could be good candidates for genetic studies of bone health.
Paraoxonase 1 (PON1), a product of the PON1 gene which is clustered in tandem on the long arms of human chromosome 7 (q21.22), is a calcium-dependent glycoprotein that is closely associated with high-density lipoprotein (HDL) in serum (Costa et al., 2005). PON1 preserves HDL functions and contributes to the antioxidant effect of HDL by hydrolyzing lipid peroxidase and thereby preventing the oxidation of low-density lipoprotein (LDL) (Mackness et al., 1991). It also metabolizes biologically active phospholipids in oxidized LDLs (Costa et al., 2003). These actions suggest a critical role for PON1 in bone metabolism in addition to its known effects on cardiovascular diseases (CVDs) and atherosclerosis. However, the possible association of PON1 polymorphisms with OFs or BMD has received little research attention. Therefore, in the present study, we performed extensive screening of the PON1 gene by direct sequencing to detect polymorphisms, and investigated their influence on OF risk and BMD in postmenopausal Korean women.
Results
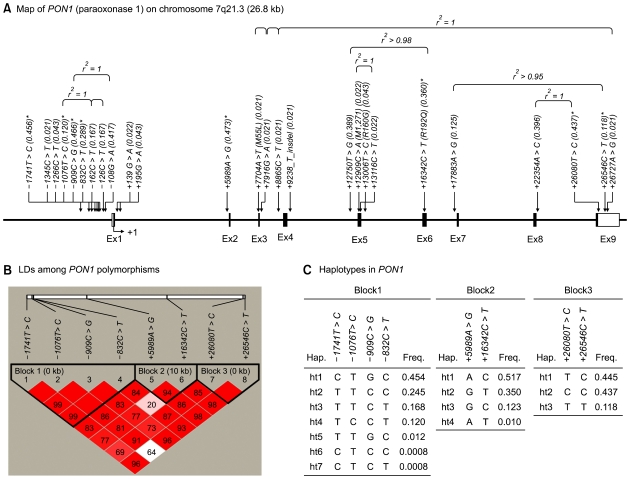
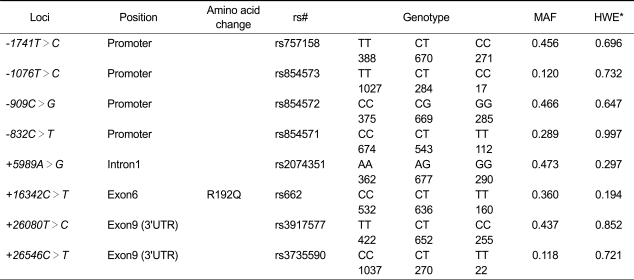
In this study, we investigated a population of Korean postmenopausal women for PON1 gene polymorphisms by directly sequencing all exons and exon-intron boundaries in the PON1 gene, including -2,000 bp of the 5' flanking region. This analysis identified 26 SNPs: nine in the promoter region, four in the coding regions of exons, three in the 3' untranslated regions (UTRs), and ten in the introns. The frequencies of these polymorphisms are shown in Figure 1A. Of the 26 polymorphisms identified, five (+139G>A, +195G>A, +9238T insdel, +12909C>A, and +13116C>T) had not been previously reported. We selected eight polymorphisms (1741T>C, 1076T>C, 909C>G, -832C>T, +5989A>G, +16342C>T, +26080T>C, and +26546C>T) for larger-scale genotyping (n = 1,329) based on minor allele frequency (MAF > 0.1), LDs, and haplotype-tagging status. All genotype distributions were in Hardy-Weinberg equilibrium (P > 0.05; Table 1). After calculating |D'| and r2 among polymorphisms, PON1 could be parsed into three haplotype blocks, with each block having strong LD spine (Figure 1B). There were four, three, and three common haplotypes (frequency > 0.1) in block1 (BL1), block2 (BL2) and block3 (BL3), respectively (Figure 1C). Among the common haplotypes in three blocks, BL1-ht1, BL1-ht4, BL2-ht1, BL2-ht2, BL3-ht2 and BL3-ht3 were not further analyzed because they were almost equivalent to -1741T>C, -1076T>C, +5989A>G, +16342C>T, +26080T>C and +26546C>T, respectively.
Figure 1.
Gene maps, haplotypes, and linkage disequilibrium (LD) coefficients of PON1. (A) Polymorphisms identified in PON1 on chromosome 7q21.3. Coding exons are marked with black rectangles, and 5'- and 3'-UTRs with white rectangles. The first base of the translation start site is denoted nucleotide '+1'. Asterisks indicate SNPs that were genotyped in a larger population (n = 1,329). The frequencies of SNPs that were not subjected to larger-scale genotyping are based on sequence data (n = 24). (B) LD blocks and correlation coefficients among PON1 polymorphisms. Red squares indicate statistically significant allelic association between the pair of SNPs, as measured by the D' statistic; darker shades of red indicate higher values of D', up to a maximum of 1. (C) Haplotype frequencies of PON1 polymorphisms.
Table 1.
Frequencies of PON1 polymorphisms in 1,329 postmenopausal Korean women
*P values of deviation from Hardy-Weinberg Equilibrium (HWE) among subjects without osteoporotic fractures (n = 1,165). MAF, minor allele frequency.
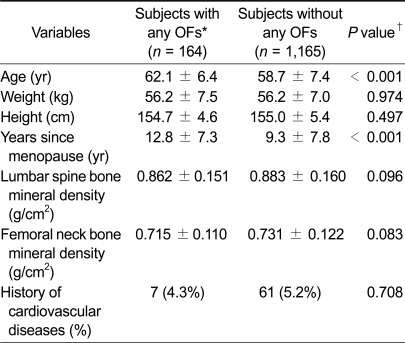
The characteristics of the study subjects are shown in Table 2. OFs of any kind (i.e., vertebral and non-vertebral fractures combined) were noted in 164 of 1,329 postmenopausal women. Of the 164 subjects with OFs, 99 exhibited vertebral fractures and 73 exhibited non-vertebral fractures. Women with OFs were significantly older (62.1 ± 6.4 yr vs. 58.7 ± 7.4 yr) and their YSM interval was longer (12.8 ± 7.3 yr vs. 9.3 ± 7.8 yr) than those without OFs. Lumbar spine and femoral neck BMD were marginally lower in women with OFs (0.862 ± 0.151 g/cm2 and 0.715 ± 0.111 g/cm2, respectively) than in those without (0.883 ± 0.160 g/cm2 and 0.731 ± 0.122 g/cm2, respectively). There were no significant differences in weight, height, or history of CVDs between the two groups. An examination of the independent effect of each variable on OF status by multiple logistic regression analysis revealed that only age was significantly associated with increased risk of OFs of any kind (P = 0.040, OR = 1.05, 95% CI = 1.01-1.11).
Table 2.
Clinical characteristics of subjects by osteoporotic fracture (OF) status
Values are mean ± SD unless otherwise specified.
*Any OFs include either vertebral or non-vertebral (wrist, hip, forearm, humerus, rib, and pelvis) fractures.
†By Student t test for continuous variables and Fisher's exact test for categorical variables.
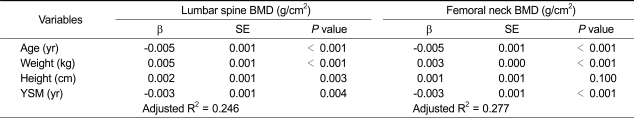
Independent associations of lumbar spine and femoral neck BMD with age, weight, height, and YSM are shown in Table 3. As expected, age and YSM were inversely associated with BMD at both the lumbar spine and femoral neck regions, whereas weight and height were positively associated with BMD at both sites. A Pearson's correlation coefficient was also calculated to examine the correlation between the BMDs at the two bone loci. The BMDs of the lumbar spine and femoral neck correlated strongly and positively with each other (correlation coefficient = 0.632, P < 0.001).
Table 3.
Multiple regression analyses on bone mineral density (BMD) (g/cm2) in 1,329 postmenopausal Korean women
Values are adjusted for all other variables in the Table.
YSM, years since menopause; β, regression coefficient; SE, standard error.
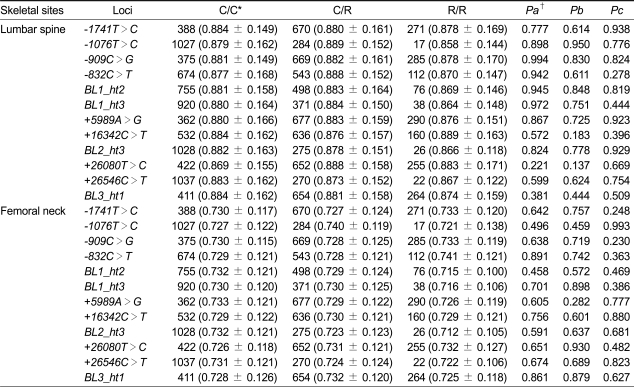
The association of PON1 polymorphisms with BMD at the lumbar spine and femoral neck was analyzed using multiple regression analysis, controlling for age, YSM, weight, and height. However, none of the polymorphisms or haplotypes showed a significant association with BMD at either site (Table 4).
Table 4.
Regression analysis of bone mineral density (BMD) (g/cm2) at lumbar spine and femoral neck with PON1 polymorphisms in postmenopausal Korean women
The number of subjects and means ± standard deviation of the BMDs are shown.
*C/C, C/R, and R/R represent homozygotes for the common allele, heterozygotes, and homozygotes for the rare allele, respectively.
†Pa, Pb, and Pc represent the P values of co-dominant, dominant, and recessive models for multiple regression analysis, respectively, after controlling for age, years since menopause, weight, and height.
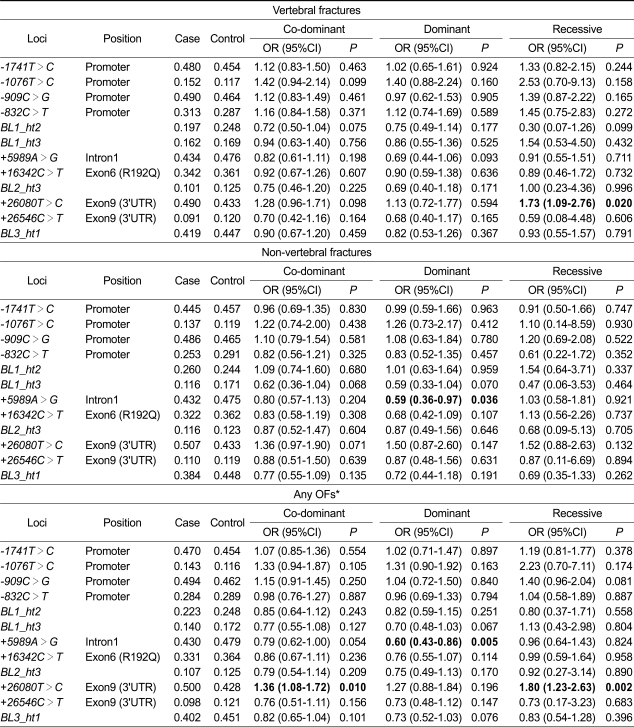
The genetic effects of PON1 polymorphisms on the risk of vertebral and non-vertebral fractures were assessed by multiple logistic regression analysis after controlling for age, YSM, weight, and height (Table 5). The minor allele of +26080T>C was more frequent in subjects with vertebral fracture (frequency = 0.490) than in those without fracture (frequency = 0.433), and was significantly associated with a higher probability for the occurrence of vertebral fracture in the recessive model (P = 0.020, OR = 1.73, 95% CI = 1.09-2.76). An extension of this analysis to a consideration of any OFs (i.e., vertebral or non-vertebral) showed that the association between the minor allele of +26080T>C and increased susceptibility to OFs persisted (co-dominant model: P = 0.010, OR = 1.36, 95% CI = 1.08-1.72; recessive model: P = 0.002, OR = 1.80, 95% CI = 1.23-2.63). On the other hand, the minor allele of +5989A>G was less frequent in subjects with non-vertebral fracture (frequency = 0.432) than in those without fracture (frequency = 0.475), and showed an association with decreased risk of non-vertebral fracture in the dominant model (P = 0.036, OR = 0.59, 95% CI = 0.36-0.97). The minor allele of +5989A>G also showed a protective effect against the occurrence of any OFs (dominant model: P = 0.005, OR = 0.60, 95% CI = 0.43-0.86), as was observed for non-vertebral fractures. Importantly, the associations of +26080T>C and +5989A>G with OFs of any kind were still significant after strictly adopting a Bonferroni correction (P = 0.016 in the recessive model and P = 0.038 in the dominant model, respectively).
Table 5.
Association of PON1 polymorphisms with the risk of osteoporotic fractures (OFs) in postmenopausal Korean women
Genotype distributions and P values for logistic analyses of three alternative models (co-dominant, dominant, and recessive) after controlling for age, weight, height, and years since menopause as covariates are shown.
*Any OFs include either vertebral or non-vertebral (wrist, hip, forearm, humerus, rib, and pelvis) fractures.
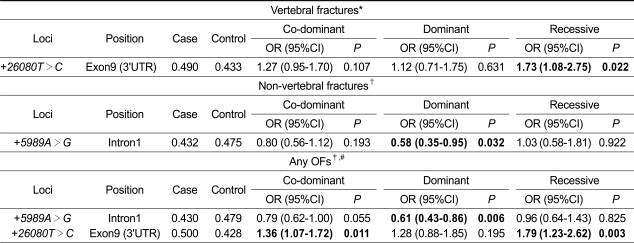
Because there was no association between BMD at the lumbar spine and femoral neck and PON1 polymorphisms, we supposed that the association of the genotypes with fracture might be independent of variations in BMD. To confirm this hypothesis, we further analyzed the association of +26080T>C and +5989A>G with OFs after additional adjustment for BMD values at the lumbar spine and/or femoral neck as covariates. As shown in Table 6, the associations of +26080T>C with vertebral fracture, +5989A>G with non-vertebral fracture, and both polymorphisms with any OFs were persistently significant even after additional adjustments for BMD values (P = 0.003-0.032 in co-dominant, dominant, and recessive models).
Table 6.
Association of PON1 polymorphisms with the risk of osteoporotic fractures (OFs) after additional adjustment for bone mineral density (BMD) (g/cm2) in postmenopausal Korean women
P values for logistic analyses of three alternative models (co-dominant, dominant, and recessive) after adjustment for BMD in addition to age, weight, height, and years since menopause as covariates, are shown.
*After additional adjustment for lumbar spine BMD.
†After additional adjustment for femoral neck BMD.
‡After additional adjustment for lumbar spine and femoral neck BMD.
#Any OFs include either vertebral or non-vertebral (wrist, hip, forearm, humerus, rib, and pelvis) fractures.
Discussion
In an effort to identify and characterize polymorphisms associated with bone metabolism in postmenopausal women, we focused on the role of PON1 polymorphisms in the determinations of OFs and bone mass. Although we could not establish that the genetic polymorphisms influenced BMD at the lumbar spine and femoral neck, direct sequencing and association analyses revealed that PON1 +5989A>G and +26080T>C polymorphisms were significantly associated with non-vertebral fractures and vertebral fractures, respectively. Specifically, the minor allele (G) of +5989A>G had a highly protective effect against non-vertebral fractures, whereas the minor allele (C) of +26080T>C increased susceptibility to vertebral fractures. To our knowledge, this is the first clinical report demonstrating a relationship between PON1 polymorphisms and OFs, and this relationship suggests that PON1 polymorphisms could be one of genetic markers with predictive value for the risk of OFs in postmenopausal women.
Recent study by a large consortium (Richards et al., 2009) showed that some of investigated genes may have effects on fracture risk that are not mediated through BMD alone, and Andrew et al.(Andrew et al., 2005) reported a small genetic correlation (~10%) between OF and BMD. These observations raise two related points. First, although there is evidence that genetic risk factors are important in the etiology of both OF (Michaelsson et al., 2005) and BMD (Arden and Spector, 1997; Eisman, 1999), such genetic factors are likely to be specifically linked to each phenotype. Of course, a low genetic correlation between OF and BMD does not exclude the possibility that these two traits might share candidate genes in common. However, more effort is required to determine the direct relationships between gene polymorphisms and OF risk in addition to their potential pleiotropic effects, and genes found to be associated with BMD need to be validated for their relationship to fractures. Second, genetic influences are known to contribute to fall-related factors, such as postural balance (Pajala et al., 2004), muscle function (Tiainen et al., 2004, 2005), cognitive abilities (Wright et al., 2001), and physical activity (Frederiksen and Christensen, 2003); thus, genetics may also contribute indirectly to OFs through increased fall risk. Accordingly, knowledge of the role of genetics in fall susceptibility in older people is useful in designing effective fall-prevention programs.
Although PON1 can affect bone metabolism through its involvement in lipid oxidation, the exact mechanism by which it influences OF risk independently of BMD in postmenopausal women is not entirely clear at present. However, bone fragility depends on factors besides BMD, including the morphology, architecture, remodeling and quality of bone (Garnero et al., 2005). Therefore, PON1 may be involved in bone metabolic pathways other than those reflected in BMD. Further studies are needed to clarify the associations between these BMD-independent OF risk factors and PON1 polymorphisms.
Despite the presumptive influence of PON1 on bone metabolism, we have been able to identify only one study that focused on the relationship between PON1 polymorphisms and osteoporosis-related phenotypes. In 2003, Yamada et al. (2003) reported that two polymorphisms in the coding region-L55M and Q192R-were associated with BMD at the lumbar spine and/or femoral neck after controlling for age in postmenopausal Japanese women. In our study, the L55M polymorphism was not prevalent (MAF = 0.021), mainly due to ethnic differences, and the Q192R polymorphism had no association with BMD at any sites in postmenopausal Korean populations. However, the Japanese study did not adjust for weight, which is an important determinant of both BMD (Kanis, 1990) and PON1 activity (Bajnok et al., 2008). Moreover, this study did not consider other identified polymorphisms besides L55M and Q192R and did not address the influence of L55M or Q192R on OF; thus, it could not completely evaluate the associations of genetic variations in PON1 with bone phenotypes.
The PON1 +5989A>G and +26080T>C polymorphisms are located in the intron and exon 9/3'UTR, respectively, and do not cause amino acid changes, suggesting that these polymorphisms may be markers that lie close to and/or in linkage disequilibrium with other functional genes. However, it has been shown previously that 3'UTRs regulate mRNA stability and thereby influence gene expression (Frittitta et al., 2001; Di Paola et al., 2002). Therefore, the +26080T>C polymorphism, which is located in the 3'UTR of the PON1 gene, may have a genetic role by regulating PON1 mRNA stability.
OFs and CVDs are known to be related in postmenopausal women (Sennerby et al., 2007). Because PON1 can affect lipid metabolism, an important risk factor for CVDs, we additionally adjusted for history of CVDs to exclude the effects of this possible confounding factor. The significance after adjustment persisted in both +26080T>C (P = 0.024 in the recessive model) and +5989A>G (P = 0.035 in the dominant model) polymorphisms, suggesting that PON1 polymorphisms and OF risk are related independently of CVDs.
In almost all of studied related to OFs, vertebral and non-vertebral fractures were analyzed separately, and there might be several reasons for that. Firstly, spine is mainly composed of trabecular architecture, whereas non-spinal skeletal part, such as femur neck, is usually composed of cortical architecture. Secondly, while at least 90% of non-vertebral fractures results from a fall, falls precede only ~25% of vertebral fractures, with many resulting from apparently insignificant everyday activities (Cooper et al., 1992; Youm et al., 1999). Thirdly, subjects with non-vertebral fractures promptly go to hospital due to the pain and disability, when the event occurs. However, those with vertebral fractures sometimes do not know the fact for a long time until undergoing radiographs for any reasons, such as routine health examination. Because of these reasons, our group also analyzed vertebral and non-vertebral fractures separately and together.
There are several potential limitations to this study. Most importantly, the power to draw any definite conclusions was limited by the small sample size due to difficulty in gathering information and relatively low incidence of OFs. Secondly, although we attempted to consider as many confounding factors as possible, there might be a possibility that the observed association could be attributable to uncontrolled factors. Thirdly, the study population, comprised of women who had visited a university hospital, may not be representative of the general community, thus possibly resulting in selection bias. Fourthly, because non-vertebral fractures were obtained by self-report and not validated by radiographs, the results could be affected by recall bias. However, self-report has previously been demonstrated to be accurate. Specificity is above 80% for self-report of fracture (Nevitt et al., 1992; Ivers et al., 2002; Hundrup et al., 2004), with underreporting rare (Ivers et al., 2002). Fifthly, because we did not measure PON1 activity, we do not know whether PON1 activity varied according to the absence or presence of polymorphisms and haplotypes. Therefore, we cannot assert that the genotypes are functionally relevant. Lastly, the genetic component of fracture may ultimately be a combination of polygenic effects and gene-gene and genetic-environmental interactions. Additional multiple analyses with other possible candidate genes would be needed to resolve these more complex relationships.
In summary, we found that PON1 +5989A>G and +26080T>C polymorphisms were significantly associated with non-vertebral fractures and vertebral fractures, respectively, without influencing BMD in postmenopausal Korean women. Our observations suggest that PON1 polymorphisms could be a genetic marker with predictive value for OF risk in postmenopausal women.
Methods
Subjects
The study population comprised apparently postmenopausal Korean women (n = 1,329) who visited the Asan Medical Center (AMC) in Seoul. Menopause was defined as the absence of menstruation for at least one yr, and was confirmed by measurement of serum follicle-stimulating hormone levels. Women displaying premature menopause (< 40 yr of age), and those taking drugs that might affect bone metabolism for more than 6 months or within the previous 12 months, such as glucocorticoid, sex hormone, bisphosphonate or other treatments for osteoporosis, were excluded. Additionally, subjects were excluded if they had suffered from any disease that might affect bone metabolism, such as diabetes, cancer, hyperparathyroidism, or rheumatoid arthritis. Women were also excluded if they had osteophyte formation above the fourth grade of the Nathan classification (Nathan, 1962) and/or severe facet joint osteoarthritis in the lumbar spine as determined by conventional spine radiographs. The study was approved by the AMC ethics review committee, and written informed consent was obtained from all subjects.
BMD measurement and osteoporotic fracture assessment
Areal BMD (g/cm2) at the lumbar spine (L2-L4) and femoral neck were measured by dual-energy X-ray absorptiometry in 834 women using Lunar equipment (Lunar, Expert XL, Madison, WI). In the remaining 495 women, BMD was estimated using Hologic equipment (Hologic, QDR 4500-A, Waltham, MA). The precision levels of the Lunar and Hologic equipment, presented as coefficients of variation, were 0.82% and 0.85% for the lumbar spine, and 1.12% and 1.20% for the femoral neck, respectively. These values were obtained by scanning 17 volunteers who were not part of the study. Each volunteer underwent five scans on the same day, getting on and off the table between examinations. Cross-calibration equations between the two systems were derived by measuring BMD values in 109 healthy Korean women (mean age, 55 ± 11 yr, range 31-75 yr) using the two machines, and were calculated as follows (Jo et al., 1999):
L2-L4 BMD (g/cm2): Lunar = 1.1287 × Hologic - 0.0027 Femoral neck BMD (g/cm2): Lunar = 1.1556 × Hologic - 0.0182
We examined prevalent morphological vertebral fractures in all study subjects by means of obtaining lateral thoracolumbar (T4-L4) radiographs. The assessment of vertebral fractures was made in accordance with the recommendations of the Working Group on Vertebral Fractures (Kiel, 1995). Radiographs were assessed at AMC by expert radiologists blinded to this study. A vertebral fracture was defined quantitatively as more than a 20% reduction in any measured vertebral height (i.e., anterior, middle, or posterior) (Genant et al., 1993). Non-vertebral fractures (i.e., wrist, hip, forearm, humerus, rib, and pelvis) were assessed using self-reported data. Fractures clearly caused by major trauma, such as motor vehicle accidents or a fall from more than a standing height, were excluded.
Sequencing analysis of the PON1 gene
We sequenced all exons, including exon-intron boundaries, and the promoter region (~1.5 Kb) to detect single nucleotide polymorphisms (SNPs) in twenty-four Korean DNA samples using the ABI PRISM 3730 DNA analyzer (Applied Biosystems, Foster City, CA). Twenty-four DNA samples from Korean subjects for initial sequencing were randomly selected from unrelated local residents with no history of familial disease (13 females and 11 males; mean ages, 55.0 ± 8.3 yr; range 41-69 yr). Fifteen primer sets were designed for amplification and sequencing analyses based on GenBank sequences (reference sequence of contig: NT_007933.15). Sequence variants were verified with automated sequencing chromatograms. SNPs were detected by multiple sequence alignment using the Phred/Phrap/Consed package and Polyphred (Ewing et al., 1998; Gordon et al., 1998).
Genotyping with fluorescence polarization detection
For genotyping of polymorphic sites, amplifying primers and probes were designed for TaqMan (Livak, 1999). Primer Express (Applied Biosystems) was used to design both the polymerase chain reaction (PCR) primers and the MGB TaqMan probes. One allelic probe was labeled with the FAM dye and the other with the fluorescent VIC dye. PCRs were run in the TaqMan Universal Master mix without UNG (Applied Biosystems), with PCR primer concentrations of 900 nM and TaqMan MGB-probe concentrations of 200 nM. Reactions were performed in a 384-well format in a total reaction volume of 5 µl using 20 ng of genomic DNA. The plates then were placed in a thermal cycler (PE 9700, Applied Biosystems) and heated at 50℃ for 2 min and 95℃ for 10 min followed by 40 cycles of 95℃ for 15 s and 60℃ for 1 min. The TaqMan assay plates were transferred to a Prism 7900HT instrument (Applied Biosystems) where the fluorescence intensity in each well of the plate was read. Fluorescence data files from each plate were analyzed using automated software (SDS 2.1, Applied Biosystems).
Statistics
The χ2 test was used to determine whether individual variants were in Hardy-Weinberg equilibrium at each locus in the subjects without OF (Chae et al., 2009). We examined Lewontin's D' (|D'|) and the linkage disequilibrium (LD) coefficient r2 between all pairs of biallelic loci (Hedrick, 1987). Haploview version 3.2 (Whitehead Institute for Biomedical Research, Cambridge, MA) was used to examine the structure of the LD block (Barrett et al., 2005). This program uses two-marker expectation maximization to estimate the maximum-likelihood values of the four gamete frequencies from which the D' and log of odds (LOD) values are derived. Haplotypes (ht) of each individual were inferred using the algorithm developed by Stephens et al. (PHASE), which uses a Bayesian approach incorporating a priori expectations of haplotypic structure based on population genetics and coalescent theory (Stephens et al., 2001). Phase probabilities of all polymorphic sites for hts were also calculated for each individual using this software. Individuals with phase probabilities of less than 97% were excluded from the analysis. The genetic effects of inferred hts were analyzed using the same method described for the analysis of polymorphisms. Associations between OF status and each clinical variable were determined using Student t test for continuous variables with a normal distribution and Fisher's exact test for categorical variables. Multiple regression analyses of BMD at the lumbar spine and femoral neck with PON1 polymorphisms were performed using age, years since menopause (YSM), weight, and height as covariates. The genotype and ht distributions between participants with and without OFs were analyzed with a logistic regression model controlling for age, YSM, weight, and height. History of CVD was additionally adjusted in this model because of the possible effect as a confounding factor. Genotypes of major homozygotes, heterozygotes, and minor homozygotes were given codes of 0, 1, and 2; 0, 1, and 1; and 0, 0, and 1 in the co-dominant, dominant, and recessive models, respectively. All statistical analyses were conducted using SAS (SAS Institute, Cary, NC).
Acknowledgements
This work was supported by a grant from the Korea Health 21 Research & Development Project, Ministry of Health & Welfare, Republic of Korea (Project No.: A010252).
Abbreviations
- BMD
bone mineral density
- ht
haplotype
- LD
linkage disequilibrium
- MAF
minor allele frequency
- OF
osteoporotic fracture
- PON1
paraoxonase 1
- YSM
years since menopause
References
- 1.Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009;284:27438–27448. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrew T, Antioniades L, Scurrah KJ, Macgregor AJ, Spector TD. Risk of wrist fracture in women is heritable and is influenced by genes that are largely independent of those influencing BMD. J Bone Miner Res. 2005;20:67–74. doi: 10.1359/JBMR.041015. [DOI] [PubMed] [Google Scholar]
- 3.Arden NK, Spector TD. Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Miner Res. 1997;12:2076–2081. doi: 10.1359/jbmr.1997.12.12.2076. [DOI] [PubMed] [Google Scholar]
- 4.Bajnok L, Seres I, Varga Z, Jeges S, Peti A, Karanyi Z, Juhasz A, Csongradi E, Mezosi E, Nagy EV, Paragh G. Relationship of serum resistin level to traits of metabolic syndrome and serum paraoxonase 1 activity in a population with a broad range of body mass index. Exp Clin Endocrinol Diabetes. 2008;116:592–599. doi: 10.1055/s-2008-1065350. [DOI] [PubMed] [Google Scholar]
- 5.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 6.Chae SC, Shim SC, Chung HT. Association of TBX21 polymorphisms in a Korean population with rheumatoid arthritis. Exp Mol Med. 2009;41:33–41. doi: 10.3858/emm.2009.41.1.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper C, Atkinson EJ, O'Fallon WM, Melton LJ., 3rd Incidence of clinically diagnosed vertebral fractures: apopulation-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res. 1992;7:221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 8.Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM, Melton LJ., 3rd Population-based study of survival after osteoporotic fractures. Am J Epidemiol. 1993;137:1001–1005. doi: 10.1093/oxfordjournals.aje.a116756. [DOI] [PubMed] [Google Scholar]
- 9.Costa LG, Cole TB, Jarvik GP, Furlong CE. Func tional genomic of the paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med. 2003;54:371–392. doi: 10.1146/annurev.med.54.101601.152421. [DOI] [PubMed] [Google Scholar]
- 10.Costa LG, Vitalone A, Cole TB, Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem Pharmacol. 2005;69:541–550. doi: 10.1016/j.bcp.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Di Paola R, Frittitta L, Miscio G, Bozzali M, Baratta R, Centra M, Spampinato D, Santagati MG, Ercolino T, Cisternino C, Soccio T, Mastroianno S, Tassi V, Almgren P, Pizzuti A, Vigneri R, Trischitta V. A variation in 3' UTR of hPTP1B increases specific gene expression and associates with insulin resistance. Am J Hum Genet. 2002;70:806–812. doi: 10.1086/339270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisman JA. Genetics of osteoporosis. Endocr Rev. 1999;20:788–804. doi: 10.1210/edrv.20.6.0384. [DOI] [PubMed] [Google Scholar]
- 13.Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998;8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 14.Faulkner KG, Cummings SR, Black D, Palermo L, Gluer CC, Genant HK. Simple measurement of femoral geometry predicts hip fracture: the study of osteoporotic fractures. J Bone Miner Res. 1993;8:1211–1217. doi: 10.1002/jbmr.5650081008. [DOI] [PubMed] [Google Scholar]
- 15.Frederiksen H, Christensen K. The influence of genetic factors on physical functioning and exercise in second half of life. Scand J Med Sci Sports. 2003;13:9–18. doi: 10.1034/j.1600-0838.2003.20219.x. [DOI] [PubMed] [Google Scholar]
- 16.Frittitta L, Ercolino T, Bozzali M, Argiolas A, Graci S, Santagati MG, Spampinato D, Di Paola R, Cisternino C, Tassi V, Vigneri R, Pizzuti A, Trischitta V. A cluster of three single nucleotide polymorphisms in the 3'-untranslated region of human glycoprotein PC-1 gene stabilizes PC-1 mRNA and is associated with increased PC-1 protein content and insulin resistance-related abnormalities. Diabetes. 2001;50:1952–1955. doi: 10.2337/diabetes.50.8.1952. [DOI] [PubMed] [Google Scholar]
- 17.Garnero P, Munoz F, Borel O, Sornay-Rendu E, Delmas PD. Vitamin D receptor gene polymorphisms are associated with the risk of fractures in postmenopausal women, independently of bone mineral density. J Clin Endocrinol Metab. 2005;90:4829–4835. doi: 10.1210/jc.2005-0364. [DOI] [PubMed] [Google Scholar]
- 18.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 19.Gordon D, Abajian C, Green P. Consed: a graphical tool for sequence finishing. Genome Res. 1998;8:195–202. doi: 10.1101/gr.8.3.195. [DOI] [PubMed] [Google Scholar]
- 20.Harris M, Nguyen TV, Howard GM, Kelly PJ, Eisman JA. Genetic and environmental correlations between bone formation and bone mineral density: a twin study. Bone. 1998;22:141–145. doi: 10.1016/s8756-3282(97)00252-4. [DOI] [PubMed] [Google Scholar]
- 21.Hedrick PW. Gametic disequilibrium measures: proceed with caution. Genetics. 1987;117:331–341. doi: 10.1093/genetics/117.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hundrup YA, Hoidrup S, Obel EB, Rasmussen NK. The validity of self-reported fractures among Danish female nurses: comparison with fractures registered in the Danish National Hospital Register. Scand J Public Health. 2004;32:136–143. doi: 10.1080/14034940310017490. [DOI] [PubMed] [Google Scholar]
- 23.Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. The accuracy of self-reported fractures in older people. J Clin Epidemiol. 2002;55:452–457. doi: 10.1016/s0895-4356(01)00518-2. [DOI] [PubMed] [Google Scholar]
- 24.Jo JM, Kim JS, Kim GS, Kim SW, Shin JW, Moon DH, Lee HK. Cross-calibration of bone mineral density between two different dual X-ray absorptiometry systems: hologic QDR 4500-A and lunar EXPERT-XL. Korean J Nucl Med. 1999;33:282–288. [Google Scholar]
- 25.Kanis JA. Osteoporosis and osteopenia. J Bone Miner Res. 1990;5:209–211. doi: 10.1002/jbmr.5650050302. [DOI] [PubMed] [Google Scholar]
- 26.Kanis JA. Diagnosis of osteoporosis. Osteoporos Int. 1997;7(Suppl 3):S108–S116. doi: 10.1007/BF03194355. [DOI] [PubMed] [Google Scholar]
- 27.Kiel D. Assessing vertebral fractures. National osteoporosis foundation working group on vertebral fractures. J Bone Miner Res. 1995;10:518–523. doi: 10.1002/jbmr.5650100403. [DOI] [PubMed] [Google Scholar]
- 28.Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int. 1985;37:594–597. doi: 10.1007/BF02554913. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ. Allelic discrimination using fluorogenic probes and the 5' nuclease assay. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 30.Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286:152–154. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- 31.Melton LJ., 3rd Hip fractures: a worldwide problem today and tomorrow. Bone. 1993;14(Suppl 1):S1–S8. doi: 10.1016/8756-3282(93)90341-7. [DOI] [PubMed] [Google Scholar]
- 32.Michaelsson K, Melhus H, Ferm H, Ahlbom A, Pedersen NL. Genetic liability to fractures in the elderly. Arch Intern Med. 2005;165:1825–1830. doi: 10.1001/archinte.165.16.1825. [DOI] [PubMed] [Google Scholar]
- 33.Nathan H. Osteophytes of the vertebral column: an anatomical study of their development according to age, race, and sex with consideration as to their etiology and significance. J Bone Joint Surg Am. 1962;44:243–268. [Google Scholar]
- 34.Nevitt MC, Cummings SR, Browner WS, Seeley DG, Cauley JA, Vogt TM, Black DM. The accuracy of self-report of fractures in elderly women: evidence from a prospective study. Am J Epidemiol. 1992;135:490–499. doi: 10.1093/oxfordjournals.aje.a116315. [DOI] [PubMed] [Google Scholar]
- 35.Pajala S, Era P, Koskenvuo M, Kaprio J, Tolvanen A, Heikkinen E, Tiainen K, Rantanen T. Contribution of genetic and environmental effects to postural balance in older female twins. J Appl Physiol. 2004;96:308–315. doi: 10.1152/japplphysiol.00660.2003. [DOI] [PubMed] [Google Scholar]
- 36.Parhami F, Jackson SM, Tintut Y, Le V, Balucan JP, Territo M, Demer LL. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res. 1999;14:2067–2078. doi: 10.1359/jbmr.1999.14.12.2067. [DOI] [PubMed] [Google Scholar]
- 37.Richards JB, Kavvoura FK, Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Zillikens MC, Wilson SG, Mullin BH, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra BA, Pols HA, Sigurdsson G, Thorsteinsdottir U, Soranzo N, Williams FM, Zhou Y, Ralston SH, Thorleifsson G, van Duijn CM, Kiel DP, Stefansson K, Uitterlinden AG, Ioannidis JP, Spector TD. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med. 2009;151:528–537. doi: 10.7326/0003-4819-151-8-200910200-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sennerby U, Farahmand B, Ahlbom A, Ljunghall S, Michaelsson K. Cardiovascular diseases and future risk of hip fracture in women. Osteoporos Int. 2007;18:1355–1362. doi: 10.1007/s00198-007-0386-0. [DOI] [PubMed] [Google Scholar]
- 39.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tiainen K, Sipila S, Alen M, Heikkinen E, Kaprio J, Koskenvuo M, Tolvanen A, Pajala S, Rantanen T. Heritability of maximal isometric muscle strength in older female twins. J Appl Physiol. 2004;96:173–180. doi: 10.1152/japplphysiol.00200.2003. [DOI] [PubMed] [Google Scholar]
- 41.Tiainen K, Sipila S, Alen M, Heikkinen E, Kaprio J, Koskenvuo M, Tolvanen A, Pajala S, Rantanen T. Shared genetic and environmental effects on strength and power in older female twins. Med Sci Sports Exerc. 2005;37:72–78. doi: 10.1249/01.mss.0000150081.04037.bb. [DOI] [PubMed] [Google Scholar]
- 42.Tintut Y, Parhami F, Tsingotjidou A, Tetradis S, Territo M, Demer LL. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J Biol Chem. 2002;277:14221–14226. doi: 10.1074/jbc.M111551200. [DOI] [PubMed] [Google Scholar]
- 43.Wright M, De Geus E, Ando J, Luciano M, Posthuma D, Ono Y, Hansell N, Van Baal C, Hiraishi K, Hasegawa T, Smith G, Geffen G, Geffen L, Kanba S, Miyake A, Martin N, Boomsma D. Genetics of cognition: outline of a collaborative twin study. Twin Res. 2001;4:48–56. doi: 10.1375/1369052012146. [DOI] [PubMed] [Google Scholar]
- 44.Yamada Y, Ando F, Niino N, Miki T, Shimokata H. Association of polymorphisms of paraoxonase 1 and 2 genes, alone or in combination, with bone mineral density in community-dwelling Japanese. J Hum Genet. 2003;48:469–475. doi: 10.1007/s10038-003-0063-x. [DOI] [PubMed] [Google Scholar]
- 45.Youm T, Koval KJ, Kummer FJ, Zuckerman JD. Do all hip fractures result from a fall? Am J Orthop. 1999;28:190–194. [PubMed] [Google Scholar]