Turner syndrome is detected in the clinical setting by high-throughput pyrosequencing using buccal swab and other DNA samples, providing an alternative to karyotype testing.
Abstract
Objective:
Turner syndrome (TS) occurs when an X-chromosome is completely or partially deleted or when X-chromosomal mosaicism is present. Girls with TS benefit from early diagnosis and treatment with GH; however, many girls with TS are not detected until after 10 yr of age, resulting in delayed evaluation and treatment.
Methods:
We developed a high-throughput test for TS, based on a quantitative method of genotyping to detect X-chromosome abnormalities. This test uses pyrosequencing to quantitate relative allele strength (RAS) from single-nucleotide polymorphisms using 18 informative single-nucleotide polymorphisms markers that span the X-chromosome and one marker for the detection of Y-chromosome material.
Results:
Cutoff ranges for heterozygous, homozygous, or out-of-range RAS values were established from a cohort of 496 males and females. Positive TS scoring criteria were defined as the presence of homozygosity for all 18 markers or the presence of at least one out-of-range RAS value. To determine the validity of this rapid test for TS detection, we undertook a large-scale study using DNA from 132 females without TS and 74 females with TS for whom karyotypes were available. TS was identified with 96.0% sensitivity and 97.0% specificity in this cohort. We also tested buccal swab DNA from a group of 19 females without TS and 69 females with TS. In this group, TS was identified with 97.1% sensitivity and 84.2% specificity.
Conclusions:
These results demonstrate the validity of a high-throughput, pyrosequencing based test for the accurate detection of TS, providing a potential alternative to karyotype testing.
Turner syndrome (TS) is one of the most common genetic conditions affecting females, with an incidence of one in 1500–2000 live births (1–3). TS occurs when an entire X-chromosome is deleted (45X), a portion of an X-chromosome is deleted, (46XdelX), or the X-chromosome is deleted in a subset of cells (TS mosaicism) (1, 3).
Clinical features include primary hypogonadism, renal abnormalities, structural cardiac problems, and short stature (1, 3). Average adult height in untreated TS is 4 ft 8 in. (143 cm) (3–5). Yet, with early diagnosis and initiation of GH therapy, normal or near-normal adult stature can be achieved (5, 6). Early diagnosis also allows for the timely management of comorbid conditions, including coarctation of the aorta and cardiac and renal problems (7).
It is estimated that one in 50–100 girls with short stature have TS (8). Thus, experts in the field recommend that short girls (≤5th percentile) be tested for this condition (7–9). Unfortunately, many girls with TS are not diagnosed until after 10 yr of age (10, 11). Furthermore, TS is not typically identified until 7 yr after short stature has been clinically evident (12). As such, final adult height will be compromised by late intervention with adjunctive growth hormone therapy (12). Estrogen and progestin therapy is also delayed, resulting in late onset of pubertal development (12, 13). Presently, cytogenetic analysis by karyotype is the standard test used to diagnose TS (14). Karyotype analysis is labor intensive and is impractical for large-scale population or high-throughput testing. Although PCR-based approaches have been proposed for TS detection (15–18), they do not effectively detect individuals with mosaicism or partial X-chromosome deletions, which account for more than 40% of karyotypes in TS (19).
We previously reported the development of a quantitative, pyrosequencing-based method for TS detection based on the genotyping of single-nucleotide polymorphisms (SNPs) (20). This approach is advantageous for detecting SNPs due to a high degree of quantitative accuracy, ease of use, and scalability (20). Our previous pyrosequencing studies based on analysis of DNA obtained from a modest number of cell lines indicated that this approach was highly specific and sensitive (21). To determine the clinical utility of this test and to establish assay specificity and sensitivity, we applied this test to a large population of control subjects and individuals with karyotype-confirmed TS.
Subjects and Methods
Human subjects
Studies were carried out under approval of the Yale Human Investigations Committee (New Haven, CT) and Chesapeake Research Review, Inc. (Columbia, MD). Buccal swab samples were obtained after parental informed consent and assent of minor subjects. All other samples were from de-identified individuals. Karyotype information was provided by the treating physician. After collection, all samples were de-identified to preserve patient confidentiality.
DNA samples
DNA samples were obtained from 1) cell lines of individuals designated as apparently healthy from the human genetic cell repository of the National Institute of General Medical Sciences (National Institutes of Health, Bethesda, MD) maintained at the Coriell Institute for Medical Research (Camden, NJ). These samples included four human diversity panels from the following populations: Caucasian (n = 200), African-American (n = 100), Han Chinese from Los Angeles (n = 100), and Mexican-American from Los Angeles (n = 100). They are summarized in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Other samples were from 2) cell lines of known TS individuals obtained from the Coriell Institute (n = 15), plus three TS samples from the human diversity panels (Supplemental Table 2); 3) purified leukocytes from 116 females with known karyotypes (113 females with 46XX karyotype and three females with TS karytoype) and 20 males with 46XY karyotype from the Yale Cytogenetics Laboratory; 4) buccal swabs (n = 70) from females evaluated for short stature (n = 62) or previously diagnosed with TS (n = 8) at Yale Pediatric Endocrinology; 5) buccal swabs (n = 39) obtained by non-Yale pediatric endocrinologists from female patients in their care; and 6) buccal swabs (n = 32) obtained from females with (n = 23) and without (n = 9) TS, who were attendees at the New Jersey and Delaware TS Society Meetings. Of the total 792 samples, 152 were from individuals with known non-TS karyotypes (46XX n = 132; 46XY n = 20), and 90 were from individuals with TS (n = 74 karyotype-confirmed; n = 16 karyotype known but unavailable to the investigators (Supplemental Table 2).
DNA from immortalized cell lines was purchased from the Coriell Institute for Medical Research. DNA from blood leukocytes was obtained from the Yale Cytogenetics Lab.
DNA extraction from buccal swabs
DNA was extracted from buccal swabs using the Sigma-Aldrich (St. Louis, MO) Red Extract method, which is a rapid approach for preparing crude genomic DNA. The amount of human DNA in the crude buccal DNA extract was measured by real-time PCR with the Applied Biosystems (Foster City, CA) Quantifiler Duo Reagent kit.
Pyrosequencing genotyping
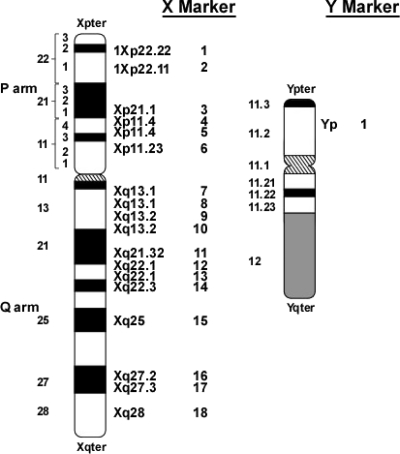
Pyrosequencing was used to genotype each genomic DNA sample for 18 X-chromosome SNP markers (Fig. 1), as reported (21). A minimum of 1 ng human buccal DNA was used for PCR amplification of each SNP marker; the resulting amplicons were purified by binding to streptavidin Sepharose and analyzed by pyrosequencing (20) (for a description of pyrosequencing, use a web browser to examine http://www.pyrosequencing.com/DynPage.aspx?id=8726&mn1=1366). The PSQ96MA pyrosequencing instrument and analysis software (version 2.0.2) was used to automatically score the quality of each reaction, measure the peak heights of each allele, and calculate relative allele strength (RAS) that represents the ratio of the signal intensity of the two alleles.
Fig. 1.
Location of X- and Y-chromosome SNP markers used in TS assay. Note that the average heterozygosity (fraction of females in a population who are heterozygous) for each of the 18 X-chromosome markers is greater than 0.35.
In addition to X-chromosome markers, one Y-chromosome marker was included with the 18 X-chromosome SNP panel (Fig. 1). The PCR primers for this marker amplify a portion of both the AMELY gene of the Y-chromosome and the AMELX gene of the X-chromosome; the amplified regions differ by a single base with the T allele derived from the Y-chromosome and the C allele from the X-chromosome. Thus, measurement of the C/T ratio using this marker provides an indication of the Y- to X-chromosome ratio.
Genotype and signal strength data were exported to Excel spreadsheets. The technician who performed the pyrosequencing and related RAS analysis was blinded to phenotype and karyotype information.
Interpretation of RAS
The RAS represents the relative signal intensity of the two alleles. When a SNP is homozygous, RAS values will be close to 0% (for aa) or 100% (for AA). When two alleles are present equally (heterozygous, Aa), RAS will be close to 50%. In individuals with mosaicism or other X-chromosomal abnormalities, RAS values will significantly diverge from 0, 50, or 100. RAS cutoff ranges for the markers were based on homozygous and heterozygous genotypes for apparently healthy individuals from the Coriell Institute for Medical Research human diversity panel (see Results). RAS values between these homozygous and heterozygous cutoffs were called out of range.
Statistical methods
Statistical analysis was performed using JMP8 (SAS, Cary, NC) software. Values in text are mean ± sd unless stated otherwise. Comparisons among groups were by ANOVA.
Results
Identification of marker RAS cutoff ranges
To establish RAS cutoffs, pyrosequencing was performed on PCR products generated from genomic DNA of apparently healthy male (n = 218) and female (n = 282) individuals derived from the Coriell Institute for Medical Research human diversity panels (Supplemental Table 1). Data were analyzed using the PSQ96MA software to assign genotypes and calculate RAS values. The data from four individuals were excluded from this analysis because three females were known to have a TS karyotype (NA17195, NA17442, and NA17457) and one male had a 46XX karyotype (NA17290). For the remaining 496 samples (217 males and 279 females), RAS data were grouped by marker and genotype and used to calculate the mean, sd, and range.
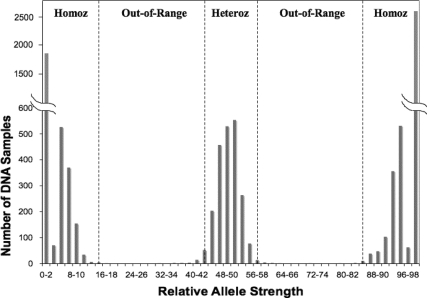
Because the data for homozygous genotypes were not normally distributed (Fig. 2), the range served as an estimate of the threshold for RAS values indicating homozygosity. The thresholds for heterozygous scores were set at the RAS mean ± 2.8 sd. RAS cutoffs were chosen to maximize the number of data points (496 samples × 18 markers) that would fall within range (Fig. 2).
Fig. 2.
Distribution of RAS values for 18 X-chromosome markers in 496 Coriell Institute for Medical Research human diversity DNA samples. The range of RAS values (0–100%) for each marker was divided into 50 bins, each 2% wide (0–2, 2–4, 4–6…98–100%), and the number of DNA samples per bin were tabulated and plotted vs. the RAS values for each bin. The cutoff ranges for 16 of the 18 X-markers (all except XM4 and XM18) are shown by dashed lines. Heteroz, Heterozygous range; Homoz, homozygous range.
For homozygous markers, a RAS range of 0–15 (or 85–100) was established for all markers with the exception of markers 4 and 18, for which the RAS range was set at 0–20 (or 80–100). For heterozygous markers, the RAS score range was set at 43–57 (mean ± 2.8 sd) for all markers with the exception of markers 4 and 18, for which the RAS range was set at 41–59. The wider RAS ranges for markers 4 and 18 reflect the distribution of RAS data for these two markers relative to the other 16 markers. These cutoff values were applied in all subsequent analyses. Marker values that did not fall within either homozygous or heterozygous ranges were called out of range. An individual was considered positive for TS if either one of two conditions was satisfied: 1) all 18 markers scored as homozygous consistent with the presence of only one X-chromosome (45X) or 2) at least one marker scored out of range, suggesting mosaicism or partial deletion of the X-chromosome.
Thirty-three of 279 female controls from the Coriell human diversity panels (Supplemental Table 1) had at least one out-of-range RAS value (11.8%). Two individuals had six and 11 out-of-range RAS values and thus probably were mosaic for the X-chromosome. Of the remaining 277 females, there were two individuals with two out-of-range RAS scores and 29 with only a single out-of-range RAS. The number of homozygous markers per female individual was 10 ± 2, range 5–16. No female was homozygous for all 18 markers. The number of heterozygous markers per individual was 8 ± 2, range 2–13. The number of out-of-range markers per individual was 0.12 ± 0.35, range 0–2.
Of the 217 males, none had any heterozygous markers, and three had at least one out-of-range RAS value (1.4%); two of these individuals had four and seven out-of-range RAS values and were probably mosaic for the X-chromosome. One individual had one out-of-range RAS value. All of the remaining RAS scores (>99.9%) were within the homozygous ranges defined above, and 99.3% of all the RAS values for the 496 non-TS female and male samples were scored as either heterozygous or homozygous (Fig. 2).
Examination of karyotype-confirmed 46XX females
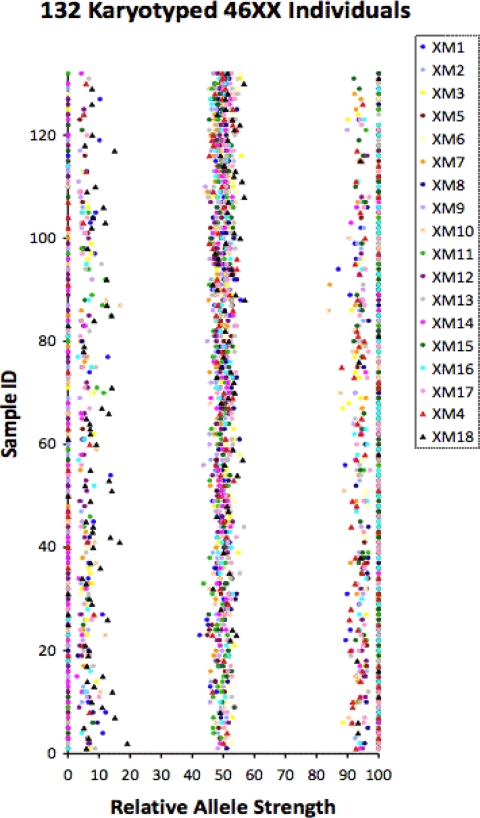
We next examined DNA obtained from 132 females with a known 46XX karyotype (Fig. 3). This cohort included 113 DNA samples from the Yale Cytogenetics Laboratory (purified leukocytes) and 19 buccal swab DNA samples (Supplemental Table 2). All females had at least two heterozygous markers. The number of homozygous markers per individual was 10.25 ± 2.34, range 4–16. The number of heterozygous markers per individual was 7.70 ± 2.37, range 2–14. The number of out-of-range markers per individual was 0.01 ± 0.09, range 0–1. Four individuals had one out-of-range RAS score resulting in a 3.03% false-positive rate, and none had two or more out-of-range values, similar to the 279 females from the human diversity panels.
Fig. 3.
RAS values for 132 females with a 46XX karyotype. The RAS data for each individual is plotted on a line parallel to the x-axis where the y-value is equal to the sample ID. A different colored symbol represents each of the 18 markers tested (inset). Sample ID was arbitrarily assigned from 1–132.
Examination of DNA from individuals with TS
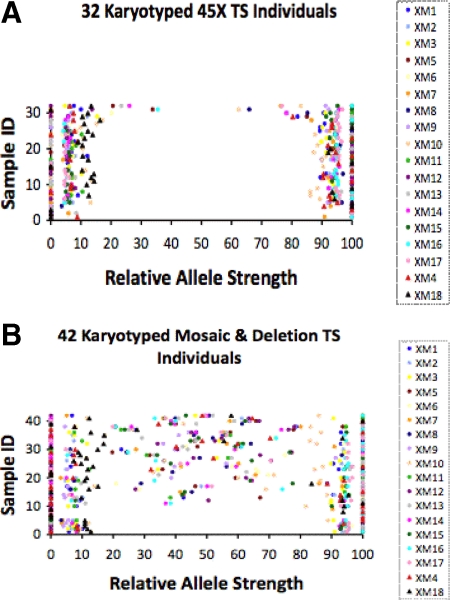
We next examined DNA samples from 74 TS individuals with known karyotypes (Fig. 4). These samples included DNA from 18 cell lines (data from 15 cell lines were previously reported) (21), three blood samples, and 53 buccal swabs (Supplemental Table 2). The karyotype for 32 samples was 45X, 35 samples had TS mosaicism (i.e. 45X/46XX), and seven samples were 46XdelX (X-chromosome deletions). For each sample we determined the number of markers called homozygous, heterozygous, and out-of-range based on the above RAS cutoffs (Fig. 2).
Fig. 4.
RAS values for 32 TS females with a 45X karyotype (A) and for 42 TS females with mosaicism or X-chromosome deletion karyotypes (B). The RAS data for each individual is plotted on a line parallel to the x-axis where the y-value is equal to the sample ID. A different colored symbol represents each of the 18 markers tested (inset). Sample ID was arbitrarily assigned from 1–32 (A) or 1–42 (B).
In this set of 74 karyotyped TS samples (Fig. 4), the number of homozygous markers per individual was 14.07 ± 4.38, range 2–18. The number of heterozygous markers per individual was 3.24 ± 4.06, range 0–15. The number of out-of-range markers per individual was 0.64 ± 1.48, range 0–6.
Of the 74 individuals with TS karyotypes, 71 were positive (Table 1) by our TS criteria (all 18 X-chromosome markers were homozygous or at least one out-of-range RAS value). Thirty-five of the samples were homozygous for all 18 markers; 36 samples had at least one out-of-range RAS value.
Table 1.
Sensitivity and specificity of TS assay for karyotyped females with and without TS
| DNA source | Buccal swab | Yale Cytogenetics Lab and Coriell TS | All combined |
|---|---|---|---|
| True positive | 51 | 20 | 71 |
| False negative | 2 | 1 | 3 |
| True negative | 16 | 112 | 128 |
| False positive | 3 | 1 | 4 |
| Sum | 72 | 134 | 206 |
| Sensitivity | 96.2 | 95.2 | 96.0 |
| Specificity | 84.2 | 99.1 | 97.0 |
| Positive predictive value | 94.4 | 95.2 | 94.7 |
| Negative predictive value | 88.9 | 99.1 | 97.7 |
Sensitivity and specificity are presented for three sample sets: 1) buccal swab (n = 72), 2) combination of Yale Cytogenetics Lab samples (n = 116) and Coriell TS (cell line) samples (n = 18), and 3) combination of buccal swab (n = 72) and Yale Cytogenetics Lab (leukocyte) samples (n = 116) and Coriell TS (cell line) samples (n = 18; Supplemental Table S1). Diagnostic criteria for TS are complete homozygosity of all 18 markers or at least one out-of-range marker.
When the karyotype was 45X (Fig. 4A), all 18 markers were homozygous in 26 of 32 individuals (Table 2), consistent with the presence of only one X-chromosome. The remaining six subjects had at least one out-of-range score; four of these had two or more out-of-range scores, suggesting a low level of mosaicism undetected by karyotype analysis. There were no heterozygous markers in any of the 45X individuals. In this group, there were no false negatives resulting in a TS detection rate of 100% (P < 0.001 TS vs. 46XX).
Table 2.
Assay sensitivity for different TS karyotypes
| TS karyotype | Total | 18 homozygous markers | 1 out-of-range marker | >1 out-of-range marker | False negatives | Sensitivity (%) |
|---|---|---|---|---|---|---|
| 45,X | 32 | 26 | 2 | 4 | 0 | 100 |
| Mosaic | 35 | 8 | 4 | 21 | 2 | 94.3 |
| Deletion | 7 | 1 | 0 | 5 | 1 | 85.7 |
| Not availablea | 16 | 5 | 5 | 6 | 0 | 100 |
Known TS individuals by medical history and confirmed by karyotype but without available karyotype data.
When the TS karyotype was other than 45X (Fig. 4B), nine of 42 individuals were homozygous for all 18 markers (Table 2). Six of the nine were known to have a 45X/46XY karyotype consistent with the presence of only one X-chromosome; an additional two had a 45X/46XX karyotype with an unreported cell ratio, suggesting that the 46XX cell type was present at a low level. At least one marker was out of range in 30 of 42 individuals, and three individuals had at least one in-range heterozygous RAS value and no markers out of range (scored TS negative). The TS detection rate for samples with karyotypes other than 45X was 92.8% (P < 0.001 vs. 46XX). Of the 74 individuals with TS and known karyotypes, 71 (96.0%) were detected (Table 1). Three individuals were scored as TS negative, resulting a in a 4.0% false-negative rate.
The three false-negative individuals had the following karyotypes: 46,X,der(X)t(X;9)(p11.4;q34.13), 45X(5%)/46XX(95%), and 46,XX/46,X,rX(ring chromosome). The first two of these samples had low numbers of heterozygous markers (two and four, respectively).
In addition to the 74 TS individuals with karyotypes known to the investigators, buccal swab samples were obtained from 16 clinically confirmed TS individuals diagnosed by karyotype but for whom karyotype reports were not available for review. In this group, five individuals were completely homozygous for all X-markers, and 11 individuals had one or more out-of-range RAS values. Thus, of 90 TS individuals, 87 scored positive by our assay for a TS detection rate of 97.8%.
Assessment of buccal swab samples
In addition to the above analysis, buccal swab data were analyzed separately. In females with confirmed TS, buccal swabs were available for 53 individuals with known karyotypes and for 16 individuals diagnosed with TS by karyotype but for whom karytoype data were not available. Of these individuals, 67 of 69 (97.1%) met the TS criteria for our test; 32 individuals were homozygous for all 18 markers, and at least one out-of-range RAS score was observed in 35 individuals. Two individuals were false negatives: one individual had low level mosaicism [45X(5%)/46XX(95%)], and the other person had a ring chromosome (46XX/46X,rX).
Buccal swabs were available for 19 non-TS 46XX karyotyped females. Of these individuals, three had one out-of-range RAS score, and none had more than a single out-of-range RAS value. Of note, the out-of-range scores were within 2% of the cutoff thresholds. All individuals had four or more heterozygous markers. Thus, for buccal swabs, the sensitivity and specificity were 97.1 and 84.2%, respectively.
Assessment of Y-chromosome material
In addition to the X-chromosome markers, we tested one Y-chromosome marker in all samples (Fig. 1). In 217 control males without karyotypes and 20 karyotype-confirmed 46XY males, Y-chromosome material was detected in all samples (RAS 44.0 ± 2.9%; range 33.6–57.8%). In 132 karyotype-confirmed 46XX females, Y-chromosome material was not detected in any sample. For TS samples, Y-chromosome material was detected in only four (11.4%) of the 35 individuals with X chromosome mosaicism, a result that matched exactly with their reported karyotype.
Assay reproducibility and precision
The reproducibility of RAS values was evaluated using two approaches. First, to evaluate intra-run precision, the RAS values for seven Centre d'Etude du Polymorphisme Humain genomic DNA samples from females without TS (obtained from the Coriell Institute) were measured in triplicate on the same pyrosequencing run for all 18 X-chromosome SNP markers. Supplemental Table 3 presents the average and sd of the triplicate RAS values from this experiment. The range of sd was 0.0–6.7 with 81.7% of the SNP-DNA combinations (seven genomic DNA samples, 18 X-chromosome SNP markers) with a sd ≤ 3.
Second, estimates of inter-run precision were generated from multiple pyrosequencing runs of the same two genomic DNA samples (NA10850 and NA10851) for all 18 X-chromosome SNP markers (Supplemental Table 4). For NA10850, the range of sd was 1.3–6.6. All heterozygous RAS values had a sd ≤ 3.5. For NA10851. the range of sd was 0.6–6.4.
Discussion
Our data confirm that high-throughput testing using quantitative genotyping by pyrosequencing allows for accurate identification of TS with clinically meaningful sensitivity and specificity (karyotype-confirmed, Table 1). The 18 X-chromosome markers used were informative, displaying a significant average heterozygosity in all four human diversity populations. The assay could detect TS in individuals with 45X, mosaic, and deletion karyotypes.
Of 90 clinically confirmed TS individuals tested, 87 (96.7%) were correctly identified by either complete homozygosity for all 18 markers or the presence of one out-of-range RAS value; 79% of total TS samples were from buccal swabs, where 67 of 69 TS individuals (97.1%) were correctly detected. When the TS individuals were grouped according to karyotype, the assay detected 100% of 45X patients (Table 2).
The sensitivity for identifying individuals with X-chromosome mosaicism and X-chromosome deletions was 94.3 and 85.7%, respectively. One of the three false-negative individuals had very low-level mosaicism [45X(5%)/46XX(95%)], indicating that 5% of 45X cells are below the lower limit of detection for mosaicism against a 46XX background. A second individual not identified as TS by pyrosequencing had a 46,X,der(X)t(X;9)(p11.4;q34.13) karyotype, which is very similar to the normal female 46XX karyotype but missing a portion of the p-arm (distal to p11.4) of one X-chromosome. Such p-arm deletions account for less than 5% of TS cases (19). The third unidentified individual was mosaic with a ring X-chromosome (46,XX/46,X,rX).
The Y-chromosome marker tested was highly specific. All normal male samples were positive for Y-chromosome sequence, and all females without TS were negative. This marker was detected in all TS individuals known to have a normal or modified Y-chromosome by karyotype analysis.
At present, a large proportion of girls with TS are not diagnosed until after 10 yr of age, which results in delayed therapy (10, 11). Increasing evidence suggests that height outcome can be markedly enhanced with early recognition of TS (22–24). Whereas it is recommended that short girls be tested for TS (8, 9), at present, a noninvasive, high-throughput diagnostic test is not available. The use of PCR for TS detection has been proposed (15, 17, 25), but such methods cannot reliably detect mosaicism or partial X-chromosome deletions, which account for more than 40% of TS karyotypes (19). In comparison, using pyrosequencing, we can readily detect TS due to either loss of an entire X-chromosome or mosaicism.
Using a pyrosequencing-based test on individuals with diverse karyotypes associated with TS, we find an overall sensitivity and specificity of 96%. As such, the vast majority of girls with TS will be identified and distinguished from girls without TS. In situations when the results of our test appear to be discordant with the clinical picture, karyotype testing would be advised. In addition, until our test has had substantially more clinical testing, it is advisable to obtain confirmatory testing.
The ability to apply this methodology to buccal swab samples and the scalability of this test to a high-throughput format confers notable advantages for the detection of TS. Considering the substantial advantages of our test, it can be readily applied to newborn screening for population-based screening to detect for TS and other sex-chromosome abnormalities. Because of the small amount of DNA needed for the test, ample DNA can be extracted from newborn screening blood spots that are routinely collected. At the very least, in the primary care and subspecialty setting, this test can be applied to all short females at or below the 5% threshold for height or with an otherwise abnormal growth pattern. This test may also be applied in the evaluation of females with delayed puberty or primary amenorrhea. As such, the penalty of a delayed or missed diagnosis of TS may be eliminated with regard to stature by the timely implementation of growth-promoting strategies. Moreover, the test would also be of clear benefit to TS individuals for early detection of potential renal and cardiac problems.
Acknowledgments
This work was supported by National Institutes of Health Grant R42HD049230.
Disclosure Summary: This manuscript describes a method for identifying sex chromosome aneuploidy. Yale University has applied for a patent covering this method. S.A.R. and J.R.G. are inventors on this patent. The patent rights have been licensed to JS Genetics, founded by S.A.R. and J.R.G. S.A.R., K.H., S.H., H.M.R., and J.R.G. are equity holders in JS Genetics. A.W. and P.L. have nothing to disclose.
Footnotes
- RAS
- Relative allele strength
- SNP
- single-nucleotide polymorphism
- TS
- Turner syndrome.
References
- 1. Saenger P. 1997. Turner's syndrome. Curr Ther Endocrinol Metab 6:239–243 [PubMed] [Google Scholar]
- 2. Gravholt CH. 2004. Epidemiological, endocrine and metabolic features in Turner syndrome. Eur J Endocrinol 151:657–687 [DOI] [PubMed] [Google Scholar]
- 3. Ranke MB, Saenger P. 2001. Turner's syndrome. Lancet 358:309–314 [DOI] [PubMed] [Google Scholar]
- 4. Saenger P. 2000. Value of growth hormone treatment in Turner's syndrome. Endocrine 12:183–187 [DOI] [PubMed] [Google Scholar]
- 5. Rosenfeld RG, Attie KM, Frane J, Brasel JA, Burstein S, Cara JF, Chernausek S, Gotlin RW, Kuntze J, Lippe BM, Mahoney CP, Moore WV, Saenger P, Johanson AJ. 1998. Growth hormone therapy of Turner's syndrome: beneficial effect on adult height. J Pediatr 132:319–324 [DOI] [PubMed] [Google Scholar]
- 6. Hull KL, Harvey S. 2003. Growth hormone therapy and quality of life: possibilities, pitfalls and mechanisms. J Endocrinol 179:311–333 [DOI] [PubMed] [Google Scholar]
- 7. Frías JL, Davenport ML. 2003. Health supervision for children with Turner syndrome. Pediatrics 111:692–702 [DOI] [PubMed] [Google Scholar]
- 8. Grote FK, Oostdijk W, De Muinck Keizer-Schrama SM, van Dommelen P, van Buuren S, Dekker FW, Ketel AG, Moll HA, Wit JM. 2008. The diagnostic work up of growth failure in secondary health care; an evaluation of consensus guidelines. BMC Pediatr 8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bondy CA. 2007. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab 92:10–25 [DOI] [PubMed] [Google Scholar]
- 10. Parker KL, Wyatt DT, Blethen SL, Baptista J, Price L. 2003. Screening girls with Turner syndrome: the National Cooperative Growth Study experience. J Pediatr 143:133–135 [DOI] [PubMed] [Google Scholar]
- 11. Massa G, Verlinde F, De Schepper J, Thomas M, Bourguignon JP, Craen M, de Zegher F, François I, Du Caju M, Maes M, Heinrichs C. 2005. Trends in age at diagnosis of Turner syndrome. Arch Dis Child 90:267–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sävendahl L, Davenport ML. 2000. Delayed diagnoses of Turner's syndrome: proposed guidelines for change. J Pediatr 137:455–459 [DOI] [PubMed] [Google Scholar]
- 13. Bertelloni S, Baroncelli GI, Fruzzetti F, Spinelli C, Simi P, Saggese G. 2003. Growth and puberty in Turner's syndrome. J Pediatr Endocrinol Metab 16(Suppl 2):307–315 [PubMed] [Google Scholar]
- 14. Longui CA, Rocha MN, Martinho LC, Gomes GG, de Miranda RE, Lima TA, Melo MB, Monte O. 2002. Molecular detection of XO: Turner syndrome. Genet Mol Res 1:266–270 [PubMed] [Google Scholar]
- 15. Fernández-Martínez FJ, Galindo A, Moreno-Izquierdo A, Gómez-Rodríguez MJ, Moreno-García M, Grañeras A, Barreiro E. 2007. Application of QF-PCR for the prenatal assessment of discordant monozygotic twins for fetal sex. Prenat Diagn 27:648–652 [DOI] [PubMed] [Google Scholar]
- 16. Rocha MN, Melo MR, Longui CA, de Oliveira DV, Figueiredo CC, Pacchi PR. 2005. A three-step molecular protocol employing DNA obtained from dried blood spots for neonatal screening for 45,X Turner syndrome. Genet Mol Res 4:749–754 [PubMed] [Google Scholar]
- 17. Ogilvie CM, Donaghue C, Fox SP, Docherty Z, Mann K. 2005. Rapid prenatal diagnosis of aneuploidy using quantitative fluorescence-PCR (QF-PCR). J Histochem Cytochem 53:285–288 [DOI] [PubMed] [Google Scholar]
- 18. Pena SD, Sturzeneker R. 2003. Fetal diagnosis of monosomy X (Turner syndrome) with methylation-specific PCR. Prenat Diagn 23:769–770 [DOI] [PubMed] [Google Scholar]
- 19. Wolff DJ, Van Dyke DL, Powell CM. 2010. Laboratory guideline for Turner syndrome. Genet Med 12:52–55 [DOI] [PubMed] [Google Scholar]
- 20. Ronaghi M. 2003. Pyrosequencing for SNP genotyping. Methods Mol Biol 212:189–195 [DOI] [PubMed] [Google Scholar]
- 21. Meng H, Hager K, Rivkees SA, Gruen JR. 2005. Detection of Turner syndrome using high-throughput quantitative genotyping. J Clin Endocrinol Metab 90:3419–3422 [DOI] [PubMed] [Google Scholar]
- 22. Davenport ML. 2006. Evidence for early initiation of growth hormone and transdermal estradiol therapies in girls with Turner syndrome. Growth Horm IGF Res 16(Suppl A):S91–S97 [DOI] [PubMed] [Google Scholar]
- 23. Davenport ML, Crowe BJ, Travers SH, Rubin K, Ross JL, Fechner PY, Gunther DF, Liu C, Geffner ME, Thrailkill K, Huseman C, Zagar AJ, Quigley CA. 2007. Growth hormone treatment of early growth failure in toddlers with Turner syndrome: a randomized, controlled, multicenter trial. J Clin Endocrinol Metab 92:3406–3416 [DOI] [PubMed] [Google Scholar]
- 24. Davenport ML, Punyasavatsut N, Stewart PW, Gunther DF, Sävendahl L, Sybert VP. 2002. Growth failure in early life: an important manifestation of Turner syndrome. Horm Res 57:157–164 [DOI] [PubMed] [Google Scholar]
- 25. Onay H, Ugurlu T, Aykut A, Pehlivan S, Inal M, Tinar S, Ozkinay C, Ozkinay F. 2008. Rapid prenatal diagnosis of common aneuploidies in amniotic fluid using quantitative fluorescent polymerase chain reaction. Gynecol Obstet Invest 66:104–110 [DOI] [PubMed] [Google Scholar]