Spontaneous thelarche and isolated menses occur in female GnRH deficiency; sequence variants in FRFR1, GNRHR, PROKR2, and KAL1 are the most commonly associated genetic abnormalities.
Abstract
Context:
GnRH deficiency is a rare genetic disorder of absent or partial pubertal development. The clinical and genetic characteristics of GnRH-deficient women have not been well-described.
Objective:
To determine the phenotypic and genotypic spectrum of a large series of GnRH-deficient women.
Design, Setting, and Subjects:
Retrospective study of 248 females with GnRH deficiency evaluated at an academic medical center between 1980 and 2010.
Main Outcome Measures:
Clinical presentation, baseline endogenous GnRH secretory activity, and DNA sequence variants in 11 genes associated with GnRH deficiency.
Results:
Eighty-eight percent had undergone pubarche, 51% had spontaneous thelarche, and 10% had 1–2 menses. Women with spontaneous thelarche were more likely to demonstrate normal pubarche (P = 0.04). In 27% of women, neuroendocrine studies demonstrated evidence of some endogenous GnRH secretory activity. Thirty-six percent (a large excess relative to controls) harbored a rare sequence variant in a gene associated with GnRH deficiency (87% heterozygous and 13% biallelic), with variants in FGFR1 (15%), GNRHR (6.6%), and PROKR2 (6.6%) being most prevalent. One woman had a biallelic variant in the X-linked gene, KAL1, and nine women had heterozygous variants.
Conclusions:
The clinical presentation of female GnRH deficiency varies from primary amenorrhea and absence of any secondary sexual characteristics to spontaneous breast development and occasional menses. In this cohort, rare sequence variants were present in all of the known genes associated with GnRH deficiency, including the novel identification of GnRH-deficient women with KAL1 variants. The pathogenic mechanism through which KAL1 variants disrupt female reproductive development requires further investigation.
Isolated GnRH deficiency is a disorder of hypogonadism attributable to low or inappropriately normal gonadotropins resulting in absent or incomplete puberty, often seen in association with nonreproductive phenotypes such as craniofacial, skeletal, neurologic, renal, and olfactory abnormalities (1). The olfactory phenotype has traditionally been used to subdivide these patients into normosmic idiopathic hypogonadotropic hypogonadism (nIHH) and anosmic [Kallmann's syndrome (KS)] variants. Although females with GnRH deficiency were included in Kallmann's original report of the familial nature of this disorder, men have been the focus of much of the subsequent scientific literature because of the significant excess of males to females reported with this condition (1).
Rare sequence variants (RSVs) in genes involved in GnRH neuronal migration (FGF8, FGFR1, KAL1, PROK2, PROKR2, and NELF), secretion (GNRH1, GPR54, TAC3, and TACR3) and receptivity (GNRHR) have been reported to contribute to GnRH deficiency in both men and women (reviewed in Ref. 1), although the relative frequency of the RSVs in each gene has not been investigated in a large female cohort. An important exception is the X-linked gene, KAL1, in which RSVs have only been found in GnRH-deficient men. However, because of the assumption that female GnRH deficiency could not be explained by a RSV in a gene associated with an X-linked recessive disorder and the absence of a reproductive phenotype in a small number of obligate KAL1 female carriers (2–4), there are fewer than 100 published cases in which KAL1 has been screened in GnRH-deficient women (3–6).
Systematic clinical investigation has broadened the phenotype of male GnRH deficiency to include not only severe congenital hypogonadism but also late pubertal arrest (7), adult-onset disease (8), and even adult reversal (9). Recent genetic studies of male probands and their families suggest that a broader phenotypic spectrum may also exist in women (10–13), but this hypothesis has not been addressed systematically.
Through detailed phenotypic and genotypic profiling of a large cohort of females with isolated GnRH deficiency, the current study demonstrates a clinical spectrum in both breast development and menses. It also reveals the relative frequency of RSVs in the genes implicated in normal GnRH function, including the unexpected finding of KAL1 RSVs in 6.2% of this entirely female cohort.
Subjects and Methods
Patient population
The cohort comprised 248 females referred to an academic medical center for presumed isolated GnRH deficiency between 1980 and 2010. Ninety-six were patients of physicians in the Reproductive Endocrine Unit (REU) of Massachusetts General Hospital. The remainder were self-referred or referred by physicians from around the world in response to a clinical trial posting and completed testing by mail (questionnaire, smell testing, blood samples for DNA isolation). All women were ≥16 yr old at the time of evaluation, had low estradiol (E2) levels in the face of low or inappropriately normal gonadotropins, no other pituitary hormone deficiencies, and no neuroanatomic or functional cause of hypogonadotropic hypogonadism. None of the women had a known eating disorder, each had achieved the minimum weight for height necessary for the onset of menstrual cycles (14), and none exercised excessively [defined as greater than 20 miles per week of running or its equivalent (15)].
Both phenotypic and genotypic information was available in 207 women, of whom 62 had baseline neuroendocrine sampling. The majority (61%) was tested for all 11 genes, while 85% were tested for ≥5 genes. The remaining women had detailed phenotypic information, but either DNA was not available or they were not included in RSV frequency calculations because they were female relatives of the proband who harbored the same RSV. Complete DNA sequencing of the 11 genes was performed in 80–160 or 200–350 alleles from female or male controls, respectively, who had normal reproductive function by history and physical examination. Each KAL1 RSV identified in GnRH-deficient women was tested in 870 X-chromosomes (from male and female controls).
This study was approved by the Massachusetts General Hospital Human Research Committee, and signed informed consent was obtained from each subject before participation.
Phenotyping
Clinical assessment
A detailed questionnaire was administered to all subjects to assess family history, ethnicity, height, weight, eating attitudes and behaviors, dysmorphic features, pubertal development, and results of brain imaging. Categorization of anosmia was based on history alone or on the results of olfactory testing (40-item University of Pennsylvania Smell Identification Test) (16). For statistical purposes, women scoring ≥5th% based on age were coded as normosmic and all others were coded as anosmic. Ovarian volumes were determined by transvaginal or transabdominal ultrasound and calculated with the formula for an ellipse (V = 0.52 × maximal longitudinal × antero-posterior × transverse diameters).
Neuroendocrine evaluation
Hormone replacement was discontinued for at least one month before initial REU evaluation. Frequent blood sampling (every 10 min overnight for 12 h) was performed to assess endogenous GnRH secretion as manifested by LH pulsatility. FSH and E2 were assayed from pools from these frequent sampling studies. Pulsatile LH was analyzed using a validated modification of the Santen and Bardin method (17, 18). Results were compared with those previously reported for 17 normally cycling women studied during the early follicular phase (EFP) of an ovulatory cycle (19, 20). Women with GnRH deficiency were classified as having low amplitude or low frequency LH pulse patterns if their levels were more than two sd's below the normal range [LH amplitude 2.3 ± 1.0, frequency 7 ± 1.8 pulses per 12-hour (mean ± sd)]. Women whose pulse frequency and amplitude were indistinguishable from controls were further evaluated for a pattern of sleep augmentation, as previously described (21).
Detection of DNA sequence variants
Genomic DNA was obtained from peripheral blood samples by standard phenol-chloroform extraction. Exonic and proximal intronic (≤15 bp from splice sites) DNA sequences of 11 genes implicated in the etiology of GnRH deficiency were amplified by PCR and determined by direct sequencing. These genes include KAL1 (anosmin-1, OMIM 308700), GNRH1 (gonadotropin-releasing hormone 1, OMIM 152760) GNRHR (GnRH receptor, OMIM 138850), GPR54 (KISS1 receptor, OMIM 604161), NELF (nasal embryonic LHRH factor, OMIM 608137), FGF8 (fibroblast growth factor 8, OMIM 600483), FGFR1 (fibroblast growth factor receptor 1, OMIM 136350), PROK2 (prokineticin 2, OMIM 607002), PROKR2 (prokineticin receptor 2, OMIM 607212), TAC3 (tachykinin 3, OMIM 162330), and TACR3 (tachykinin receptor 3, OMIM 162332). One KS woman with features of CHARGE syndrome was also tested for CHD7 (chromodomain helicase DNA-binding protein 7, OMIM 608892). PCR primers and amplification conditions for each gene have been described previously (3, 22–30). All sequence variations were observed on both DNA strands and were confirmed in a separate PCR. Homozygosity for the KAL1 Q131H variant was confirmed by multiplex ligation-dependent probe amplification (MLPA, MRC-Holland, The Netherlands). Genes and proteins are described using standard nomenclature (31). Presentation of results is restricted to sequence variants that were 1) at splice junctions within 5 bp of coding sequence, or 2) in coding sequence and nonsynonymous; and 3) present in <1% of control alleles. Rare synonymous changes were also compared between cases and controls for internal validation.
Functional analysis
The in vitro functional data reported has been previously described (10, 12, 23–26, 30, 32–36). Where in vitro data were not available, five different prediction programs were used to determine the potential significance of missense variants and one prediction program was used for intronic changes. These included PolyPhen (37), Mutation Taster (38), Panther (39), SIFT (40), pMUT (41), and Human Splicing Finder (42). In vitro data and prediction program results are presented in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org/).
Mode of inheritance
Phenotypic characterizations of the proband and family members were used to determine the mode of inheritance as previously described (7). No families in this cohort met the definition of X-linked recessive or X-linked dominant inheritance, although the latter could not always be distinguished from autosomal dominant inheritance (i.e., in cases of small pedigrees with transmission of the trait through the female). A family was classified as sporadic if no other relatives were affected and as unknown if no pedigree information was available.
Assays
Serum LH and FSH were measured using a two-site monoclonal nonisotopic system (Axsym; Abbott Laboratories, Abbott Park, IL) as previously described (43–46), and expressed in international units per liter (IU/liter) of the Pituitary 2nd International Standard 80/552. Estradiol was measured by two different RIAs using highly specific antisera with a functional sensitivity of ≤20 pg/ml (73.4 pmol/liter) which were cross-referenced (47, 48).
Statistical methods
Data are expressed as the mean ± se unless otherwise indicated. Because of the association of a more severe reproductive phenotype with anosmia in men with GnRH deficiency, comparisons were performed between nIHH and KS women using independent samples t tests (for parametric data) and Wilcoxon Rank Sum tests (for nonparametric data) for continuous variables. χ2 or Fischer's exact test were used as tests of association and to compare categorical variables between nIHH and KS women. A P value of <0.05 was considered to be statistically significant.
Results
Phenotype studies
Clinical presentation
The clinical and biochemical features of the 248 women are summarized in Table 1. Forty-seven percent of women were anosmic. As the women presented for evaluation at a relatively advanced age (mean 28.5 yr), nearly all had undergone at least some treatment with hormone replacement before presentation. However, before hormone replacement, 88% had undergone normal pubarche, 51% had some degree of breast development, and 10% had one or two spontaneous menses with no difference noted between nIHH and KS women. Women with thelarche were more likely to demonstrate pubarche than those without (97% vs. 77%, P = 0.04) but did not have higher FSH (P = 0.3) or E2 levels (P = 0.09), or larger ovarian volumes (P = 0.6).
Table 1.
Clinical presentation of GnRH-deficient women
| Full cohort (n = 248) | KS women (n = 116) | nIHH women (n = 132) | P value | |
|---|---|---|---|---|
| Age, yr | 28.5 ± 0.9 | 27.2 ± 1.3 | 29.8 ± 1.3 | 0.16 |
| BMI, kg/m2 | 25.4 ± 2 | 26.5 ± 2.6 | 23.1 ± 2.4 | 0.43 |
| Thelarche, % | 51% (44/86) | 50% (20/40) | 52% (24/46) | 1 |
| Menarche, % | 10% (10/98) | 8% (4/48) | 12% (6/50) | 0.7 |
| Pubarche, % | 88% (49/56) | 84% (21/25) | 90% (28/31) | 0.7 |
| Ovarian volume, cc | 2.8 ± 0.3 (n = 39) | 2.0 ± 0.4 (n = 18) | 3.4 ± 0.4 (n = 21) | 0.02 |
| LH, IU/liter | 0.6 ± 0.1 (n = 85) | 0.5 ± 0.1 (n = 44) | 0.7 ± 0.2 (n = 41) | 0.13 |
| FSH, IU/liter | 1.5 ± 0.2 (n = 85) | 1.2 ± 0.3 (n = 44) | 1.7 ± 0.2 (n = 41) | 0.12 |
| Baseline study | ||||
| Apulsatile, % | 73% (51/70) | 70% (24/34) | 75% (27/36) | 0.8a |
| Pulsatile, % | 27% (19/70) | 30% (10/34) | 25% (9/36) | |
| Low frequency and/or amplitude, % | 19% (13/70) | 21% (7/34) | 17% (6/36) | |
| Normal frequency and amplitude, % | 8% (6/70) | 9% (3/34) | 8% (3/36) |
Represents pulsatile vs. apulsatile patterns in KS vs. nIHH.
Neuroendocrine and ultrasonographic studies
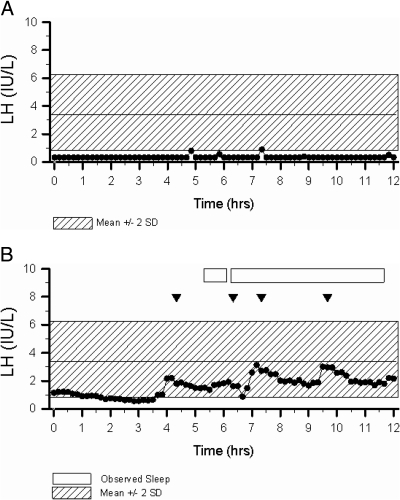
E2 levels were low, nearly all being undetectable [<20 pg/ml (<73.4 pmol/liter)], with normal to low gonadotropins (Table 1). Mean ovarian volumes were smaller than in normal adult women [mean 9.5 cc; 95% confidence interval (3.9–15.9 cc)] (49), and nIHH women had larger ovarian volumes than KS women (P = 0.02) with a tendency for higher gonadotropin levels (Table 1). Normosmic and KS women had similar patterns of LH secretion with the majority (75 and 70%, respectively) having an apulsatile pattern (Fig. 1A) and a smaller number demonstrating a low amplitude and/or low frequency pattern. In 8% of women (n = 6), the mean frequency and amplitude of LH pulses was within 2 sd of the EFP mean in normal women. Two of these women with evidence of pulsatile GnRH release exhibited a pattern of sleep augmentation that is characteristic of children in early puberty (Fig. 1B). The six women with LH pulse frequency and amplitude within the EFP range demonstrated a similar spectrum of clinical reproductive phenotypes, nonreproductive phenotypes [olfaction, age, body mass index (range 19–23)], and genetic variation as the overall cohort, but had higher gonadotropin levels (LH 2.0 ± 0.6 vs. 0.3 ± 0.07, P < 0.001; and FSH 4.2 ± 0.4 vs. 1.0 ± 0.14, P < 0.001) compared with women with absent pulses. Despite their mild phenotype, none of the six women with a more robust pattern of pulsatile LH secretion has developed menstrual cycles after up to twenty years of follow-up (Table 2). The overall group of women with evidence of some underlying GnRH secretion was no more likely to have experienced thelarche or menarche than women without LH pulses (P = 0.8 and P = 0.07, respectively). Women with menses (n = 10) were also not different from the overall cohort in terms of reproductive and nonreproductive phenotypes, body mass index, age, or LH secretory pattern.
Fig. 1.
A, Representative 12-h pattern of LH secretion in a 25-year-old woman with KS (Subject 17) who harbors two KAL1 RSVs demonstrates an absence of LH pulses. Shaded region represents the normal range for LH in healthy women with normal menstrual cycles in the EFP of their ovulatory menstrual cycles (19, 20). B, Representative 12-h pattern of LH secretion in a 27-year-old woman with nIHH (Subject 5) who carries a TACR3 RSV demonstrates pulses of similar amplitude and frequency to that seen in normal women during the EFP but with amplified pulses during sleep. Shaded region represents the normal range for LH in healthy women with normal ovulatory menstrual cycles in the EFP (19, 20). Arrowheads signify LH pulses, and boxes represent periods of observed sleep.
Table 2.
Phenotypic and genotypic characteristics of the six GnRH-deficient women with LH pulse patterns indistinguishable from normal early follicular phase women [previously shown to have a mean LH pulse amplitude of 2.3 IU/liter (95% CI 0.3–4.3), mean pulse frequency of 7 pulses/12 h (95% CI 3.4–10.6), and mean ovarian volume of 9.5 cc (95% CI 3.9–15.9)] (19, 20, 49)
| Subject | KS/nIHH | Thelarche/menarche | LH (IU/liter) | FSH (IU/liter) | Mean LH pulse amplitude (IU/liter) | LH pulse frequency (pulses/12 h) | Ovarian vol (cc) | Nonreproductive phenotype | Rare sequence variant | Follow-up (yrs) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | KS | +/+ | 0.6 | 5.2 | 0.6 | 5 | N.A. | N.A. | 16 | |
| 2 | KS | −/− | 3.8 | 5.3 | 0.5 | 9 | 8.8 | Agenesis of the CC, short 4th MC, high-arched palate | NEG | 6 |
| 3 | nIHH | +/− | 3.5 | 4.7 | 1.5 | 10 | 8.8 | PROKR2 het (R85Ca) | 4 | |
| 4 | KS | +/− | 2.6 | 4.3 | 0.4 | 5 | 1.4 | Hypoplastic olfactory bulbs and sulci, hearing loss | KAL1 het (K185N) | 20, pregnant with GnRH pump |
| 5 | nIHH | +/+ | 1.0 | 3.2 | 1.1 | 4 | 8 | 3 frontal incisors | TACR3 het (A449T) | 2 |
| 6 | nIHH | +/− | 0.6 | 2.8 | 2.7 | 5 | 4 | Gap between teeth, otosclerosis | NEG | 2, pregnant with gonadotropins |
CC, corpus callosum; MC, metacarpals; het, heterozygous; N.A., not assessed. Subject numbers are consistent across tables.
Loss of function variant.
Genetic studies
Detailed family histories were available in 148 women, revealing a familial pattern of GnRH deficiency in 66% (64% autosomal dominant, 36% autosomal recessive, 0% X-linked). Normosmic IHH cases were more likely to be familial (and autosomal recessive), whereas KS cases were more likely to be sporadic (P < 0.005; Supplemental Table 2).
Thirty-six percent of GnRH-deficient women harbored at least one RSV in a gene known to be associated with GnRH deficiency. This was significantly more than in control men and women (14%; P < 0.001) and suggests that most of the RSVs detected confer susceptibility to the GnRH deficiency phenotype. This difference was in large part attributable to an increased frequency of RSVs in FGFR1, GNRHR, PROKR2, and KAL1 in GnRH-deficient women compared with controls (Supplemental Table 3). As expected, the frequency of rare synonymous variants was not different between GnRH-deficient women and controls (8% and 12%, respectively, P = 0.1).
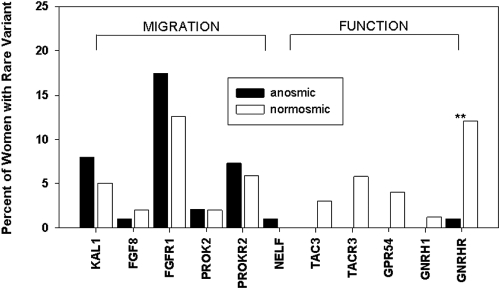
Seventy-six different RSVs were identified in GnRH-deficient women compared with 23 in controls (data not shown). Sixty-five of the GnRH-deficient women had an alteration in a single gene (86% heterozygous, 14% biallelic), whereas 10 had alterations in more than one gene (nine digenic, one trigenic) (Table 3). Fifty percent of RSVs were loss of function [frameshift or by previously reported in vitro testing (Supplemental Table 1)]. The remainder were either missense variants that have not been tested in vitro or intronic changes within 5 bps of the exon. Sixty-nine percent of these missense variants are predicted to be deleterious by two or more prediction programs. RSVs were most frequent in FGFR1, GNRHR, and PROKR2 (Fig. 2, Supplemental Table 3), and these genes also harbored the greatest number of unique RSVs. RSVs were also identified in FGF8 (1.5%), GNRH1 (0.7%), GPR54 (2%), NELF (1%), PROK2 (2%), TAC3 (2%), and TACR3 (3.6%). Although not every woman was sequenced for all 11 genes, the RSV frequency remained unchanged when limiting the analysis to the 120 women who were completely assessed.
Table 3.
Women with rare sequence variants in more than one gene associated with GnRH deficiency
| Subject | Variant gene #1 | Nucleotide change | Amino acid change | Homo/het | Variant gene #2 | Nucleotide change | Amino acid change | Homo/het | Variant gene #3 | Nucleotide change | Amino acid change | Homo/het |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | FGFR1 | c.716T>C | I239Ta | Het | GNRH1 | c.91C>T | R31Ca frame-shift | Het | PROKR2 | c.604A>G | S202G | Het |
| 8 | FGFR1 | c.1549-2A>G | Intronic | Het | KAL1 | c.1759G>T | V587L | Het | ||||
| 9 | FGFR1 | c.1409G>T | R470La | Het | GNRHR | c.317A>G | Q106Ra | Het | ||||
| GNRHR | c.785C>A | R262Qa | Het | |||||||||
| 10 | FGFR1 | c.350A>G | N117Sa | Het | GNRHR | c.247C>G | L83V | Het | ||||
| GNRHR | c.317A>G | Q106Ra | Het | |||||||||
| 11 | KAL1 | c.1464A>G | T472A | Het | PROKR2 | c.151G>A | A51T | Het | ||||
| 12 | FGFR1 | c.682T>G | Y228Da | Het | GPR54 | c.565G>A | A189T | Het | ||||
| 13 | PROK2 | c.70G>C | A24Pa | Het | PROKR2 | c.343G>A | V115ma | Het | ||||
| 14 | FGFR1 | c.854C>G | P285R | Het | KAL1 | c.1464A>G | T472A | Het | ||||
| 15 | KAL1 | c.1627G>A | V543I | Het | CHD7 | c.2440C>T | Q814Xa | Het | ||||
| 16 | FGFR1 | c165_171 deletion | Frame-shift | Het | PROKR2 | c.518T>G | L173Ra | Het |
Het, heterozygous. Subject numbers are consistent across tables.
Loss-of-function variant.
Fig. 2.
Frequency of rare sequence variants in genes involved in neuronal migration and function of GnRH in KS vs. nIHH women. **, GnRHR variants were more common in nIHH women than in KS women (P < 0.01). Variants in NELF were only identified in KS women, and variants in GNRH1, GPR54, TAC3, and TACR3 were only identified in nIHH women.
Notably, we have also identified KAL1 RSVs in 10 women with GnRH deficiency (6.2%; nine heterozygous, one biallelic) at a frequency that is similar to that of PROKR2. Seven KAL1 missense variants were identified, one of which is novel (T649M). The remaining RSVs in KAL1 were identified in a mixed cohort of men and women with GnRH deficiency, however detailed phenotypic information was not provided (50) (Tables 4 and 5, Supplemental Table 1). To date there is no validated functional in vitro assay for KAL1, and none of the women with KAL1 RSVs had affected male relatives. However, as in males, the majority of RSVs identified in this cohort fall within the fibronectin III domains (51). In addition, five of the seven RSVs (Q131H, K185N, P277T, V587L, and T659M) are predicted to be deleterious by at least one prediction program (Table 4). While not predicted to be deleterious, one of the RSVs (V543I) has previously been described in an unrelated man with severe KS in the absence of other genetic variants (50). Like the KS woman with this RSV (subject 15, Tables 4 and 5), this patient did not have synkinesia or renal agenesis. T472A was present in five GnRH-deficient women. T472A and V587L were present in 0.30% and 0.47%, respectively, of 870 control X-chromosomes; however, neither was present in any of the genomes screened thus far in the 1000 Genomes Project (www.1000genomes.org), arguing that each may be a susceptibility factor for GnRH deficiency that is not fully penetrant. Men with RSVs in KAL1 are more likely to demonstrate a more severe reproductive phenotype compared with other GnRH-deficient men (3, 7), however female KAL1 carriers displayed a similar spectrum of reproductive features to those not harboring these RSVs. Sixty percent of women with KAL1 RSVs were anosmic, whereas none demonstrated synkinesia or renal agenesis. Six women had a RSV only in KAL1, whereas four also harbored RSVs in other genes associated with GnRH deficiency (PROKR2, FGFR1, and CHD7).
Table 4.
Genotypic characterization of women with KAL1 variants
| Subject | Gene | Exon and domain | Nucleotide change | Amino acid change | Homo/het | Mono/oligogenic | Inheritance | Polyphen | SIFT | pMUT | Panther | Mutation taster | Control frequency |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | KAL1 | 5 (FNIII) | c.555G>C | K185N | Het | Mono | Sporadic | + | − | − | + | + | 0% |
| 8 | KAL1 | 12 (FNIII) | c.1759G>T | V587L | Het | FGFR1 | Autosomal dominant (surrogate marker)a | − | − | + | − | − | 0.47% |
| 11 | KAL1 | 10 (FNIII) | c.1464A>G | T472A | Het | PROKR2 | Unknown | + | − | − | − | − | 0.3% |
| 14 | KAL1 | 10 (FNIII) | c.1464A>G | T472A | Het | FGFR1 | Unknown | + | − | − | − | − | 0.3% |
| 15 | KAL1 | 12 (FNIII) | c.1627G>A | V543I | Het | CHD7 | Sporadic | − | − | − | − | − | 0% |
| 17 | KAL1 | 4 (WAP) | c.393G>T | Q131H | Homo | Mono | Sporadic | + | + | − | + | + | 0% |
| KAL1 | 6 (FNIII) | c.829C>A | P277T | Het | + | + | − | + | + | 0% | |||
| 18 | KAL1 | 10 (FNIII) | c.1464A>G | T472A | Het | Mono | Unknown | + | − | − | − | − | 0.3% |
| 19 | KAL1 | 10 (FNIII) | c.1464A>G | T472A | Het | Mono | Unknown | + | − | − | − | − | 0.3% |
| 20 | KAL1 | 10 (FNIII) | c.1464A>G | T472A | Het | Mono | Unknown | + | − | − | − | − | 0.3% |
| 21 | KAL1 | 13 (FNIII) | c.1946C>T | T649 m | Het | Mono | Sporadic | + | − | + | − | − | 0% |
Homo, Homozygous; Het, heterozygous; WAP, whey acidic protein; FNIII, fibronectin type III; +, not tolerated, pathological, probable, possible, deleterious, or disease causing. −, tolerated, neutral, benign, not deleterious. Subject numbers are consistent across tables.
Subject 8's mother harbored the same KAL1 variant and had delayed puberty, a validated surrogate marker of inheritance of GnRH deficiency.
Table 5.
Phenotypic characterization of women with KAL1 variants
| Subject | Reproductive phenotype | Nonreproductive phenotype | Olfaction |
|---|---|---|---|
| 4 | Tanner II breasts, normal pubarche, 1° amenorrhea, pulses of normal frequency and amplitude on baseline | Normal head CT | KS |
| 8 | No thelarche, 1° amenorrhea | Dental abnormalities, short fourth metacarpals | nIHH |
| 11 | Spontaneous thelarche, 1° amenorrhea | Retinitis pigmentosa | nIHH (not formally tested) |
| 14 | 1° amenorrhea | KS | |
| 15 | Spontaneous thelarche, 1° amenorrhea | CHARGE association: bilateral coloboma, ASD, developmental delay, hearing loss, ataxia, cleft lip (negative for 22q deletion) | KS |
| 17 | Tanner III breasts, 1° amenorrhea, apulsatile baseline | clinodactyly, flat nasal bridge, cannot fully extend elbows, normal sella turcica film | KS |
| 18 | 1° amenorrhea | nIHH (not formally tested) | |
| 19 | 1° amenorrhea | nIHH (not formally tested) | |
| 20 | 1° amenorrhea | KS | |
| 21 | KS |
Also of note, one woman with anosmia had a heterozygous GNRHR missense variant (Q106R) which has been identified in nIHH patients and shown to be pathogenic in in vitro studies (32, 24). While this woman had no additional defects in genes known to be associated with anosmia including FGF8, FGFR1, KAL1, PROK2, PROKR2, and NELF, her presentation suggests digenicity with an as yet undiscovered gene involved in neuronal migration.
Genotype/phenotype correlations
RSVs in GNRHR were more prevalent in nIHH compared with KS women (P < 0.01), and RSVs in GPR54, TAC3, and TACR3 were only present in nIHH women. Women with thelarche, isolated menses, or endogenous LH pulses did not exhibit a unique genetic signature, although these analyses are limited by the relatively small number of subjects with each RSV. Similarly, there was no specific phenotypic signature for any of the individual genes assessed.
The 10 women with RSVs in more than one gene did not appear to be more severely affected than those with a single gene RSV [50% vs. 62% with thelarche (P = 0.2), 11% vs. 16% with isolated menses (P = 1), respectively]. Although only three of the women with RSVs in more than one gene underwent frequent sampling to assess LH secretion, a spectrum of GnRH deficiency was observed (one low amplitude and low frequency pattern of LH secretion and two apulsatile). In digenic pedigrees, individuals with a larger number of affected genes were more likely to manifest GnRH deficiency as opposed to milder defects such as delayed puberty, anosmia, or cleft lip/palate as has been noted previously (50). For example, in one pedigree, the father carried a FGFR1 RSV (C55fsX45) and had anosmia, cleft lip/palate, and missing teeth, whereas his two daughters, who had RSVs in both FGFR1 (C55fsX45) and PROKR2 (L173R), were GnRH-deficient.
Discussion
Recent studies in patients with GnRH deficiency have provided remarkable insight into the genes that control GnRH neuronal development and function and have suggested that the clinical phenotype of GnRH deficiency may be broader than previously thought. Because of their minority status among GnRH-deficient patients, women have often been overlooked in focused studies of the genetics and clinical presentation of GnRH deficiency. In the current study, RSVs in all of the genes known to be associated with GnRH ontogeny and function, including KAL1, were identified in a large cohort of GnRH-deficient women. The large excess of RSVs in cases relative to controls argues strongly that the majority of these RSVs contribute to the clinical GnRH deficiency phenotype, which has been found to include both thelarche and occasional menses.
The traditional clinical description of the reproductive phenotype of female GnRH-deficiency has included absent thelarche and primary amenorrhea. In the current series of 248 women with GnRH-deficiency, the majority of women exhibited some degree of breast development and a small percent experienced isolated menses. As thelarche and menses are signs of early and prolonged estrogen production, respectively, neither would be expected to be highly prevalent in women with GnRH deficiency. While adrenarche and gonadarche are thought to proceed independently, it is noteworthy that women with spontaneous thelarche were more likely to have undergone pubarche, perhaps suggesting that aromatization of adrenal androgens contributes to early breast development in these patients. Alternatively, the association of thelarche and pubarche may reflect a permissive role of estrogen on pubic hair development (52).
There was no association between a history of thelarche and/or isolated menses and either FSH levels, estradiol levels, or evidence of pulsatile LH secretion. Our ability to ascertain an association between estradiol levels and thelarche may be limited by the sensitivity of the assay. Furthermore, frequent sampling studies were performed at the time of initial presentation which in most cases occurred several years after breast development had occurred by history. Finally, the possibility that this phenotypic discordance reflects a temporal decline in GnRH activity from the initial time of thelarche to the time of evaluation, as reported in GnRH-deficient men (7, 8), cannot be excluded.
Frequent sampling studies were consistent with absent pulsatile GnRH secretion in the majority of GnRH-deficient women, whereas some suggested enfeebled or more robust GnRH secretion. Of the six women with an LH pulse frequency and amplitude that was indistinguishable from EFP control women, none met criteria for functional hypothalamic amenorrhea, three were anosmic, four had primary amenorrhea, four had associated phenotypes, and three had RSVs in the genes associated with GnRH deficiency, suggesting that they should not be excluded from this cohort. Two of these women had augmentation of LH pulse amplitude during sleep, which is a feature of early puberty (53) that has also been observed in GnRH-deficient men (54). While it is possible that these women were assessed in the early stages of a reversal from a state of GnRH deficiency to normal GnRH production, none subsequently manifested any clinical signs of reproductive axis recovery in follow-up, arguing against this possibility. It has also been shown that genes implicated in GnRH deficiency may predispose to functional hypothalamic amenorrhea (55), raising the possibility that this subset of GnRH-deficient women with a more robust LH pulse pattern may bridge the gap between hypothalamic amenorrhea and more profound GnRH deficiency.
Genotypic analysis of this large female GnRH-deficient cohort identified a RSV in one or more of the genes known to be involved in GnRH neuron migration or function in more than a third of patients. To achieve an unbiased assessment of the phenotypic spectrum of female GnRH deficiency, we excluded women who appeared to be GnRH-deficient but in whom a diagnosis of functional hypothalamic amenorrhea could not be discounted, and thus this is likely an underestimate. FGFR1, GNRHR, and PROKR2 are the most commonly altered genes in GnRH-deficient women. RSVs in more than one gene were identified in 13% of women, providing further support for the importance of gene–gene interactions in the pathogenesis of GnRH deficiency (50). Interestingly, women with digenic or trigenic RSVs did not appear to be more severely affected than those with monogenic RSVs, although this conclusion is preliminary as not every woman has been screened for all 11 genes. This finding contrasts with that of a recent small study which reported a more severe phenotype among women with biallelic compared with monoallelic mutations in PROK2/PROKR2 (13). Further studies which include larger groups of digenic GnRH-deficient men and women will be necessary to determine how these genes interact to produce a given phenotype.
Our analysis also led to the unexpected identification of 10 GnRH-deficient women who harbor RSVs in KAL1. One KS woman (subject 17, Tables 4 and 5) was identified with a heterozygous and a homozygous KAL1 RSV, where the presence of two copies was confirmed using MLPA. Neither of these RSVs was present in 870 X-chromosomes from control men and women, and both are predicted to be deleterious by four prediction programs. In addition, no other genetic defects were identified in this patient. Taken together, these findings provide strong evidence that the KAL1 RSVs are pathogenic in this patient.
The heterozygous KAL1 RSVs found in the nine other women may also be pathogenic. Four of the five RSVs are predicted to be deleterious by at least one prediction program, an additional RSV has previously been identified as the sole genetic defect in a man with a severe form of KS (50), and all RSVs were seen in <1% of 870 control X-chromosomes. In addition, five of these women tested negative for RSVs in all other genes.
These findings raise the question of the mechanism through which a heterozygous variant on the X-chromosome can cause GnRH deficiency in women, because the KAL1 gene has been thought to escape X-inactivation in females. This supposition has been based upon the location of the KAL1 gene in the pseudoautosomal region of the X chromosome, the absence of a reproductive phenotype in a small number of obligate KAL1 female carriers (2–4), and the ability of oligonucleotide primers to amplify anosmin mRNA transcripts in mouse/human hybrid cell lines containing either the active or inactive X-chromosome (57). KAL1 mutations in males are then thought to involve functional inactivation of KAL1 and the failure of KAL1-related sequences on the Y chromosome to compensate for this loss of function.
One possibility is that KAL1 RSVs in these females may act in a dominant negative fashion rather than through the simple loss of function usually associated with recessive inheritance. However, the hypothesis of X-linked dominant inheritance runs counter to the X-linked recessive inheritance pattern observed in published KAL1 pedigrees. A second possibility is that KAL1 may undergo X-inactivation that varies with developmental stage or by tissue, in line with recent reports that have suggested that a gene's ability to escape inactivation is not “all-or-none” but may instead be incomplete and may vary between women and across tissues (56–58). In this scenario, the reproductive phenotype of KAL1 female carriers could be attributable to skewed (nonrandom) X-inactivation in these particular individuals, whereas the previously reported absence of such a phenotype in female KAL1 carriers (2–4) might reflect nonskewed (random) inactivation or inactivation skewed to favor continued expression of the normal allele. A final possibility is that GnRH-deficient women with KAL1 heterozygous variants carry additional genetic defects. While digenicity was confirmed in only four of the nine heterozygotes, additional, as yet undiscovered genetic defects may exist in the other five women. Further work will be required to distinguish these possibilities.
In summary, the phenotypic spectrum of isolated GnRH deficiency in women is broader than previously appreciated and does not differ between nIHH and KS women. In light of this phenotypic variability, dismissing the diagnosis of GnRH deficiency in women with spontaneous thelarche and isolated menses is not appropriate, as GnRH function may change over time and/or adrenarche may provide the substrates for early breast and endometrial development. Women with GnRH deficiency harbor RSVs in all of the genes implicated in this disorder, including KAL1. Further studies of KAL1 X-inactivation are necessary to fully understand the role of KAL1 in GnRH and olfactory neuron development in women.
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through R01 HD42708 and cooperative agreement U54 HD028138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and M01-RR-01066 National Institutes of Health/National Center for Research Resources, General Clinical Research Centers Program. Dr. Gusella received support from the Developmental Genome Anatomy Project (GM061354). Dr. Shaw received fellowship support from the National Institutes of Health (5T32 HD007396) and from the Scholars in Clinical Science program of Harvard Catalyst [The Harvard Clinical and Translational Science Center (Award #UL1 RR 025758) and financial contributions from Harvard University and its affiliated academic health care centers]. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Study registered with www.ClinicalTrials.gov ID# NCT00392756.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- E2
- Estradiol
- EFP
- early follicular phase
- KS
- Kallmann's syndrome
- nIHH
- normosmic idiopathic hypogonadotropic hypogonadism
- RSV
- rare sequence variant.
References
- 1. Semple RK, Topaloglu AK. 2010. The recent genetics of hypogonadotrophic hypogonadism – novel insights and new questions. Clin Endocrinol (Oxf) 72:427–435 [DOI] [PubMed] [Google Scholar]
- 2. Georgopoulos NA, Pralong FP, Seidman CE, Seidman JG, Crowley WF, Jr, Vallejo M. 1997. Genetic heterogeneity evidenced by low incidence of KAL-1 gene mutations in sporadic cases of gonadotropin-releasing hormone deficiency. J Clin Endocrinol Metab 82:213–217 [DOI] [PubMed] [Google Scholar]
- 3. Oliveira LM, Seminara SB, Beranova M, Hayes FJ, Valkenburgh SB, Schipani E, Costa EM, Latronico AC, Crowley WF, Jr, Vallejo M. 2001. The importance of autosomal genes in Kallmann syndrome: genotype-phenotype correlations and neuroendocrine characteristics. J Clin Endocrinol Metab 86:1532–1538 [DOI] [PubMed] [Google Scholar]
- 4. Sato N, Katsumata N, Kagami M, Hasegawa T, Hori N, Kawakita S, Minowada S, Shimotsuka A, Shishiba Y, Yokozawa M, Yasuda T, Nagasaki K, Hasegawa D, Hasegawa Y, Tachibana K, Naiki Y, Horikawa R, Tanaka T, Ogata T. 2004. Clinical assessment and mutation analysis of Kallmann syndrome 1 (KAL1) and fibroblast growth factor receptor 1 (FGFR1, or KAL2) in five families and 18 sporadic patients. J Clin Endocrinol Metab 89:1079–1088 [DOI] [PubMed] [Google Scholar]
- 5. Albuisson J, Pecheux C, Carel JC, Lacombe D, Leheup B, Lapuzina P, Bouchard P, Legius E, Matthijs G, Wasniewska M, Delpech M, Young J, Hardelin JP, Dode C. 2005. Kallmann syndrome: 14 novel mutations in KAL1 and FGFR1 (KAL2). Hum Mutat 25:98–99 [DOI] [PubMed] [Google Scholar]
- 6. Bhagavath B, Xu N, Ozata M, Rosenfield RL, Bick DP, Sherins RJ, Layman LC. 2007. KAL1 mutations are not a common cause of idiopathic hypogonadotrophic hypogonadism in humans. Mol Hum Reprod 13:165–170 [DOI] [PubMed] [Google Scholar]
- 7. Pitteloud N, Hayes FJ, Boepple PA, DeCruz S, Seminara SB, MacLaughlin DT, Crowley WF., Jr 2002. The role of prior pubertal development, biochemical markers of testicular maturation, and genetics in elucidating the phenotypic heterogeneity of idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 87:152–160 [DOI] [PubMed] [Google Scholar]
- 8. Nachtigall LB, Boepple PA, Pralong FP, Crowley WF., Jr 1997. Adult-onset idiopathic hypogonadotropic hypogonadism–a treatable form of male infertility. N Engl J Med 336:410–415 [DOI] [PubMed] [Google Scholar]
- 9. Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, Cole LW, Pearce SH, Lee H, Boepple P, Crowley WF, Jr, Pitteloud N. 2007. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med 357:863–873 [DOI] [PubMed] [Google Scholar]
- 10. Beranova M, Oliveira LM, Bedecarrats GY, Schipani E, Vallejo M, Ammini AC, Quintos JB, Hall JE, Martin KA, Hayes FJ, Pitteloud N, Kaiser UB, Crowley WF, Jr, Seminara SB. 2001. Prevalence, phenotypic spectrum, and modes of inheritance of gonadotropin-releasing hormone receptor mutations in idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 86:1580–1588 [DOI] [PubMed] [Google Scholar]
- 11. Bhagavath B, Ozata M, Ozdemir IC, Bolu E, Bick DP, Sherins RJ, Layman LC. 2005. The prevalence of gonadotropin-releasing hormone receptor mutations in a large cohort of patients with hypogonadotropic hypogonadism. Fertil Steril 84:951–957 [DOI] [PubMed] [Google Scholar]
- 12. Raivio T, Sidis Y, Plummer L, Chen H, Ma J, Mukherjee A, Jacobson-Dickman E, Quinton R, Van VG, Lavoie H, Hughes VA, Dwyer A, Hayes FJ, Xu S, Sparks S, Kaiser UB, Mohammadi M, Pitteloud N. 2009. Impaired fibroblast growth factor receptor 1 signaling as a cause of normosmic idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 94:4380–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarfati J, Dodé C, Young J. 2010. Kallmann syndrome caused by mutations in the PROK2 and PROKR2 genes: pathophysiology and genotype-phenotype correlations. Front Horm Res 39:121–132 [DOI] [PubMed] [Google Scholar]
- 14. Frisch RE, McArthur JW. 1974. Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science 185:949–951 [DOI] [PubMed] [Google Scholar]
- 15. Feicht CB, Johnson TS, Martin BJ, Sparkes KE, Wagner WW., Jr 1978. Secondary amenorrhoea in athletes. Lancet 2:1145–1146 [DOI] [PubMed] [Google Scholar]
- 16. Doty RL, Shaman P, Kimmelman CP, Dann MS. 1984. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 94:176–178 [DOI] [PubMed] [Google Scholar]
- 17. Santen RJ, Bardin CW. 1973. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest 52:2617–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE. 1999. Free alpha-subunit is superior to luteinizing hormone as a marker of gonadotropin-releasing hormone despite desensitization at fast pulse frequencies. J Clin Endocrinol Metab 84:1028–1036 [DOI] [PubMed] [Google Scholar]
- 19. Filicori M, Santoro N, Merriam GR, Crowley WF., Jr 1986. Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab 62:1136–1144 [DOI] [PubMed] [Google Scholar]
- 20. Hall JE, Schoenfeld DA, Martin KA, Crowley WF., Jr 1992. Hypothalamic gonadotropin-releasing hormone secretion and follicle-stimulating hormone dynamics during the luteal-follicular transition. J Clin Endocrinol Metab 74:600–607 [DOI] [PubMed] [Google Scholar]
- 21. Perkins RB, Hall JE, Martin KA. 1999. Neuroendocrine abnormalities in hypothalamic amenorrhea: spectrum, stability, and response to neurotransmitter modulation. J Clin Endocrinol Metab 84:1905–1911 [DOI] [PubMed] [Google Scholar]
- 22. Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. 2002. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417:405–410 [DOI] [PubMed] [Google Scholar]
- 23. Cole LW, Sidis Y, Zhang C, Quinton R, Plummer L, Pignatelli D, Hughes VA, Dwyer AA, Raivio T, Hayes FJ, Seminara SB, Huot C, Alos N, Speiser P, Takeshita A, Van VG, Pearce S, Crowley WF, Jr, Zhou QY, Pitteloud N. 2008. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J Clin Endocrinol Metab 93:3551–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Roux N, Young J, Misrahi M, Genet R, Chanson P, Schaison G, Milgrom E. 1997. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med 337:1597–1602 [DOI] [PubMed] [Google Scholar]
- 25. Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SH, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N. 2008. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest 118:2822–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonca BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. 2010. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab 95:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lalani SR, Safiullah AM, Fernbach SD, Harutyunyan KG, Thaller C, Peterson LE, McPherson JD, Gibbs RA, White LD, Hefner M, Davenport SL, Graham JM, Bacino CA, Glass NL, Towbin JA, Craigen WJ, Neish SR, Lin AE, Belmont JW. 2006. Spectrum of CHD7 mutations in 110 individuals with CHARGE syndrome and genotype-phenotype correlation. Am J Hum Genet 78:303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miura K, Acierno JS, Jr, Seminara SB. 2004. Characterization of the human nasal embryonic LHRH factor gene, NELF, and a mutation screening among 65 patients with idiopathic hypogonadotropic hypogonadism (IHH). J Hum Genet 49:265–268 [DOI] [PubMed] [Google Scholar]
- 29. Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, Crowley WF., Jr 2007. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 104:17447–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 31. Antonarakis SE. 1998. Recommendations for a nomenclature system for human gene mutations. Nomenclature Working Group. Hum Mutat 11:1–3 [DOI] [PubMed] [Google Scholar]
- 32. Costa EM, Bedecarrats GY, Mendonca BB, Arnhold IJ, Kaiser UB, Latronico AC. 2001. Two novel mutations in the gonadotropin-releasing hormone receptor gene in Brazilian patients with hypogonadotropic hypogonadism and normal olfaction. J Clin Endocrinol Metab 86:2680–2686 [DOI] [PubMed] [Google Scholar]
- 33. de Roux N, Young J, Brailly-Tabard S, Misrahi M, Milgrom E, Schaison G. 1999. The same molecular defects of the gonadotropin-releasing hormone receptor determine a variable degree of hypogonadism in affected kindred. J Clin Endocrinol Metab 84:567–572 [DOI] [PubMed] [Google Scholar]
- 34. Meysing AU, Kanasaki H, Bedecarrats GY, Acierno JS, Jr, Conn PM, Martin KA, Seminara SB, Hall JE, Crowley WF, Jr, Kaiser UB. 2004. GNRHR mutations in a woman with idiopathic hypogonadotropic hypogonadism highlight the differential sensitivity of luteinizing hormone and follicle-stimulating hormone to gonadotropin-releasing hormone. J Clin Endocrinol Metab 89:3189–3198 [DOI] [PubMed] [Google Scholar]
- 35. Pitteloud N, Acierno JS, Jr, Meysing A, Eliseenkova AV, Ma J, Ibrahimi OA, Metzger DL, Hayes FJ, Dwyer AA, Hughes VA, Yialamas M, Hall JE, Grant E, Mohammadi M, Crowley WF., Jr 2006. Mutations in fibroblast growth factor receptor 1 cause both Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 103:6281–6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pitteloud N, Meysing A, Quinton R, Acierno JS, Jr, Dwyer AA, Plummer L, Fliers E, Boepple P, Hayes F, Seminara S, Hughes VA, Ma J, Bouloux P, Mohammadi M, Crowley WF., Jr 2006. Mutations in fibroblast growth factor receptor 1 cause Kallmann syndrome with a wide spectrum of reproductive phenotypes. Mol Cell Endocrinol 254–255:60–69 [DOI] [PubMed] [Google Scholar]
- 37. Sunyaev S, Ramensky V, Koch I, Lathe W, 3rd, Kondrashov AS, Bork P. 2001. Prediction of deleterious human alleles. Hum Mol Genet 10:591–597 [DOI] [PubMed] [Google Scholar]
- 38. Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. 2010. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 7:575–576 [DOI] [PubMed] [Google Scholar]
- 39. Thomas PD, Kejariwal A, Campbell MJ, Mi H, Diemer K, Guo N, Ladunga I, Ulitsky-Lazareva B, Muruganujan A, Rabkin S, Vandergriff JA, Doremieux O. 2003. PANTHER: a browsable database of gene products organized by biological function, using curated protein family and subfamily classification. Nucleic Acids Res 31:334–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ng PC, Henikoff S. 2003. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res 31:3812–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferrer-Costa C, Gelpí JL, Zamakola L, Parraga I, de la Cruz X, Orozco M. 2005. PMUT: a web-based tool for the annotation of pathological mutations on proteins. Bioinformatics 21:3176–3178 [DOI] [PubMed] [Google Scholar]
- 42. Desmet FO, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. 2009. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res 37:e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Welt CK, Adams JM, Sluss PM, Hall JE. 1999. Inhibin A and inhibin B responses to gonadotropin withdrawal depends on stage of follicle development. J Clin Endocrinol Metab 84:2163–2169 [DOI] [PubMed] [Google Scholar]
- 44. Welt CK, Pagan YL, Smith PC, Rado KB, Hall JE. 2003. Control of follicle-stimulating hormone by estradiol and the inhibins: critical role of estradiol at the hypothalamus during the luteal-follicular transition. J Clin Endocrinol Metab 88:1766–1771 [DOI] [PubMed] [Google Scholar]
- 45. Sykiotis GP, Hoang XH, Avbelj M, Hayes FJ, Thambundit A, Dwyer A, Au M, Plummer L, Crowley WF, Jr, Pitteloud N. 2010. Congenital idiopathic hypogonadotropic hypogonadism: evidence of defects in the hypothalamus, pituitary, and testes. J Clin Endocrinol Metab 95:3019–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taylor AE, Khoury RH, Crowley WF., Jr 1994. A comparison of 13 different immunometric assay kits for gonadotropins: implications for clinical investigation. J Clin Endocrinol Metab 79:240–247 [DOI] [PubMed] [Google Scholar]
- 47. Sluss PM, Hayes FJ, Adams JM, Barnes W, Williams G, Frost S, Ramp J, Pacenti D, Lehotay DC, George S, Ramsay C, Doss RC, Crowley WF., Jr 2008. Mass spectrometric and physiological validation of a sensitive, automated, direct immunoassay for serum estradiol using the Architect. Clin Chim Acta 388:99–105 [DOI] [PubMed] [Google Scholar]
- 48. Crowley WF, Jr, Beitins IZ, Vale W, Kliman B, Rivier J, Rivier C, McArthur JW. 1980. The biologic activity of a potent analogue of gonadotropin-releasing hormone in normal and hypogonadotropic men. N Engl J Med 302:1052–1057 [DOI] [PubMed] [Google Scholar]
- 49. Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, Hall JE. 1997. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab 82:2248–2256 [DOI] [PubMed] [Google Scholar]
- 50. Sykiotis GP, Plummer L, Hughes VA, Au M, Durrani S, Nayak-Young S, Dwyer AA, Quinton R, Hall JE, Gusella JF, Seminara SB, Crowley WF, Jr, Pitteloud N. 2010. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci USA 107:15140–15144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trarbach EB, Baptista MT, Garmes HM, Hackel C. 2005. Molecular analysis of KAL-1, GnRH-R, NELF and EBF2 genes in a series of Kallmann syndrome and normosmic hypogonadotropic hypogonadism patients. J Endocrinol 187:361–368 [DOI] [PubMed] [Google Scholar]
- 52. Turan S, Bereket A, Guran T, Akcay T, Papari-Zareei M, Auchus RJ. 2009. Puberty in a case with novel 17-hydroxylase mutation and the putative role of estrogen in development of pubic hair. Eur J Endocrinol 160:325–330 [DOI] [PubMed] [Google Scholar]
- 53. Boyar RM, Rosenfeld RS, Kapen S, Finkelstein JW, Roffwarg HP, Weitzman ED, Hellman L. 1974. Human puberty. Simultaneous augmented secretion of luteinizing hormone and testosterone during sleep. J Clin Invest 54:609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Spratt DI, Carr DB, Merriam GR, Scully RE, Rao PN, Crowley WF., Jr 1987. The spectrum of abnormal patterns of gonadotropin-releasing hormone secretion in men with idiopathic hypogonadotropic hypogonadism: clinical and laboratory correlations. J Clin Endocrinol Metab 64:283–291 [DOI] [PubMed] [Google Scholar]
- 55. Dhruvakumar S, Petko K, Plummer L, Sidis Y, Hall JE, Crowley WF, Jr, Martin KA, Pitteloud N, Genetic defects contribute to susceptibility to hypothalamic amenorrhea. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008 [Google Scholar]
- 56. Anderson CL, Brown CJ. 1999. Polymorphic X-chromosome inactivation of the human TIMP1 gene. Am J Hum Genet 65:699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carrel L, Willard HF. 2005. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434:400–404 [DOI] [PubMed] [Google Scholar]
- 58. Lyon MF. 2005. No longer ‘all-or-none.’ Eur J Hum Genet 13:796–797 [DOI] [PubMed] [Google Scholar]