An oral challenge of glucose and fructose gives a robust lipogenic response as measured from the increase in VLDL triglyceride palmitate over 4 hours.
Abstract
Context:
Increased hepatic de novo lipogenesis (DNL) in response to dietary sugar is implicated in dyslipidemia, fatty liver, and insulin resistance.
Objective:
The aim of the study was to develop a simple outpatient tolerance test for lipogenic sensitivity to dietary sugar.
Design and Setting:
In inpatients given repeated doses of fructose, protocol 1 compared the acute increase in DNL determined from the percentage of palmitate (“new palmitate”) and the percentage of isotopically labeled palmitate (“%DNL”) in very low-density lipoprotein triglyceride (TG). Protocol 2 compared the increase in new palmitate in outpatients given three different sugar beverages in a randomized crossover design.
Participants:
There were 15 lean and overweight volunteers in protocol 1 and 15 overweight volunteers in protocol 2.
Interventions:
In protocol 1, subjects received 1.4 g/kg fructose in divided oral doses over 6 h; in protocol 2, subjects received 0.5 g/kg fructose, 0.5 g/kg fructose plus 0.5g/kg glucose, or 1 g/kg fructose plus 1g/kg glucose each as a single oral bolus.
Main Outcome Measures:
We measured the increase in DNL by two methods.
Results:
After repeated doses of fructose, new palmitate was significantly correlated with the increase in %DNL (Δ, r = 0.814; P < 0.001) and with fasting insulin levels (area under the curve, r = 0.754; P = 0.001). After a single sugar dose, new palmitate showed a dose effect and was greater after fructose plus glucose. Very low-density lipoprotein TG and total TG significantly increased in both protocols.
Conclusions:
A single oral bolus of fructose and glucose rapidly increases serum TG and TG palmitate in overweight subjects. A dual sugar challenge test could prove useful to identify individuals at risk for carbohydrate-induced dyslipidemia and other adverse effects of increased DNL.
The serious metabolic complications that increasingly plague overweight children and adults include dyslipidemia, fatty liver, and insulin resistance that may promote cardiovascular disease and diabetes. One dietary pattern that may worsen the complications of obesity is excess dietary sugar (1, 2). A possible mechanism is stimulation of hepatic synthesis of the saturated fatty acid, palmitate, and the secretion of palmitate-enriched triglyceride (TG) in very low-density lipoprotein (VLDL) (3–7). This process, de novo lipogenesis (DNL), occurs after the flux of glucose and/or fructose through the glycolytic and lipogenic pathways in the liver. DNL is most potently stimulated by dietary fructose, which, unlike glucose, increases hepatic DNL within hours (8–10). It is important to carefully evaluate the lipogenic effects of fructose because evidence is mounting that increased hepatic DNL causes dyslipidemia, fatty liver, and insulin resistance (11–15).
In this report, we describe our first efforts to develop an outpatient test that could identify, even in childhood, the individuals most susceptible to dyslipidemia and other adverse metabolic sequelae of DNL induced by dietary fructose. We show a good correlation between the increase in DNL after repeated oral doses of fructose measured by an established stable isotope-mass spectrometric methodology [mass isotopomer distribution analysis (MIDA) (8, 16)] and by the increase in percentage of palmitate in serum VLDL TG. We then compare the increase in VLDL TG palmitate in overweight volunteers who ingest beverages containing fructose alone or fructose combined with glucose. Our findings support the hypothesis that measuring the increase in serum VLDL TG palmitate after ingesting a single beverage containing glucose and fructose is a surrogate test of lipogenic sensitivity to fructose-rich foods and beverages.
Subjects and Methods
Protocol 1
Study design
Fifteen healthy lean and overweight adult volunteers were each studied at The Rockefeller University Center for Clinical and Translational Science (CCTS). Participants were eligible if they were males or females 18–75 yr of age with a fasting low-density lipoprotein (LDL) cholesterol (LDL-C) concentration below 160 mg/dl, high-density lipoprotein cholesterol (HDL-C) above 30 mg/dl, and TG below 250 mg/dl. Exclusion criteria included a body mass index (BMI) above 35 kg/m2, weight less than 90% of maximum weight, a weight change of more than 10% usual weight in the previous 6 months, blood pressure above 140/90 mm Hg, diabetes, other systemic illnesses, prescription medications, aspirin, fish oil, high-sensitivity C-reactive protein (hsCRP) above 5 mg/liter, known alcohol abuse, and cigarette smoking greater than half a pack per day. There were nine males and six females with mean (range) for age, 35 (21–64) yr; weight, 77 (63–92) kg; BMI, 26.2 (21.6–30.7) kg/m2; LDL-C, 110 (78–148) mg/dl; HDL-C, 52 (32–72) mg/dl; TG, 81 (46–138) mg/dl; and hsCRP, 1.4 (0.2–3.6) mg/liter. The ethnic/racial backgrounds of the subjects were self-reported as follows: five white, six black, three Hispanic, and one Asian. Written consent was obtained from all participants after approval by The Rockefeller University Institutional Review Board.
Diet stabilization
After admission, volunteers consumed a single day menu of solid food as three meals per day for 5 d. The macronutrient composition was 35% fat, 7% saturated fat, 50% carbohydrate, 15% protein, 30% starch, 15% sugar, 3% fructose, and 25 g/d fiber. The Harris-Benedict equation was used to calculate total calories needed to maintain body weight. Body weight was stable for all participants.
Oral fructose challenge
On d 6, fructose was orally administered as 1.4 g/kg dissolved in 360 ml water and divided into 13 doses (each 1 ounce) every half hour between 0800 and 1400 h, as previously described (8). The average total dose of fructose was 108 g or 432 kcal. Blood samples were obtained after an overnight 12-h fast at −2, 0, 2, 4, 6, 8, and 24 h relative to the start of fructose ingestion. Urinary fructose excretion was measured in the first morning void and between 0800 and 1600 h. Water, but no other food or beverage, was permitted during this period, and physical activity was restricted to reading or watching videos. Lunch was consumed at 1600 h, dinner was finished by 2000 h, and a final fasting blood sample was obtained at 0800 h the next morning.
Adipose tissue biopsy
On the first or second study day, small samples of sc adipose tissue (∼10 mg) were obtained from the buttock and abdomen through an 18-gauge needle after anesthetizing each area with 1 ml of 1% lidocaine.
Fatty acid analysis
At all time points, plasma VLDL was isolated after the overlay of plasma with saline/EDTA using an Optima Max Ultracentrifuge and a TLA 120.2 rotor (Beckman Coulter, Inc., Brea, CA) spun at 120,000 rpm for 30 min at 20 C. The fatty acid composition of VLDL TG was measured after lipid extraction, thin layer chromatography, and transmethylation to form fatty acid methyl esters quantified by capillary gas chromatography, as previously described (5). The fatty acid composition of the total lipid extracts of adipose tissue samples and diet homogenate (∼98% TG) were also analyzed (5).
DNL measured by MIDA
On the day before the oral fructose challenge, at 1800 h, sodium [1-13C1]acetate (>99% pure and >98% enriched; Cambridge Isotope Laboratories, Andover, MA) dissolved in 1 liter of sterile water was infused by vein at 370 mg/h until 2 h after the last dose of fructose for a total of 22 h (4). The VLDL TG fatty acid methyl esters were analyzed by gas chromatography/mass spectrometry using the MIDA method (16). In brief, the fraction of de novo synthesized palmitate in VLDL TG was calculated from the 13C enrichment of the acetate precursor pool determined from the ratio of double- to single-labeled 13C-palmitate in VLDL TG. The calculated enrichments of precursor acetate were stable over time within subjects (data not shown), averaging 6.4 ± 1.7%.
Other laboratory assays
At every time point, the concentrations of VLDL and total TG, cholesterol, glucose, lactate (Roche Diagnostics, Indianapolis, IN), fructose (Sigma Aldrich, St. Louis, MO), and nonesterified fatty acids (NEFA; Wako Chemicals, Richmond, VA) were measured by enzymatic methods. HDL-C was measured after precipitation of β-lipoproteins with dextran sulfate and magnesium chloride (Fisher Scientific, Pittsburgh, PA). Apolipoprotein A1 (apoA1) and apolipoprotein B (apoB) were measured by immunoturbidimetric methods (Roche and Randox Laboratories Ltd., Crumlin, UK, respectively). LDL-C was calculated by the Friedewald equation. Insulin was measured by ELISA [Siemens (formerly DPC), Los Angeles, CA]. At time point 0800 h, after an overnight fast, LDL size and particle number were measured by nuclear magnetic resonance spectroscopy (LipoScience, Inc., Raleigh, NC), fibrinogen by a clot-based functional assay (Diagnostica Stago Inc., Parsippany, NJ), and cortisol by enzyme-amplified chemiluminescence (Siemens). hsCRP was measured by a latex-enhanced immunoturbidimetric method (Siemens) at 0800 and 1600 h. Results below the detection limit were recorded as the detection limit/2.
Protocol 2
Study design
The acute lipogenic effects of fructose were assessed in the outpatient setting with a simplified dosing regimen and measurement of DNL. In an attempt to maximize the lipogenic signal, minimize gastrointestinal tolerance, and mimic a soft drink, equal amounts of glucose were added to fructose, and the results were compared with the results after fructose alone. The addition of glucose to fructose had been reported to enhance fructose absorption and decrease gastrointestinal intolerance that is common when fructose doses exceed 50 g (17).
Two hypotheses were tested: 1) the combination of glucose with fructose enhances palmitate response in VLDL (representing DNL) compared with fructose alone; and 2) DNL shows a dose-response to fructose. Fifteen mild to moderately overweight, otherwise healthy adults were recruited. The eligibility criteria were similar to protocol 1, except that the BMI range was higher (25–35 kg/m2) and the only lipid exclusion was a fasting TG above 800 mg/dl. There were nine males and six females with mean (range) for age, 44 (28–65) yr; BMI, 31.0 (25.8–35.6) kg/m2; weight, 90.0 (80.3–117.3) kg; LDL-C, 133 (84–164) mg/dl; HDL-C, 44 (28–62) mg/dl; TG, 115 (67–286) mg/dl; and hsCRP, 4.1 (0.3–17.1) mg/liter. The ethnic/racial backgrounds were self-reported as eight white, three black, three Hispanic, and one “other.” Written consent was obtained from all volunteers after approval by The Rockefeller University Institutional Review Board.
Oral dual sugar challenge test
In the Rockefeller CCTS outpatient clinic, enrolled volunteers received in randomized, crossover design one of the following three drinks: 1) fructose (0.5 g/kg); 2) fructose (0.5 g/kg) + glucose (0.5 g/kg); and 3) fructose (1 g/kg) + glucose (1 g/kg). These drinks are abbreviated as “F,” “F:G,” and “2X F:G,” respectively. The sugar was dissolved in 12 ounces of water and consumed in 15 min. After a 12-h overnight fast, blood was sampled before and 1, 2, 3, and 4 h after sugar ingestion. To confirm the lack of an increase in DNL after glucose, the fatty acid composition of VLDL TG was also measured in blood sampled during the 3-h oral glucose tolerance test (OGTT) screening test (75 g glucose, average 0.8 g/kg, similar to the amount of sugar in F:G and glucose in 2X F:G). Study visits were at least 1 wk and no more than 3 months apart. Body weight did not significantly change during the study (90.7 ± 11.2 vs. 90.1 ± 10.5 kg).
During the testing period, no food or other beverage (except water) was permitted, and physical activity was restricted to reading or watching videos. Unlike protocol 1, the diets preceding the screening and study visits were unrestricted, except that participants were instructed to continue their usual diet, omit alcohol for 24 h before each visit, and omit fish oil and other supplements for 1 wk before the first study visit until study completion.
Laboratory assays
At every time point, the fatty acid composition of VLDL TG and concentrations of VLDL and total TG, cholesterol, apoB, NEFA, glucose, fructose (except during the OGTT), insulin, and lactate were measured with methods described for protocol 1. In addition, uric acid was measured with an enzymatic assay (Roche). At time point 0 h, HDL-C and hsCRP were measured, and LDL-C was calculated as described for protocol 1. hsCRP was also measured at 4 h.
Statistical methods
Student's paired t test or Wilcoxon's signed-rank test was used to compare peak fractional DNL or percentage palmitate vs. baseline values. Repeated measures one-way ANOVA or the Friedman repeated measures ANOVA was used to compare the peak lipogenic response among each experimental meal and the control (OGTT). For variables changing over time, the area under the curve from baseline (ΔAUC) was calculated using the trapezoid rule. Pearson correlation analysis or Spearman's rank test was used to assess the relationship between fractional DNL and changes in percentage palmitate and between DNL and other continuous variables. Scatter plots were drawn to check for nonlinear relationships and outliers. The CRP values were not normally distributed and were logarithmically transformed. Excel (Microsoft, Redmond, WA) and Sigma Stat (Systat Software, San Jose, CA) statistical software was used. Data are shown as mean ± sd, except in Figs. 1 and 4 and Supplemental Figs. 1–4 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org), where se is displayed for clarity. P values < 0.05 were considered statistically significant after adjustment when necessary for multiple comparisons using the Holm-Sidak or Tukey test.
Fig. 1.
Increase in percentage DNL by the MIDA method (top) and percentage palmitate (16:0, bottom) in VLDL TG before and after repeated oral doses of fructose given over 6 h (mean ± se).
Fig. 4.
Time course of changes in percentage palmitate (16:0) in VLDL TG after single oral boluses of fructose 0.5 g/kg (open circles); fructose 0.5 g/kg + glucose 0.5 g/kg (closed triangles); fructose 1 g/kg + glucose 1 g/kg (open triangles); and glucose 75 g, ∼1 g/kg (closed circles). Data are shown as mean ± se.
Results
Protocol 1
Acute induction of DNL by repeated oral doses of fructose
This study successfully reproduced the previously reported acute stimulatory effect of DNL by fructose given orally in multiple small doses (8). All subjects completed the study without gastrointestinal complaints or other side effects. Fractional DNL measured by MIDA markedly increased 2.4-fold from baseline fasting levels to a plateau between 6 and 8 h (Fig. 1, upper panel; time (T) = 0 h vs. T = 8 h, mean ± sd, 10.2 ± 7.5 to 24.2 ± 10.3%; P < 0.001). After discontinuation of the 13C-acetate infusion at 1600 h (T = 8 h), DNL decreased to below baseline values in all subjects by 0800 h the next morning. This reflects the complete clearance of labeled VLDL and washout with unlabeled preformed palmitate.
Changes in percentage palmitate correlate with changes in DNL
The increase in DNL by MIDA after oral fructose was qualitatively similar to the increase in the percentage of palmitate (% 16:0) of total fatty acids in VLDL TG for the group (Fig. 1, lower panel; T = 0 h vs. T = 8 h, 21.2 ± 3.7 to 25.0 ± 4.2%; P < 0.001), although with considerably higher variability of the estimate (see error bars). From this point on for simplicity, the increase in the percentage palmitate measured by fatty acid analysis will be called “new palmitate.” To statistically compare the increase in percentage de novo palmitate measured by MIDA and the new palmitate measured by fatty acid analysis, the percentage de novo palmitate was first expressed as a percentage of total VLDL TG fatty acids (% de novo palmitate × % palmitate of total VLDL TG fatty acids). The difference between T0 and T8 for MIDA palmitate synthesis values and new palmitate were then compared.
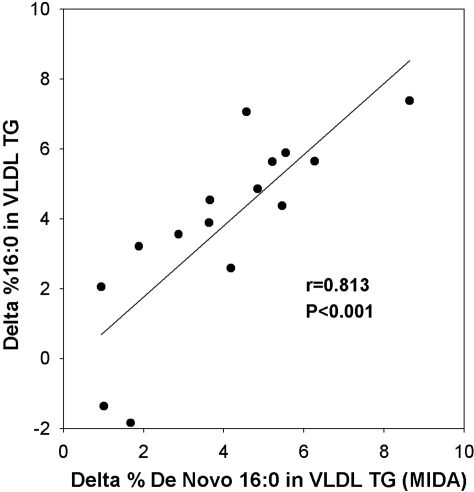
Figure 2 shows the statistically significant positive correlation (r = 0.813) between these values. This good correlation makes it highly likely that the increase in new palmitate after fructose was principally due to an increase in synthesized palmitate in the liver and not to a selective metabolism of preformed palmitate. In all cases, the peak percentage palmitate in VLDL TG exceeded the percentages of the two main sources of preformed palmitate: the dietary fat (11%), and adipose tissue (mean of two sites ± sd, 19.8 ± 1.5%). Other fatty acids or ratios of fatty acids did not show a better correlation with MIDA results.
Fig. 2.
Significant positive correlation between the absolute difference (Δ) in DNL between T = 0 and 8 h measured by MIDA and by fatty acid analysis of the percentage palmitate (16:0) in VLDL TG.
Changes in VLDL TG, total TG, and other metabolites
Between T = 0 and 8 h, the concentrations of VLDL TG (mean ± sd, 47 ± 49 to 56 ± 39 mg/dl; P = 0.100) and total TG (110 ± 97 to 132 ± 103 mg/dl; P < 0.001) increased in parallel (Supplemental Fig. 1). Interestingly, levels of apoB and total cholesterol did not change, indicating that the VLDL particles secreted by the liver were most likely palmitate- and TG- enriched, but were not increased in number (Supplemental Fig. 2). Levels of HDL-C, LDL-C, and apoA1 also did not significantly change. As expected, fructose produced only small (but statistically significant) increases in peak levels of glucose (86 ± 6 to 89 ± 6 mg/dl; P = 0.03) and insulin (5.0 ± 5.3 to 8.5 ± 6.6 μU/ml; P < 0.001). Also as expected, there was a decrease in NEFA levels (0.52 ± 0.18 to 0.23 ± 0.07 meq/liter; P < 0.001) and an increase in lactate levels (1.53 ± 0.57 to 2.75 ± 0.58 mmol/liter; P < 0.001). Baseline serum fructose levels were below detection in all but one subject and peaked at 40.8 ± 12.3 μg/ml. Baseline urinary fructose was below detection and minimal after fructose (52 ± 41 mg/8 h). There was no change in hsCRP (0.7 ± 0.6 and 0.7 ± 0.7 mg/liter).
Correlates of DNL
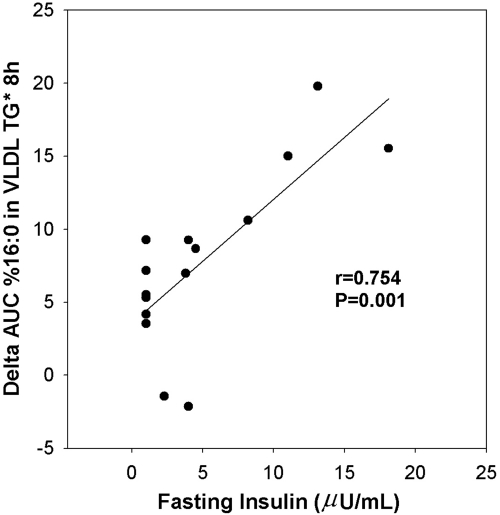
There was a strong, significant positive correlation between the ΔAUC for new palmitate and fasting insulin (Fig. 3) and the homeostasis model of assessment score (r = 0.756; P = 0.001; data not shown). New palmitate was positively associated with fasting LDL particle number (for AUC, r = 0.620; P = 0.014) and inversely associated with fasting HDL-C and apoA1 (for fold increase, r = −0.691, P = 0.004; and −0.730, P = 0.002, respectively). The waist to hip circumference ratio showed a positive correlation with new palmitate at borderline statistical significance (r = 0.474; P = 0.074).
Fig. 3.
Statistically significant positive correlation between AUC percentage palmitate (16:0) in VLDL TG and fasting insulin.
Of note is the lack of a significant relationship between fasting or postfructose DNL (measured as absolute and percentage change and AUC from baseline to plateau at 1600 h) and fasting or postfructose change in VLDL TG or total TG, indicating that levels of TG may have also been determined by differences in TG synthesis and clearance. As expected with the high first-pass uptake by the liver, the peak serum levels of fructose and the amount of urinary fructose did not correlate with the increase in DNL. Fasting or postfructose DNL was also not significantly correlated with fasting or postfructose glucose, insulin, NEFA, lactate, age, BMI, body weight, adipose tissue palmitate, cholesterol, apoB, LDL particle size, fibrinogen, cortisol, or hsCRP.
Protocol 2
Rapid increase in VLDL TG palmitate after a single dose of sugar
This protocol was designed to explore the feasibility of a simple outpatient fructose dosing regimen that was well-tolerated and gave a prompt, robust lipogenic response. All enrolled participants completed the study. One volunteer with premenstrual abdominal symptoms before the test vomited half an hour after ingesting the highest dose (results excluded). The mean baseline lab values were similar among visits.
The ingestion of all three drinks was followed by a rapid, significant increase in VLDL TG palmitate (Fig. 4). The greatest increase from baseline occurred after 2X F:G containing the largest dose of fructose (26.4 ± 4.4 to 29.1 ± 5.0%; P < 0.001 by repeated measures ANOVA). A plateau was not reached at the highest dose (2X F:G). In contrast to the lipogenic effects of fructose-containing drinks, there was no increase and, in fact, a borderline significant decrease in VLDL TG percentage palmitate from baseline after the OGTT (26 ± 1.1 to 25.6 ± 1.1%; P = 0.06).
Figure 5 shows that, as hypothesized, the addition of an equal amount of glucose to fructose increased new palmitate 2-fold (F vs. F:G). When the extreme outlier was removed, new palmitate increased 3-fold and reached statistical significance. Fructose levels were higher after F:G compared with F, as might occur if absorption were increased (72 ± 32 vs. 57 ± 26 μg/ml; P = 0.06). Also, as hypothesized, new palmitate significantly doubled with a doubling of the sugar dose (F:G vs. 2X F:G). These results further support the more potent lipogenic effect of fructose compared with glucose.
Fig. 5.
Δ for percentage palmitate (16:0) in VLDL TG before and after OGTT screening test (glucose, 75 g; average, 0.8 g/kg), F (fructose, 0.5 g/kg), F:G (fructose, 0.5 g/kg, glucose 0.5 g/kg), and 2X F:G (fructose, 1 g/kg, and glucose, 1 g/kg). Data are presented as mean ± sd. All fructose-containing test means were significantly different from the OGTT mean (repeated measures ANOVA, P < 0.05). One outlier was excluded from the statistical comparisons of F vs. F:G and F:G vs. 2X F:G (paired Student's t test).
Changes in VLDL TG, total TG, and other metabolites
Table 1 and Supplemental Figs. 3 and 4 show the levels of markers of lipid and carbohydrate metabolism before and after the sugar boluses. For all doses of fructose (but not glucose), there were significant increases in VLDL TG, total TG, and uric acid. Lactate was significantly increased to a greater extent after fructose compared with glucose drinks. hsCRP did not change (2.9 ± 3.4 and 2.8 ± 2.3 mg/liter for 2X F:G).
Table 1.
Markers of lipid and carbohydrate metabolism before (pre) and after (post) an oral challenge with a single drink of glucose or glucose + fructose
| OGTT |
2X F:G |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| VLDL TG palmitate (%) | 26.1 ± 4.4 | 25.6 ± 4.1 | 26.4 ± 4.4 | 29.1 ± 5.0b |
| VLDL TG (mg/dl) | 57 ± 41 | 58 ± 43 | 61 ± 54 | 75 ± 66b |
| TG (mg/dl) | 115 ± 53 | 118 ± 65 | 126 ± 71 | 159 ± 95a |
| Apo B (mg/dl) | 102 ± 16 | 101 ± 17 | 101 ± 16 | 100 ± 17 |
| NEFA nadir (meq/liter) | 0.59 ± 0.30 | 0.08 ± 0.06b | 0.55 ± 0.26 | 0.05 ± 0.01b |
| Insulin peak (μU/ml) | 18.8 ± 15.4 | 83.1 ± 43.6b | 12.1 ± 12.5 | 94.5 ± 85.3a |
| Glucose peak (mg/dl) | 99 ± 10 | 158 ± 26b | 93 ± 12 | 117 ± 22a |
| Fructose peak (μg/ml) | 13.9 ± 19.4 | 93.3 ± 23.6b | ||
| Lactate peak (mmol/liter) | 1.46 ± 0.34 | 1.83 ± 0.47a | 1.71 ± 0.68 | 3.79 ± 0.77b |
| Uric acid peak (mg/dl) | 6.0 ± 1.8 | 6.0 ± 1.8 | 5.8 ± 1.4 | 6.5 ± 1.4b |
There were no significant differences in baseline values between OGTT and 2X F:G.
P < 0.01;
P < 0.001, pre vs. post.
Correlates of DNL
As in protocol 1, for 2X F:G, the lipogenic response was associated with markers of insulin resistance. There were positive trends between the ΔAUC for new palmitate and fasting insulin (r = 0.513; P = 0.061) and homeostasis model of assessment score (r = 0.505; P = 0.066). The ΔAUC significantly correlated with waist circumference (r = 0.715; P = 0.004). New palmitate, whether expressed as absolute or fold change or ΔAUC, was not significantly correlated with age, BMI, weight, baseline palmitate, baseline or change in VLDL and total TG, HDL-C, LDL-C, NEFA, glucose, fructose, lactate, uric acid, or hsCRP.
Discussion
In these studies, we demonstrate a rapid stimulation of hepatic DNL by fructose in response to two different oral dosing methods in lean and obese volunteers. The good correlation between the increase in DNL measured by MIDA and the increase in percentage of palmitate in VLDL TG after repeated oral doses of fructose makes it highly likely that the latter method also reflects hepatic lipogenic sensitivity to fructose. The significant correlation of fructose-induced DNL with markers of insulin resistance in both protocols supports the increasing evidence for an important role of DNL in the metabolic complications of obesity. With further validation, the outpatient measurement of new palmitate after a sugar bolus may directly reflect both the central mechanism responsible for these complications and the potential response to therapy.
In protocol 2 in obese volunteers, all fructose-containing beverages, but not glucose, induced new palmitate. These results contrast with the lack of an increase in VLDL TG percentage palmitate after F:G 50:50 or 75:25, 1 g/kg, in lean, normolipidemic volunteers who likely were more insulin sensitive (9). As predicted, new palmitate was increased when fructose was combined with glucose (F vs. F:G). One potential mechanism for this effect is a more rapid and complete gastrointestinal absorption of fructose when ingested with glucose (17–21). This may be due to the facilitation of binding of fructose to the glucose transporter 5 receptor, an intestinal fructose-specific hexose transporter (20, 21), or the stimulation glucokinase and glucose entry into the glycolytic pathway by fructose (22, 23). Also as predicted, the magnitude of the lipogenic response was enhanced with doubling of the dose (2X F:G vs. F:G). The doubling of the dose suggests that a higher dose of sugar, if tolerated, might yield a more pronounced increase in new palmitate and thus a better signal to noise ratio. A sampling time beyond 4 h to a maximum response might also increase the signal to noise ratio, but it has the disadvantage of prolonged fasting and confinement to the clinic. Clearly, further testing is needed to determine the optimal dose, blood sampling schedule, and within-subject reproducibility.
The estimated intake of added sugar in U.S. individuals at least 2 yr of age has increased to 17% of energy, and the 80th percentile of intake is 220 g/d or 900 kcal/d, a significant proportion of total energy intake (24). The major sources of dietary sugar are calorically sweetened beverages and desserts that contain combinations of fructose and glucose 1:1 as monosaccharides (high fructose corn syrup) or disaccharide (table sugar, sucrose). In protocol 2, the amount of sugar at the highest dose (2X F:G, 2 g/kg) was similar to the amount of sugar in a commercial “supersize” soft drink consumed by a 60-kg person. It is likely that a beverage made with high fructose corn syrup or sucrose would produce a similar lipogenic response, as previously shown in normal volunteers (9, 25). For those who consume large quantities of calorically sweetened beverages, our results indicate that in addition to glycemia and insulinemia, a potential immediate adverse consequence is the stimulation of DNL and secretion of palmitate- and triglyceride-enriched VLDL.
These data suggest that the new palmitate method may be a useful surrogate for the stable isotopic MIDA method for assessing DNL sensitivity to fructose. Further studies will be necessary to confirm this result in broader settings in subjects excluded here (e.g. BMI > 35 kg/m2, alcohol use, cigarette smoking, recent weight change). Moreover, the ability to distinguish among individuals with intermediate values of DNL sensitivity to fructose stimulation (e.g. Fig. 2, for 11 MIDA Δ DNL values between ∼2 and 7%, for which the correlation with new palmitate was r = 0.625; P = 0.04) will need to be established. The magnitude and range of the lipogenic response to fructose is likely to increase when tested in a larger population with a broader range of insulin sensitivity.
In addition to adverse acute effects, it has been recently postulated that excessive chronic hepatic synthesis of palmitate contributes to the development of dyslipidemia, insulin resistance, fatty liver, and other prodiabetic and proatherogenic metabolic consequences (11–15). This tendency may be present early in the development of diabetes and foster the progression of insulin resistance. In view of the increasing recognition of the important role of dietary sugar in the metabolic complications of obesity, the acute and chronic lipogenic effects need better definition. Once a sugar-dosing regimen that gives a reproducible and rapid lipogenic response is optimized, it must be determined whether the acute lipogenic response predicts long-term detrimental effects of excess dietary sugar. If this is demonstrated, then it may be possible to identify the children and adults who are most sensitive to the lipogenic effects of dietary sugar and who need more intensive dietary counseling, monitoring of cardiovascular risk factors, and medication targeted to inhibit DNL. Thus, this dual sugar challenge test has the potential to become an important screening tool in efforts to prevent early in life diabetes and cardiovascular disease.
Acknowledgments
We thank the nursing and dietary staff of The Rockefeller University Center for Clinical and Translational Science. The excellent technical assistance of Aline Baday, Shai Rosenfeld, Jaqueline Baumgartner, and The Rogosin Institute Iris and B. Gerald Cantor Clinical Research Laboratory is much appreciated.
This work was supported in part by a General Clinical Research Center grant (M01-RR00102); a Clinical Center for Translational Science award (UL1RR024143) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research; The Robert C. Atkins Foundation; funds from William and Linda Macaulay; and an award from the College of Natural Resources, University of California at Berkeley, California. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Clinical Trial registration nos. NCT00234156 and NCT00535535.
Disclosure Summary: L.C.H., T.S.P., and D.M.L. have nothing to declare. M.K.H. is chairman of the scientific advisory board, has equity interests in, and has consulted and lectured for KineMed. He also is an inventor of U.S. Patent no. 5,338,686.
Footnotes
- apoA1
- Apolipoprotein A1
- apoB
- apolipoprotein B
- AUC
- area under the curve
- BMI
- body mass index
- DNL
- de novo lipogenesis
- HDL-C
- high-density lipoprotein-cholesterol
- hsCRP
- high-sensitivity C-reactive protein
- LDL
- low-density lipoprotein
- LDL-C
- LDL-cholesterol
- MIDA
- mass isotopomer distribution analysis
- NEFA
- nonesterified fatty acid
- OGTT
- oral glucose tolerance test
- T
- time
- TG
- triglyceride
- VLDL
- very low-density lipoprotein.
References
- 1. Vartanian LR, Schwartz MB, Brownell KD. 2007. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health 97:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J. 2009. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 120:1011–1020 [DOI] [PubMed] [Google Scholar]
- 3. Parks EJ, Hellerstein MK. 2000. Carbohydrate-induced hypertriacylglycerolemia: historical perspective and review of biological mechanisms. Am J Clin Nutr 71:412–433 [DOI] [PubMed] [Google Scholar]
- 4. Hudgins LC, Hellerstein MK, Seidman CE, Neese RA, Tremaroli JD, Hirsch J. 2000. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J Lipid Res 41:595–604 [PubMed] [Google Scholar]
- 5. Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. 1996. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest 97:2081–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hudgins LC, Seidman CE, Diakun J, Hirsch J. 1998. Human fatty acid synthesis is reduced after the substitution of dietary starch for sugar. Am J Clin Nutr 67:631–639 [DOI] [PubMed] [Google Scholar]
- 7. Aarsland A, Wolfe RR. 1998. Hepatic secretion of VLDL fatty acids during stimulated lipogenesis in men. J Lipid Res 39:1280–1286 [PubMed] [Google Scholar]
- 8. Schwartz JM, Neese RA, Shackleton C, Hellerstein MK. 1993. De novo lipogenesis during fasting and oral fructose in lean and obese hyperinsulinemic subjects. Diabetes 42(Suppl 1):39A [Google Scholar]
- 9. Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. 2008. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr 138:1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chong MF, Fielding BA, Frayn KN. 2007. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr 85:1511–1520 [DOI] [PubMed] [Google Scholar]
- 11. Stanhope KL, Havel PJ. 2008. Fructose consumption: potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr Opin Lipidol 19:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sánchez-Lozada LG. 2007. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86:899–906 [DOI] [PubMed] [Google Scholar]
- 13. Bray GA. 2010. Soft drink consumption and obesity: it is all about fructose. Curr Opin Lipidol 21:51–57 [DOI] [PubMed] [Google Scholar]
- 14. Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, Shulman GI. 2007. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA 104:12587–12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. 2005. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parks EJ, Hellerstein MK. 2006. Thematic review series: patient-oriented research. Recent advances in liver triacylglycerol and fatty acid metabolism using stable isotope labeling techniques. J Lipid Res 47:1651–1660 [DOI] [PubMed] [Google Scholar]
- 17. Truswell AS, Seach JM, Thorburn AW. 1988. Incomplete absorption of pure fructose in healthy subjects and the facilitating effect of glucose. Am J Clin Nutr 48:1424–1430 [DOI] [PubMed] [Google Scholar]
- 18. Choi YK, Johlin FC, Jr, Summers RW, Jackson M, Rao SS. 2003. Fructose intolerance: an under-recognized problem. Am J Gastroenterol 98:1348–1353 [DOI] [PubMed] [Google Scholar]
- 19. Ravich WJ, Bayless TM, Thomas M. 1983. Fructose: incomplete intestinal absorption in humans. Gastroenterology 84:26–29 [PubMed] [Google Scholar]
- 20. Kneepkens CM, Vonk RJ, Fernandes J. 1984. Incomplete intestinal absorption of fructose. Arch Dis Child 59:735–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rumessen JJ, Gudmand-Høyer E. 1986. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut 27:1161–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bizeau ME, Pagliassotti MJ. 2005. Hepatic adaptations to sucrose and fructose. Metabolism 54:1189–1201 [DOI] [PubMed] [Google Scholar]
- 23. Moore MC, Cherrington AD, Mann SL, Davis SN. 2000. Acute fructose administration decreases the glycemic response to an oral glucose tolerance test in normal adults. J Clin Endocrinol Metab 85:4515–4519 [DOI] [PubMed] [Google Scholar]
- 24. Duffey KJ, Popkin BM. 2008. High-fructose corn syrup: is this what's for dinner? Am J Clin Nutr 88:1722S–1732S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. 2008. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr 87:1194–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]