Room light exerts a profound suppressive effect on melatonin levels and shortens the body's internal representation of night duration.
Abstract
Context:
Millions of individuals habitually expose themselves to room light in the hours before bedtime, yet the effects of this behavior on melatonin signaling are not well recognized.
Objective:
We tested the hypothesis that exposure to room light in the late evening suppresses the onset of melatonin synthesis and shortens the duration of melatonin production.
Design:
In a retrospective analysis, we compared daily melatonin profiles in individuals living in room light (<200 lux) vs. dim light (<3 lux).
Patients:
Healthy volunteers (n = 116, 18–30 yr) were recruited from the general population to participate in one of two studies.
Setting:
Participants lived in a General Clinical Research Center for at least five consecutive days.
Intervention:
Individuals were exposed to room light or dim light in the 8 h preceding bedtime.
Outcome Measures:
Melatonin duration, onset and offset, suppression, and phase angle of entrainment were determined.
Results:
Compared with dim light, exposure to room light before bedtime suppressed melatonin, resulting in a later melatonin onset in 99.0% of individuals and shortening melatonin duration by about 90 min. Also, exposure to room light during the usual hours of sleep suppressed melatonin by greater than 50% in most (85%) trials.
Conclusions:
These findings indicate that room light exerts a profound suppressive effect on melatonin levels and shortens the body's internal representation of night duration. Hence, chronically exposing oneself to electrical lighting in the late evening disrupts melatonin signaling and could therefore potentially impact sleep, thermoregulation, blood pressure, and glucose homeostasis.
The pineal gland hormone melatonin is released during the biological night and provides the body's internal biological signal of darkness. Exposure to light both resets the circadian rhythm of melatonin and acutely inhibits melatonin synthesis (1, 2). In some mammals, light regulation of melatonin gives rise to photoperiodic responses including patterns of seasonal breeding and changes in pelage (3, 4). The duration of nocturnal melatonin secretion in humans is likewise dependent on photoperiod (5), but effects on the reproductive system remain controversial. Several groups have shown seasonality in births, but few studies have examined the potential link between melatonin duration and reproductive hormones that determine the likelihood of conception (6–8).
Because the onset of melatonin secretion is associated with an increase in sleep propensity, and exogenous administration of melatonin can facilitate sleep (9–12), melatonin has long been hypothesized as a sleep-promoting factor in humans. Melatonin treatment reduces sleep onset latency when endogenous levels of melatonin are low during the biological daytime (12). Melatonin receptors are located on circadian clock neurons in the suprachiasmatic nucleus in the anterior hypothalamus (13), suggesting that feedback regulation by melatonin signaling may contribute to circadian regulation, including the timing of sleep. Consistent with this hypothesis, daily ingestion of melatonin has been shown to synchronize circadian rhythms of behavior and physiology in blind individuals (14, 15). In addition to its hypnotic and circadian phase resetting effects, exogenous melatonin has been shown to lower blood pressure and body temperature (16, 17), and recent genome-wide association studies have established a putative link between signaling at the melatonin 1B receptor and risk for type 2 diabetes (18–20). With melatonin receptors located in several sites of the central nervous system and in peripheral tissues including the heart, kidney, pancreatic islets, adrenal glands, stomach, and gonads (21), melatonin has been explored as a treatment option for various human disease states including insomnia, hypertension, and cancer (22).
Despite the potential therapeutic benefits of melatonin treatment, the physiological consequences of chronically inhibiting melatonin synthesis are unknown. Recent studies have shown that indoor room light (i.e. <500 lux) can elicit strong melatonin suppression and phase shift responses (23–25), suggesting that individuals who habitually expose themselves to light during nighttime hours could have reduced melatonin levels and perturbed rhythms. In a study that examined the dose response for melatonin suppression and phase resetting responses to white light given at night, half-maximal responses were observed at about 100 lux (25), which is substantially dimmer than recommended office lighting (∼350–500 lux) (26). In that study, however, participants were kept in relatively dim light (<15 lux) for 3 d preceding the light stimulus, which may have sensitized the circadian system to light (27, 28). Nonetheless, in other studies, exposure to room light suppressed the onset of melatonin secretion even when preceded by room light levels during the daytime (24, 28). Appropriately timed exposure to indoor light (∼380 lux) has also been shown to accelerate entrainment to a rapid 5-h advance of the sleep-wake cycle (29). Taken together, these studies indicate that melatonin suppression and phase shift responses are sensitive to ordinary room light levels regardless of previous light history.
These findings suggest that exposure to room light before bedtime, a common practice in modern society, may inhibit melatonin production and, as a result, alter physiological processes regulated by melatonin signaling. To address this possibility, we examined melatonin responses to room light vs. dim light in 116 research volunteers studied in the laboratory under a fixed sleep-wake schedule (8 h asleep, 16 h awake). Here, we report that exposure to electrical light between dusk and bedtime strongly suppresses melatonin levels, leading to an artificially shortened melatonin duration and disruption of the body's biological signal of night.
Subjects and Materials
Participants
Healthy research volunteers (n = 116) aged 18–30 yr were enrolled into one of two inpatient studies (see below) at the General Clinical Research Center (GCRC), Brigham and Women's Hospital (BWH) (Boston, MA). Physical health was evaluated by medical history and physical examination, blood biochemistry and hematology, and electrocardiogram. Sleep and circadian rhythm disorders were exclusionary. Mental health was assessed by interview with a staff psychologist or psychiatrist, and normal sight was confirmed by an ophthalmological examination and/or the Ishihara test for color blindness. For at least 2 wk before the inpatient study, participants were required to keep a fixed sleep-wake schedule (8 h asleep, 16 h awake) of their choice, and compliance was verified by continuous actigraphy monitoring (Actiwatch-L; Minimitter, Inc., Bend, OR). To ensure that participants had refrained from the use of drugs, a comprehensive toxicology screen was performed on the day of admission to the GCRC. Informed consent was obtained from all volunteers, and research procedures were approved by the Institutional Review Board at BWH and were in compliance with HIPAA regulations and the Declaration of Helsinki.
General inpatient procedures
Research volunteers lived individually in a laboratory free of time cues. During the first 3 d of each study, participants were scheduled to sleep and wake at their habitual prestudy sleep-wake times (8 h asleep, 16 h awake). Ambient light was provided by ceiling-mounted 4100K fluorescent lamps (Philips Lighting, Eindhoven, The Netherlands) and transmitted through an UV-stable filter (Lexan, General Electric Plastics, Pittsfield, MA). Illuminance was measured with an IL1400 radiometer fitted with an SEL-033/Y/W detector (International Light Inc., Peabody, MA). For the under 200 lux and under 3 lux settings, lighting levels were defined by the maximum illuminance measured in the horizontal plane, with the detector aimed directly at the ceiling lamps at a height of 187 cm. For the under 200 lux light setting, illuminance in the horizontal angle of gaze was less than 150 lux (i.e. measured in the vertical plane), and typical illumination at the eyes ranged from 60–130 lux, as reported previously (25, 30). For the approximately 200 lux light setting, overhead lighting was adjusted such that when participants were in bed they received approximately 200 lux of corneal illuminance (28).
On the second baseline day of each study, an indwelling iv catheter was inserted into a forearm vein to allow for continuous collection of blood plasma every 30–60 min for melatonin assay. In the present investigation, melatonin data were analyzed only across study d 2–5. During sleep episodes and the constant routine procedure (see below), blood was drawn from outside the research suite through a porthole in the bedroom wall. Melatonin concentration was determined by standard RIA by laboratories that were blind to the experimental intervention (Pharmasan, Osceola, WI; BWH GCRC Core Laboratory, Boston, MA). Plasma melatonin intraassay and interassay coefficients of variation for each of the studies were cited in the original reports (28, 30–32).
Protocol design
Study 1
Plasma melatonin was examined in 104 volunteers who participated in a 9- to 10-day research study (31, 32). Participants slept in darkness and were exposed to room light (<200 lux) until midway through study d 3, after which the light was dimmed to under 3 lux. The following morning, participants awoke to a constant routine procedure consisting of wakefulness enforced by technician monitors (30–50 h), semirecumbent bed rest, consumption of hourly equicaloric snacks, and constant exposure to dim light (<3 lux) (33).
Study 2
Twelve volunteers completed a 14-d research protocol (28), including one person who completed the study twice under different lighting conditions (see below). We analyzed a 4-d segment of the protocol that was randomized to occur either on study d 6–9, or study d 10–13. Over the first 3 d, participants slept in darkness and were exposed to room light during the daytime (<200 lux, n = 5; ∼200 lux, n = 8). After 8 h of scheduled sleep in darkness, participants underwent a 40-h constant routine procedure in room light. Melatonin levels in room light during the habitual hours of sleep were compared with melatonin levels during sleep on the previous night. To determine percent suppression of melatonin, the area under the curve (AUC, trapezoidal method) was calculated for exposure to room light (AUCRL), and compared with the AUC for the melatonin rhythm during the preceding sleep episode in darkness (AUCD) at the same relative clock times. Hence, percent melatonin suppression was calculated as [1 − (AUCRL) × (AUCD)−1] × 100, with higher values indicating stronger suppression of the melatonin rhythm.
Determination of melatonin phase, duration, and phase angle
For each subject in study 1, the melatonin rhythm during the constant routine procedure was fit by a three-harmonic regression model to estimate the amplitude. Dim light melatonin onset (DLMOn25%) and offset (DLMOff25%) were defined as the clock times at which the melatonin rhythm crossed a threshold value of 25% of the peak-to-trough fitted amplitude (half the standard amplitude). The same 25% threshold was used for determining melatonin onset and offset on d 2–3, during which individuals were exposed to either room light or dim ambient light. Melatonin duration was defined as the number of consecutive hours that melatonin levels exceeded the 25% threshold between melatonin onset and offset. Phase angle was defined as the difference in timing between melatonin onset or offset vs. bedtime, with positive values indicating that the event occurred before each participant's regular bedtime. To determine the percent reduction in melatonin concentration due to exposure to room light before bedtime, the AUC was calculated between melatonin onset and bedtime on d 3 (<3 lux) and compared with the AUC on the preceding day (<200 lux) at the same relative clock times. Local clock times of sleep and wake during inpatient studies were scheduled based on each subject's habitual sleep hours during the prestudy screening procedures. For purposes of illustration, data were aligned by scheduled sleep in Figs. 1–4 and plotted vs. relative clock time. We set relative clock times of sleep from 2400 to 0800 h. The distribution of wake times, melatonin onset, and melatonin offset vs. local time are shown in Supplemental Fig. 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
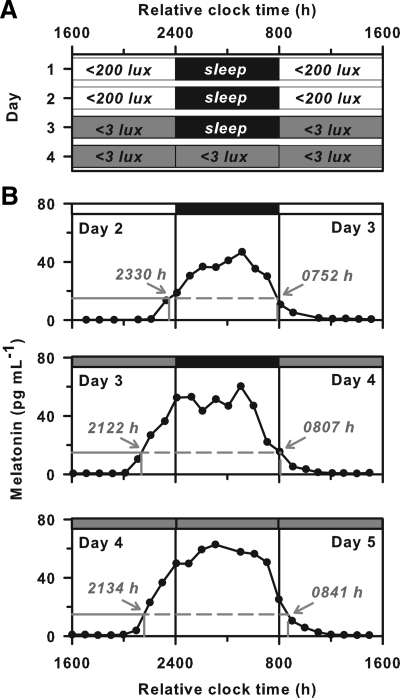
Fig. 1.
Melatonin onset occurs later in room light than in dim light. A, In study 1, participants lived in room light (<200 lux) and slept in darkness for the first two baseline days. Upon awakening on the morning of d 3, participants were exposed to 8 h of room light, followed by 8 h of dim light (<3 lux) before bedtime. After sleep, individuals underwent a constant routine procedure in dim light. B, The melatonin rhythm is shown for a representative subject over three consecutive cycles (d 2–5). In this subject, melatonin onset occurred about 2 h earlier on d 3 and 4 in dim light, compared with d 2 in room light. The timing of melatonin onset and offset are shown by the labeled arrows in gray. White and gray bars at the top of each plot indicate exposure to room light and dim light, respectively, and black bars indicate sleep in darkness.
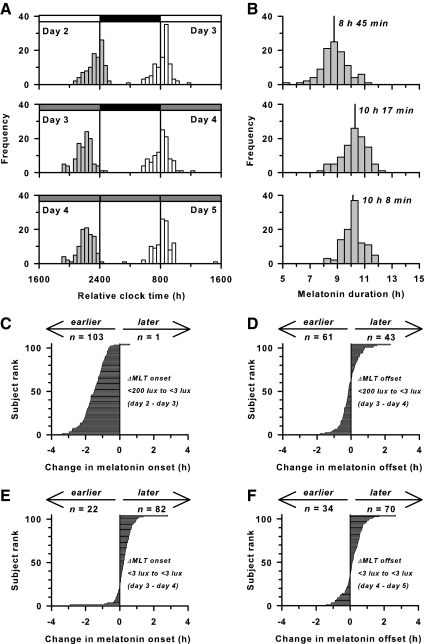
Fig. 2.
Exposure to room light before bedtime shortens melatonin (MLT) duration. A, Histograms show the timing of melatonin onset (gray bars) and offset (white bars) in participants (n = 104) living in room light vs. dim light. White and gray bars at the top of each plot indicate exposure to room light (<200 lux) and dim light (<3 lux), respectively, and black bars indicate scheduled sleep in darkness. B, Histograms show melatonin duration in the same participants over three consecutive cycles corresponding to A. Median melatonin duration is indicated by the vertical line with label. Melatonin duration is longest when the onset and offset occur under dim light. C, Horizontal bar chart showing the change in timing of melatonin onset in individual participants from d 2 in room light to d 3 in dim light. In 99% of individuals, melatonin onset occurred earlier in dim light relative to room light. Data are ranked in ascending order of magnitude. D–F, Similar plots are shown for changes in the timing of melatonin offset from the morning of d 3 in room light to d 4 in dim light (D), melatonin onset from d 3 in dim light to d 4 in dim light (E), and melatonin offset from the morning of d 4–5 in dim light (F).
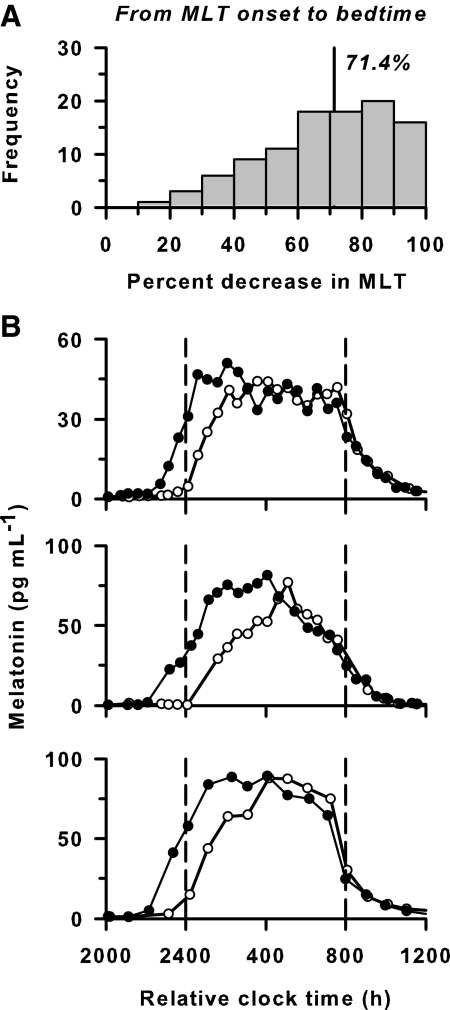
Fig. 3.
Exposure to room light before bedtime decreases melatonin (MLT) levels. A, Histogram showing the percent decrease in melatonin from melatonin onset to bedtime when participants were exposed to room light (<200 lux) vs. dim light (<3 lux) until scheduled sleep. The AUC of the melatonin profile was determined in dim light and compared with the AUC on the preceding day at the same relative clock times. Median percent decrease in melatonin is indicated by the vertical line with label. B, The melatonin rhythm is shown for three representative volunteers exposed to room light (○) before and after scheduled sleep in darkness (enclosed by the vertical dashed lines) vs. exposure to dim light (●) at the same relative clock times on the following day.
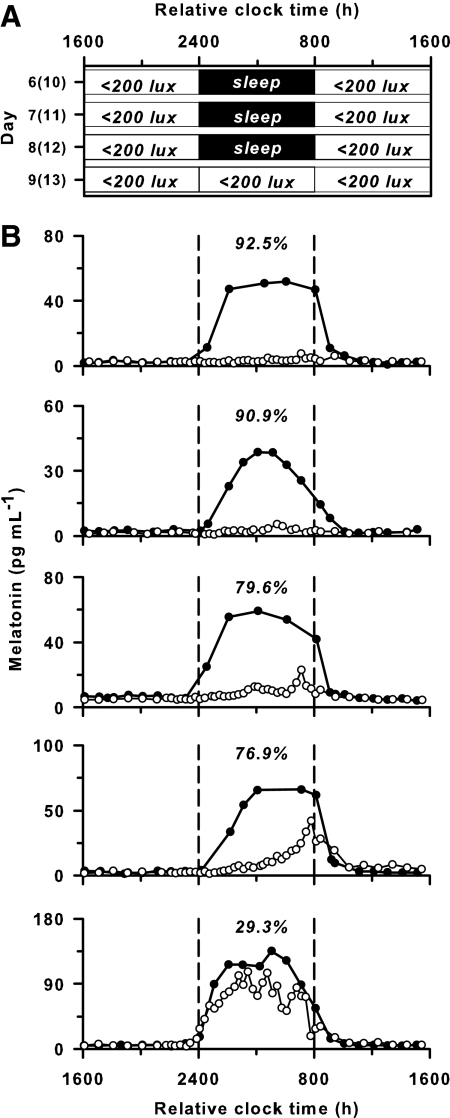
Fig. 4.
Exposure to room light elicits strong suppression of melatonin during the usual hours of sleep. A, Participants lived in ambient room light (<200 lux) and slept in darkness for 3 baseline days, after which they underwent a constant routine procedure in room light (<200 lux). Data are shown from either d 6–9 or 10–13 of a 14-d research protocol (See Subjects and Methods). B, The melatonin rhythm is shown for five individuals exposed to room light (○) during the constant routine vs. darkness during sleep (●) on the preceding day. Percent melatonin suppression by room light is indicated at the top of each plot for the 8 h corresponding to habitual sleep (enclosed by the vertical dashed lines).
Data analysis and statistics
Within-subjects differences in the timing of melatonin onset, offset, duration, and phase angle were compared by Friedman's repeated-measures ANOVA on ranks (SigmaPlot 11; Systat Software, Inc., San Jose, CA). We chose to use nonparametric statistics because the distribution of melatonin onsets and offsets did not always pass the Kolmogorov-Smirnov test for normality (P < 0.05). In each comparison, the melatonin outcome (onset, offset, duration, or phase angle) was the dependent variable, and ambient lighting, which varied over three consecutive days, was the repeated factor (i.e. the treatment group). For those comparisons in which the difference in median values among the treatment groups was greater than that expected by chance (P < 0.05), all pairwise multiple comparisons were tested for significance using Tukey's method (α = 0.05). Within-subjects differences in AUC for melatonin levels in dim light (<3 lux) vs. room light (<200 lux) were compared using the Wilcoxon signed rank test. Median values for melatonin outcomes are reported in the text with the interquartile range (IQR); the 25th and 75th percentiles are shown in Table 1.
Table 1.
Melatonin responses to room light vs. dim light in study 1 (n = 104)
| Median (25%, 75%) |
|||
|---|---|---|---|
| Light (<200 lux), d 2-3 | Dim (<3 lux), d 3-4 | Dim (<3 lux), d 4-5 | |
| MLT onset (h min) | 23 39 (22 18, 00 55) | 21 54 (20 58, 23 14)a | 22 09 (21 13, 23 21)a,b |
| MLT offset (h min) | 08 16 (07 20, 09 34) | 08 23 (07 12, 09 22) | 08 26 (07 17, 09 30)b |
| MLT duration (h) | 8.75 (8.25, 9.42) | 10.28 (9.68, 10.85)a | 10.13 (9.68, 10.60)a |
| Phase angle MLT onset (h) | 0.38 (−0.32, 1.22) | 1.95 (1.37, 2.63)a | 1.68 (1.07, 2.45)a,b |
| Phase angle MLT offset (h) | −8.52 (−8.92, −7.55) | −8.33 (−8.87, −7.50) | −8.38 (−8.87, −7.77)b |
Melatonin (MLT) outcomes are reported for participants over three consecutive days. Individuals lived in room light (<200 lux) and slept in darkness until midway through d 3 (column 2). Over the next 24 h, participants lived in dim light (<3 lux) and slept in darkness (column 3), followed by a constant routine procedure on d 4 and 5 conducted in under 3 lux light (column 4). Phase angle is defined as the relative timing of melatonin onset or offset vs. scheduled bedtime. A positive phase angle indicates that the event happened before scheduled sleep, whereas negative values indicate that the event happened after bedtime.
Significant differences for comparisons with the first melatonin cycle (column 2; d 2-3)
Significant differences for comparisons with the second melatonin cycle (column 3; d 3-4).
Results
Melatonin onset and duration are affected by evening exposure to room light
In study 1, changes in the melatonin profile were compared within subjects (n = 104) exposed to either room light or dim light before sleep (Fig. 1). In room light, melatonin onset occurred 23 min (IQR, 1 h 36 min) before scheduled sleep, whereas in dim light, melatonin onset occurred 1 h 57 min (IQR, 1 h 16 min) before scheduled bedtime (P < 0.05, Fig. 2A and Table 1). In contrast, the timing of melatonin offset did not differ significantly between room light and dim light conditions. Thus, due to its effect on melatonin onset, exposure to room light before bedtime shortened melatonin duration by 1 h 32 min (IQR, 1 h 6 min) compared with exposure to dim light (8 h 45 min vs. 10 h 17 min, P < 0.05; Fig. 2B and Table 1).
Next, we examined changes in the timing of melatonin onset and offset in individual participants. We found that 99.0% of participants (103 of 104) exhibited an earlier melatonin onset in dim light (d 3) vs. room light (d 2), and 78.6% of these individuals exhibited an earlier onset by more than an hour (Fig. 2C). In contrast, only 58.6% of participants showed an earlier melatonin offset in dim light vs. room light, indicating that melatonin offset was not affected by the difference in lighting conditions (Table 1 and Fig. 2D). During two cycles of exposure to dim light, most participants showed a small daily delay (∼12 min) in the timing of melatonin onset and offset, 78.9 and 67.3% of individuals, respectively (Fig. 2, E and F), which is consistent with the longer-than-24-h intrinsic period of the human circadian system reported in previous studies (34).
To determine the effect of room light exposure on melatonin concentration before sleep, we compared the AUC for melatonin measured on d 2 (<200 lux) vs. d 3 (<3 lux). In dim light, the onset of nocturnal melatonin secretion occurred before scheduled sleep in 98% of participants (102 of 104). In these individuals, exposure to room light from the onset of melatonin until bedtime reduced melatonin concentration by 71.4% (IQR, 32.2%) relative to exposure to dim light 24 h later (P < 0.001, Fig. 3). In contrast, exposure to room light after awakening did not reduce melatonin levels (P = 0.802) in participants whose melatonin offset occurred after scheduled wake time in dim light (n = 64).
Exposure to room light suppresses melatonin during the usual hours of sleep
To test directly whether the later melatonin onset that we observed in room light was due to melatonin suppression, in study 2, we examined the melatonin rhythm under constant routine conditions in room light (n = 5, <200 lux) (Fig. 4). Compared with the melatonin rhythm observed when participants slept in darkness, exposure to room light during the normal hours of sleep suppressed melatonin strongly in four of five individuals (percent suppression: 92.5, 90.9, 79.6, 76.9, and 29.3%). In another group of participants (n = 8) who were exposed to a slightly higher level of ambient light (∼200 lux at the level of the eyes), there was robust melatonin suppression in seven of eight individuals (percent suppression: 87.6, 87.1, 73.7, 62.7, 54.2, 53.0, 51.1, and −1.3%). Hence, in 11 of 13 trials, exposure to room light in participants who were kept awake during the usual hours of sleep suppressed melatonin by more than half the amount measured during sleep in darkness.
Discussion
Our results demonstrate that the melatonin profile is truncated by exposure to room light before bedtime. Specifically, we show that exposure to room light (<200 lux) in the late evening suppresses the onset of melatonin synthesis, thereby shortening melatonin duration by about 90 min compared with exposure to dim light (<3 lux). As a result of this exposure to electrical light between dusk and bedtime, presleep levels of melatonin were reduced by 71.4% and total daily levels of melatonin were reduced by about 12.5%. When room light exposure continues for the entire night, total daily melatonin is suppressed by more than 50% in most individuals, with median suppression of 73.7%. These findings suggest that exposure to electrical room light before bedtime and during the normal hours of sleep (e.g. during shift work) may impact physiological processes regulated by melatonin signaling, such as sleepiness, thermoregulation, blood pressure, and perhaps even glucose homeostasis.
Room light suppresses melatonin and shortens melatonin duration
We hypothesize that the later melatonin onset we observed during exposure to room light in the evening was due primarily to melatonin suppression, rather than phase shifting of the circadian system. In study 1, the earlier melatonin onset we observed on d 3 could be attributed, in part, to a net phase advance of the circadian system, because participants were exposed to room light in the morning and early afternoon and dim light in the late afternoon and evening. Hence, individuals were exposed to higher light levels during the predicted phase-advance region of the phase-response curve, compared with the phase-delay region (30) (Fig. 1A). If phase shifting were principally responsible for the change in melatonin onset, we would expect a comparable shift in the timing of melatonin offset in the same direction. Rather, our results show that the timing of melatonin offset was unchanged. To examine this question in greater detail, we examined results from an additional set of 58 participants who completed a similar research protocol under different lighting conditions (see Supplemental Data). In that study, volunteers were exposed to room light until the end of the third baseline day, after which time the circadian system was released into constant conditions. Whereas 70.7% of individuals showed a later melatonin offset (41 of 58 participants) in dim light, consistent with drift of the circadian pacemaker (34), 86.2% of individuals showed an earlier melatonin onset (50 of 58) when exposed to dim light (<15 lux) on d 4, vs. room light on d 3 (Supplemental Figs. 2 and 3). These data suggest that the underlying circadian phase of melatonin onset on d 3 was masked by light exposure, presumably due to photic melatonin suppression. Consistent with this interpretation, in study 2, we observed that exposure to room light during the usual hours of sleep resulted in strong melatonin suppression in 84.6% of trials (Fig. 4). Some participants showed partial recovery from melatonin suppression during room light exposure, whereas others showed complete suppression of melatonin such that melatonin onset or offset could not be measured. By comparison, two participants showed weak melatonin suppression responses to room light. This inter-individual variability in melatonin suppression sensitivity is consistent with the dose-response function for melatonin suppression reported previously; room light (100–500 lux) falls on the steep linear part of the dose-response curve, such that small differences in corneal illuminance can result in large differences in melatonin suppression magnitude (25, 32).
A limitation of the present study is that the dim light and room light conditions were not balanced for order of presentation. We obtained similar results in a previous study, however, in which the order of light conditions was reversed, such that participants were exposed to dim light on d 1 (<3 lux), followed by 2 d in room light (<200 lux). In that study, melatonin onset also occurred substantially later in room light compared with dim light (by about 90 min), suggesting that the order of room light vs. dim light in the present study did not affect the primary outcomes (35). Another limitation of our study is that participants were exposed to long durations of room light or dim light (16 or 8 h) before sleep, whereas in the real world, individuals often choose to turn on, or turn off, electrical lights closer to bedtime. Also, illumination levels that people are exposed to during the daytime could potentially modulate melatonin suppression responses to electrical light at night (e.g. by sensitizing or desensitizing melatonin suppression responses). Previously, we showed that the suppressive effect of room light on melatonin synthesis was reduced by about 15% when participants were exposed to room light, instead of dim light, during the daytime (28). In the present study, participants were also exposed to room light during the day, which may have decreased melatonin suppression sensitivity at night; nonetheless, melatonin levels were strongly suppressed by room light (by about 70%) before bedtime and during the usual hours of sleep (Figs. 2–4).
Potential implications of melatonin suppression by electrical lighting
In modern society, people are routinely exposed to electrical lighting during evening hours, after the onset of melatonin production, to partake in work, recreational, and social activities. Our results demonstrate that this indoor room light profoundly alters the timing, duration, and amount of melatonin synthesis, the health consequences of which are unknown. Melatonin is the body's internal representation of night duration, or scotoperiod, and is sensitive to changes in season in humans (5, 36). Chronic exposure to evening electrical lighting extends the photoperiod and shortens the scotoperiod, which is equivalent to placing modern humans in a continual biological summer. This could, in turn, have effects on metabolic function via alteration of melatonin secretion directly (18–20) or indirectly via altered sleep duration (37).
The effects of exogenous melatonin on human physiology suggest that this hormone plays a role in regulating body temperature, blood pressure, and sleepiness (12, 17, 38). Given the ability of melatonin to inhibit linoleic acid uptake, melatonin has also been proposed as a treatment option for inhibiting cancer progression (39). In an animal model for human cancer in which nude rats were implanted with breast cancer xenografts, perfusion with blood taken from women exposed to dim light at night, when melatonin is released, reduced tumor growth markedly (40). In contrast, blood taken from women who had been exposed to bright light at night, which drastically reduced levels of plasma melatonin, did not affect growth rate of xenografts. Rates of breast cancer are especially high in chronic shift workers (41–43), most of whom are exposed to light that is of sufficient intensity to suppress nighttime melatonin levels. This, coupled with the finding that the rate of breast cancer is lower in blind women without light perception (44–46), raises the possibility that chronic light suppression of melatonin may increase the relative risk for some types of cancer (47), an idea that was proposed nearly 25 yr ago by Richard Stevens as part of his light-at-night theory (48, 49). Chronically exposing oneself to light at night could also increase cancer risk by disrupting circadian clock function; continuous exposure to light disrupts behavioral rhythms and increases tumor malignity in C57BL/6 mice, even though these animals do not secrete melatonin (50). If further research substantiates melatonin suppression as a significant risk factor for breast cancer, our results demonstrating strong suppression of melatonin with evening room light could have important health implications. Moreover, in future studies, it will be important to determine whether small, chronic changes in sleep, melatonin, and circadian phase, as experienced every day in many nonshift workers, might present a health risk in vulnerable individuals (49). Given that melatonin receptor genes have recently been linked to the pathogenesis of type 2 diabetes (18–20, 51), it is possible that disruption of melatonin signaling by exposure to light at night could contribute to the increased risk for developing metabolic syndrome and type 2 diabetes in shift workers. Hence, future work should focus on determining the mechanisms by which melatonin regulates glucose metabolism and the consequences of inhibiting melatonin receptor signaling on blood glucose and insulin levels (51, 52).
In conclusion, our findings demonstrate that melatonin levels are remarkably sensitive to room light levels, with exposure before bedtime resulting in strong suppression of melatonin synthesis. As a result, exposure to room light into the late evening has the effect of shortening melatonin duration, thereby truncating the body's internal representation of solar night. With growing evidence that melatonin receptor signaling plays an important role in regulating human physiology, in future studies, it will be important to determine the impact of chronic nighttime exposure to electrical lighting on melatonin suppression and morbidity.
Acknowledgments
We thank research volunteers, subject recruiters, and research staff at the Division of Sleep Medicine, BWH. We also thank the technicians and medical staff of the General Clinical Research Center for their assistance in carrying out the inpatient protocols.
This work was supported by M01 RR02635, Brigham and Women's Hospital General Clinical Research Center and 1 UL1 RR025758 Harvard Clinical and Translational Science Center, from the National Center for Research Resources; NIH/NHLBI T32-HL07901 (to J.J.G.); NIMH R01 MH45130 (to C.A.C.); NCCAM R01 AT002129 (to S.W.L.); NINDS R01 NS36590 (George C. Brainard, Ph.D.); E.V.R., C.A.C., and S.W.L. are supported in part by the National Space Biomedical Research Institute through NASA NCC 9-58. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Clinical Trial Registration Number: NCT00200863.
Disclosure Summary: J.J.G., K.C., K.A.S., S.B.S.K., E.V.R., and J.M.Z. have nothing to declare. S.M.W.R. consults for Vanda Pharmaceuticals, Inc., through Monash University. C.A.C. has received consulting fees from or served as a paid member of scientific advisory boards for Cephalon, Inc.; Eli Lilly and Co.; Johnson & Johnson; Koninklijke Philips Electronics, N.V./Philips Respironics, Inc.; Sanofi-Aventis Groupe; Sepracor, Inc.; Somnus Therapeutics, Inc.; Vanda Pharmaceuticals, Inc.; and Zeo, Inc. CAC has equity interests in Lifetrac, Inc.; Somnus Therapeutics, Inc.; Vanda Pharmaceuticals, Inc.; and Zeo, Inc. C.A.C. has received lecture fees from the Accreditation Council of Graduate Medical Education; American Academy of Sleep Medicine; Duke University School of Medicine; Harvard School of Public Health; Mount Sinai School of Medicine; National Academy of Sciences; National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK/NIH); North East Sleep Society; Office of Rare Diseases Research (NIH); Sanofi-Aventis, Inc.; Society for Obstetric Anesthesia and Perinatology (SOAP); St. Luke's Roosevelt Hospital; University of Virginia Medical Center; University of Washington Medical Center; and University of Wisconsin Medical School. C.A.C. has received royalties from McGraw Hill, the New York Times, and Penguin Press. C.A.C. has received research prizes with monetary awards from the American Academy of Sleep Medicine and the American Clinical and Climatologic Association. C.A.C. has received clinical trial research contracts from Cephalon, Inc., and his research laboratory at the Brigham and Women's Hospital has received unrestricted research and education funds and/or support for research expenses from Cephalon, Inc.; Koninklijke Philips Electronics, N.V.; and ResMed. The Harvard Medical School Division of Sleep Medicine (HMS/DSM), which C.A.C. directs, has received unrestricted research and educational gifts and endowment funds from Boehringer Ingelheim Pharmaceuticals, Inc.; Cephalon, Inc.; George H. Kidder, Esq.; Gerald McGinnis; GlaxoSmithKline; Herbert Lee; Hypnion; Jazz Pharmaceuticals; Jordan's Furniture; Merck & Co., Inc.; Peter C. Farell, Ph.D.; Pfizer; ResMed; Respironics, Inc.; Sanofi-Aventis, Inc.; Sealy, Inc.; Sepracor, Inc.; Simmons; Sleep Health Centers LLC; Spring Aire; Takeda Pharmaceuticals; and Tempur-Pedic. The HSM/DSM Sleep and Health Education Program has received Educational Grant funding from Cephalon, Inc.; Takeda Pharmaceuticals; Sanofi-Aventis, Inc.; and Sepracor, Inc. C.A.C. is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc., and holds a number of process patents in the field of sleep/circadian rhythms (e.g. photic resetting of the human circadian pacemaker). S.W.L. has received federal research grants to study the effects of light on circadian rhythms and is Principal Investigator on investigator-initiated grants from Apollo Lighting; Philips Lighting; Alcon, Inc.; and Philips Respironics. S.W.L. has a service agreement with Vanda Pharmaceuticals and consults for a federally funded project at Thomas Jefferson University. S.W.L. has received honoraria and/or travel support from the American Society for Photobiology; Harvard University Summer School; Illinois Coalition for Responsible Outdoor Lighting; International Graduate School of Neuroscience; Lightfair; New York Academy of Science; North East Sleep Society; Philips Lighting; Thomas Jefferson University; Utica College; Velux; Woolcock Institute of Medical Research; and Wyle Integrated Science and Engineering (NASA). S.W.L. is a coinventor on a process patent for the use of short-wavelength light for resetting the human circadian pacemaker and improving alertness and performance which is assigned to the Brigham and Women's Hospital. S.W.L. has received unrestricted equipment gifts from Philips Lighting and Bionetics Corp. Lamps obtained from Philips Lighting were used to provide standard ambient lighting for the experiments described in the manuscript. S.W.L. has received unrestricted monetary gifts to support research from Swinburne University of Technology, Australia; and Optalert, Pty, Ltd. S.W.L. has received an advance author payment from Oxford University Press for writing a book on sleep.
Footnotes
- AUC
- Area under the curve
- GCRC
- General Clinical Research Center
- IQR
- interquartile range.
References
- 1. Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. 1980. Light suppresses melatonin secretion in humans. Science 210:1267–1269 [DOI] [PubMed] [Google Scholar]
- 2. Shanahan TL, Czeisler CA. 1991. Light exposure induces equivalent phase shifts of the endogenous circadian rhythms of circulating plasma melatonin and core body temperature in men. J Clin Endocrinol Metab 73:227–235 [DOI] [PubMed] [Google Scholar]
- 3. Bittman EL, Karsch FJ, Hopkins JW. 1983. Role of the pineal gland in ovine photoperiodism: regulation of seasonal breeding and negative feedback effects of estradiol upon luteinizing hormone secretion. Endocrinology 113:329–336 [DOI] [PubMed] [Google Scholar]
- 4. Reiter RJ. 1980. The pineal and its hormones in the control of reproduction in mammals. Endocr Rev 1:109–131 [DOI] [PubMed] [Google Scholar]
- 5. Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C, Bender C. 1993. Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol 265:R846–R857 [DOI] [PubMed] [Google Scholar]
- 6. Kauppila A, Kivelä A, Pakarinen A, Vakkuri O. 1987. Inverse seasonal relationship between melatonin and ovarian activity in humans in a region with a strong seasonal contrast in luminosity. J Clin Endocrinol Metab 65:823–828 [DOI] [PubMed] [Google Scholar]
- 7. Roenneberg T, Aschoff J. 1990. Annual rhythm of human reproduction. II. Environmental correlations. J Biol Rhythms 5:217–239 [DOI] [PubMed] [Google Scholar]
- 8. Roenneberg T, Aschoff J. 1990. Annual rhythm of human reproduction. I. Biology, sociology, or both? J Biol Rhythms 5:195–216 [DOI] [PubMed] [Google Scholar]
- 9. Hughes RJ, Badia P. 1997. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep 20:124–131 [PubMed] [Google Scholar]
- 10. Rajaratnam SM, Middleton B, Stone BM, Arendt J, Dijk DJ. 2004. Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans. J Physiol 561:339–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stone BM, Turner C, Mills SL, Nicholson AN. 2000. Hypnotic activity of melatonin. Sleep 23:663–669 [PubMed] [Google Scholar]
- 12. Wyatt JK, Dijk DJ, Ritz-de Cecco A, Ronda JM, Czeisler CA. 2006. Sleep-facilitating effect of exogenous melatonin in healthy young men and women is circadian-phase dependent. Sleep 29:609–618 [DOI] [PubMed] [Google Scholar]
- 13. Vanĕcek J, Pavlík A, Illnerová H. 1987. Hypothalamic melatonin receptor sites revealed by autoradiography. Brain Res 435:359–362 [DOI] [PubMed] [Google Scholar]
- 14. Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. 2000. Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol 164:R1–R6 [DOI] [PubMed] [Google Scholar]
- 15. Sack RL, Brandes RW, Kendall AR, Lewy AJ. 2000. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med 343:1070–1077 [DOI] [PubMed] [Google Scholar]
- 16. Arangino S, Cagnacci A, Angiolucci M, Vacca AM, Longu G, Volpe A, Melis GB. 1999. Effects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am J Cardiol 83:1417–1419 [DOI] [PubMed] [Google Scholar]
- 17. Reid K, Van den Heuvel C, Dawson D. 1996. Day-time melatonin administration: effects on core temperature and sleep onset latency. J Sleep Res 5:150–154 [DOI] [PubMed] [Google Scholar]
- 18. Bouatia-Naji N, Bonnefond A, Cavalcanti-Proença C, Sparsø T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, De Graeve F, Chèvre JC, Borch-Johnsen K, Hartikainen AL, Ruokonen A, Tichet J, Marre M, Weill J, Heude B, Tauber M, Lemaire K, Schuit F, Elliott P, Jørgensen T, Charpentier G, Hadjadj S, Cauchi S, Vaxillaire M, Sladek R, Visvikis-Siest S, Balkau B, Lévy-Marchal C, Pattou F, Meyre D, Blakemore AI, Jarvelin MR, Walley AJ, Hansen T, Dina C, Pedersen O, Froguel P. 2009. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet 41:89–94 [DOI] [PubMed] [Google Scholar]
- 19. Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. 2009. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 41:82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, Potter SC, Erdos MR, Sanna S, Hottenga JJ, Wheeler E, Kaakinen M, Lyssenko V, Chen WM, Ahmadi K, Beckmann JS, Bergman RN, Bochud M, Bonnycastle LL, Buchanan TA, Cao A, Cervino A, Coin L, Collins FS, Crisponi L, de Geus EJ, Dehghan A, Deloukas P, Doney AS, Elliott P, Freimer N, Gateva V, Herder C, Hofman A, Hughes TE, Hunt S, Illig T, Inouye M, Isomaa B, Johnson T, Kong A, Krestyaninova M, Kuusisto J, Laakso M, Lim N, Lindblad U, Lindgren CM, McCann OT, Mohlke KL, Morris AD, Naitza S, Orrù M, Palmer CN, Pouta A, Randall J, Rathmann W, Saramies J, Scheet P, Scott LJ, Scuteri A, Sharp S, Sijbrands E, Smit JH, Song K, Steinthorsdottir V, Stringham HM, Tuomi T, Tuomilehto J, Uitterlinden AG, Voight BF, Waterworth D, Wichmann HE, Willemsen G, Witteman JC, Yuan X, Zhao JH, Zeggini E, Schlessinger D, Sandhu M, Boomsma DI, Uda M, Spector TD, Penninx BW, Altshuler D, Vollenweider P, Jarvelin MR, Lakatta E, Waeber G, Fox CS, Peltonen L, Groop LC, Mooser V, Cupples LA, Thorsteinsdottir U, Boehnke M, Barroso I, Van Duijn C, Dupuis J, Watanabe RM, Stefansson K, McCarthy MI, Wareham NJ, Meigs JB, Abecasis GR. 2009. Variants in MTNR1B influence fasting glucose levels. Nat Genet 41:77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Drew JE, Barrett P, Mercer JG, Moar KM, Canet E, Delagrange P, Morgan PJ. 2001. Localization of the melatonin-related receptor in the rodent brain and peripheral tissues. J Neuroendocrinol 13:453–458 [DOI] [PubMed] [Google Scholar]
- 22. Sánchez-Barceló EJ, Mediavilla MD, Tan DX, Reiter RJ. 2010. Clinical uses of melatonin: evaluation of human trials. Curr Med Chem 17:2070–2095 [DOI] [PubMed] [Google Scholar]
- 23. Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. 1996. Dose-response relationships for resetting of human circadian clock by light. Nature 379:540–542 [DOI] [PubMed] [Google Scholar]
- 24. Laakso ML, Hätönen T, Stenberg D, Alila A, Smith S. 1993. One-hour exposure to moderate illuminance (500 lux) shifts the human melatonin rhythm. J Pineal Res 15:21–26 [DOI] [PubMed] [Google Scholar]
- 25. Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. 2000. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol 526(Pt 3):695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Osterhaus W, Office lighting: a review of 80 years of standards and recommendations. IEEE Industry Applications Society Annual Meeting, Toronto, Ontario, Canada, 1993 [Google Scholar]
- 27. Jasser SA, Hanifin JP, Rollag MD, Brainard GC. 2006. Dim light adaptation attenuates acute melatonin suppression in humans. J Biol Rhythms 21:394–404 [DOI] [PubMed] [Google Scholar]
- 28. Smith KA, Schoen MW, Czeisler CA. 2004. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab 89:3610–3614 [DOI] [PubMed] [Google Scholar]
- 29. Boivin DB, James FO. 2002. Phase-dependent effect of room light exposure in a 5-h advance of the sleep-wake cycle: implications for jet lag. J Biol Rhythms 17:266–276 [DOI] [PubMed] [Google Scholar]
- 30. Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. 2003. A phase response curve to single bright light pulses in human subjects. J Physiol 549:945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. 2010. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med 2:31ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lockley SW, Brainard GC, Czeisler CA. 2003. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab 88:4502–4505 [DOI] [PubMed] [Google Scholar]
- 33. Duffy JF, Dijk DJ. 2002. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms 17:4–13 [DOI] [PubMed] [Google Scholar]
- 34. Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. 1999. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284:2177–2181 [DOI] [PubMed] [Google Scholar]
- 35. Wright KP, Jr, Gronfier C, Duffy JF, Czeisler CA. 2005. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms 20:168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Owen J, Arendt J. 1992. Melatonin suppression in human subjects by bright and dim light in Antarctica: time and season-dependent effects. Neurosci Lett 137:181–184 [DOI] [PubMed] [Google Scholar]
- 37. Spiegel K, Tasali E, Leproult R, Van Cauter E. 2009. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol 5:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simko F, Paulis L. 2007. Melatonin as a potential antihypertensive treatment. J Pineal Res 42:319–322 [DOI] [PubMed] [Google Scholar]
- 39. Blask DE, Sauer LA, Dauchy RT, Holowachuk EW, Ruhoff MS, Kopff HS. 1999. Melatonin inhibition of cancer growth in vivo involves suppression of tumor fatty acid metabolism via melatonin receptor-mediated signal transduction events. Cancer Res 59:4693–4701 [PubMed] [Google Scholar]
- 40. Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, Sauer LA, Rivera-Bermudez MA, Dubocovich ML, Jasser SA, Lynch DT, Rollag MD, Zalatan F. 2005. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res 65:11174–11184 [DOI] [PubMed] [Google Scholar]
- 41. Hansen J. 2001. Light at night, shiftwork, and breast cancer risk. J Natl Cancer Inst 93:1513–1515 [DOI] [PubMed] [Google Scholar]
- 42. Hansen J. 2001. Increased breast cancer risk among women who work predominantly at night. Epidemiology 12:74–77 [DOI] [PubMed] [Google Scholar]
- 43. Tokumaru O, Haruki K, Bacal K, Katagiri T, Yamamoto T, Sakurai Y. 2006. Incidence of cancer among female flight attendants: a meta-analysis. J Travel Med 13:127–132 [DOI] [PubMed] [Google Scholar]
- 44. Feychting M, Osterlund B, Ahlbom A. 1998. Reduced cancer incidence among the blind. Epidemiology 9:490–494 [PubMed] [Google Scholar]
- 45. Flynn-Evans EE, Stevens RG, Tabandeh H, Schernhammer ES, Lockley SW. 2009. Total visual blindness is protective against breast cancer. Cancer Causes Control 20:1753–1756 [DOI] [PubMed] [Google Scholar]
- 46. Hahn RA. 1991. Profound bilateral blindness and the incidence of breast cancer. Epidemiology 2:208–210 [DOI] [PubMed] [Google Scholar]
- 47. Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L, Cogliano V. 2007. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol 8:1065–1066 [DOI] [PubMed] [Google Scholar]
- 48. Stevens RG. 1987. Electric power use and breast cancer: a hypothesis. Am J Epidemiol 125:556–561 [DOI] [PubMed] [Google Scholar]
- 49. Stevens RG. 2009. Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. Int J Epidemiol 38:963–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Otálora BB, Madrid JA, Alvarez N, Vicente V, Rol MA. 2008. Effects of exogenous melatonin and circadian synchronization on tumor progression in melanoma-bearing C57BL6 mice. J Pineal Res 44:307–315 [DOI] [PubMed] [Google Scholar]
- 51. Contreras-Alcantara S, Baba K, Tosini G. 2010. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity (Silver Spring) 18:1861–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mühlbauer E, Gross E, Labucay K, Wolgast S, Peschke E. 2009. Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur J Pharmacol 606:61–71 [DOI] [PubMed] [Google Scholar]