The time span and patterns of change in serum FSH and E2 across the menopausal transition do not vary by age at the final menstrual period.
Abstract
Background and Objective:
To determine whether patterns of change in serum estradiol (E2) and FSH across the menopausal transition were associated with age at the final menstrual period (FMP).
Design and Setting:
The Study of Women's Health Across the Nation (SWAN) is a seven-site, multiethnic, longitudinal study of the menopausal transition being conducted in 3302 menstruating women who were aged 42–52 yr at the 1996 study baseline.
Measurements:
Annually collected serum was assayed for E2 and FSH levels. Patterns of hormone change were evaluated in the 1215 women with a documented natural FMP by follow-up visit 9 (2006) using semiparametric stochastic and piecewise linear mixed modeling.
Results:
The FSH pattern across the menopausal transition began with an increase 6.10 yr before the FMP, an acceleration 2.05 yr before the FMP, deceleration beginning 0.20 yr before the FMP, and attainment of stable levels 2.00 yr after the FMP, independent of age at the FMP, race/ethnicity, or smoking status. Obesity attenuated the FSH rise and delayed the initial increase to 5.45 yr before the FMP. The mean E2 concentration did not change until 2.03 yr before the FMP when it began decreasing, achieving maximal rate of change at the FMP, then decelerating to achieve stability 2.17 yr after the FMP. Obesity, smoking behavior, and being Chinese or Japanese were associated with some variation in E2 levels but not the pattern of E2 change.
Conclusions:
Time spans and overall patterns of change in serum FSH and E2 across the menopausal transition were not related to age at FMP or smoking, whereas time spans but not overall patterns were related to obesity and race/ethnicity.
Ovarian aging, as manifested by the clinically defined menopausal transition (MT), is characterized by the progressive loss of competent oocytes. The stages of the MT are primarily described by patterns of uterine bleeding resulting from progressive dysregulation of the hypothalamic-pituitary-ovarian system and anchored to the termination of bleeding, the final menstrual period (FMP) (1, 2). The duration of the MT and age at FMP may be quite variable (3, 4).
The endocrinology underlying the uterine bleeding that characterizes the MT has been described in longitudinal (5–12) and cross-sectional (13–19) observational studies. However, revealing this endocrinology has been challenging because of the marked variability in the hormones measured and the variable rates and onsets of change in those hormones over time. Seminal work indicated that, on average, FSH increased and estradiol (E2) decreased monotonically across the MT (20), with the midpoint of the FSH rise occurring a year before the midpoint of the E2 fall (10), and that the FSH rise began before the E2 fall (11). Two recent analyses (21, 22) examined change in mean serum E2 and FSH concentrations over the MT with respect to chronological age and anchored to the FMP. Based on annual hormone measurements over 15 yr in a population-based sample traversing the MT, E2 was stable until a rapid decline began 2 yr before the FMP and achieved stability at a low level by 2 yr after the FMP (22). FSH, in contrast, was already rising 10 yr before the FMP, with the rise accelerating 7 yr before the FMP and again 2 yr before the FMP, and decelerating to relative stability 1 yr after the FMP (21). These trajectories of E2 and FSH change were not related to age of menarche, parity, or smoking behavior. These data suggest a more orderly and predictable process of ovarian aging when normalized to a biologically relevant clinical endpoint, the FMP, rather than to chronological age as the point of reference.
It has been reported that women with a later FMP have a shorter MT (3). We tested the hypothesis that women with a later FMP would have a shorter MT, as revealed by differences in FSH and E2 hormone patterns in relation to age at FMP using 9 yr of data from 1215 women with documented natural FMPs enrolled in the Study of Women's Health Across the Nation (SWAN).
Subjects and Methods
Study population
The data are from the participants of SWAN, a longitudinal cohort of community-based groups of women whose recruitment and enrollment have been described in detail (23). Eligibility criteria for entry into the longitudinal cohort were age 42–52 yr, intact uterus and at least one ovary, no current use of estrogens or other medications known to affect ovarian function, at least one menstrual period in the 3 months before screening, not pregnant or lactating, and self-identification as a member of one of the five eligible ethnic groups. Each of the seven clinical sites enrolled Caucasian women as well as women belonging to one prespecified racial/ethnic group. African-American women were enrolled in Boston, Chicago, the Detroit area, and Pittsburgh, whereas Japanese, Chinese, and Hispanic women were enrolled in Los Angeles, Oakland, and Hudson County, NJ, respectively. This resulted in the recruitment of 3302 women in one of five ethnic/racial groups: Caucasian (n = 1550), African-American (n = 935), Japanese (n = 281), Chinese (n = 250), and Hispanic (n = 286). Primary languages were Cantonese, English, Japanese, and Spanish. Institutional Review Board approval was obtained at each study site, and all participants provided signed, written informed consent. This report is based on data from SWAN participants with a documented natural FMP during 9 yr of follow-up.
Interview and collection of biological specimens at each of the seven clinical sites was undertaken with a single common assessment protocol. Measured reproductive hormones were the primary independent variables and included E2 and FSH. Optimally, women were scheduled for venipuncture before 1000 h on d 2–5 of a spontaneous menstrual cycle occurring within 60 d of recruitment at the baseline visit and annually thereafter. Two attempts were made to obtain the d 2–5 sample. If a follicular phase sample could not be obtained, a random fasting sample was taken within a 90-d window of the anniversary of the baseline visit. Blood was refrigerated 1–2 h after phlebotomy, and after centrifugation, the serum was aliquoted, frozen, and batched for shipment to the central laboratory.
FSH assays were conducted in singlicate and E2 assays in duplicate using an ACS-180 automated analyzer (Bayer Diagnostics Corp., Norwood, MA). E2 concentrations were measured with a modified, off-line ACS-180 (E2–6) immunoassay. Inter- and intraassay coefficients of variation averaged 10.6 and 6.4%, respectively, over the assay range, and the lower limit of detection was 1 pg/ml. Serum FSH concentrations were measured with a two-site chemiluminometric immunoassay. Inter- and intraassay coefficients of variation were 12.0 and 6.0%, respectively, and the lower limit of detection was 1.1 IU/liter. Menopausal status was based on self-report of bleeding patterns. Bleeding in the 3 months before examination with no decrease in the predictability of menses in the year before examination was considered premenopausal; bleeding in the past 3 months with a decrease in predictability in the past year was considered early perimenopausal; no menses for 3–11 months was classified as late perimenopausal; and no menses for 12 or more months was categorized as postmenopausal (24). For data analyses, mean age in years at observable FMP was stratified into approximate quartiles defined by the following cutpoints: less than 49.5, 49.5 to less than 51, 51 to less than 53, and 53 or higher.
Age was calculated based on the participant's date of birth and the date of the visit. Height (centimeters) and weight (kilograms) were measured using a stadiometer and calibrated scales, respectively. Body mass index (BMI) was calculated as weight (kilograms)/height2 (square meters). Hormone trajectory data were stratified by baseline BMI according to nonobese and obese subgroups [<30 kg/m2 n = 813 (67% of sample), and [≥30 kg/m2 n = 399 (33% of sample), respectively]. Women were categorized as never, former, or current smokers based on seven smoking questions adapted from the American Thoracic Society standard questions (25). Health status, referencing the 12 months before the respective visit, was classified as worse, same, and better health compared with the previous visit.
Data analysis
Variable distributions were examined for normality, the presence of nonplausible outliers, and changing variability over time. E2 and FSH values were natural log transformed for statistical modeling but back transformed using the Δ-method Taylor series approach for clarity and comprehension in reporting results.
From the 1215 women with an observable natural FMP, 9435 hormone observations collected over the 9-yr follow-up period were available (mean of 6.9 observations per woman) for data analyses. A multiple-step process was used to organize these data into trajectories of mean hormone levels and rates of change profiles in relation to the FMP (21, 22). To describe the trajectories of mean serum hormone levels over time in relation to the number of years before and after the FMP, semiparametric stochastic mixed modeling was used to estimate a cubic spline function, and bootstrapping was used to identify the 95% confidence intervals (CI) around the mean hormone trajectories (21, 22, 26). Then, to describe patterns of change in serum hormone levels and to estimate the timing of significant changes in these patterns in relation to age and the FMP, the instantaneous rate of change and acceleration/deceleration of the log(hormone) were estimated with first- and second-order derivatives. The 95% confidence bands were obtained using bootstrapping with 1000 bootstrap samples (27, 28). To quantify the MT time spans based on different rates of change in hormone levels, turning points were identified in the patterns of change along with acceleration/deceleration and piecewise linear mixed modeling used to compare adjacent slopes from two contiguous segments around that turning point (29). The time spans between turning points were determined using the Bayesian model averaging method (30–32).
Data were stratified to examine the effects of obesity, smoking, and race/ethnicity in quantifying the association of hormone profiles with age at FMP using tests for interactions. Models were adjusted for the following additional covariates from the baseline assessment: overall health, difficulty in paying for basics, and physical activity (33).
Analyses were implemented in Matlab 7.0 (The MathWorks, Inc., Natick, MA), SAS version 9.1, SAS macro language, and SAS/IML (SAS Institute, Cary, NC).
Results
The median age of women with an observable spontaneous FMP was 47.2 yr at baseline (n = 1215), slightly less than a year older than the mean baseline age of the entire SWAN population. Women with an observable spontaneous FMP had higher baseline and visit 9 FSH levels than the entire SWAN population but did not differ from the entire SWAN population by baseline BMI, mean E2 level, race/ethnicity, health status, ability to pay for basics, smoking status, or physical activity level.
Mean serum FSH increased from a baseline value of 19.25 to 99.30 IU/liter at visit 9, whereas mean serum E2 decreased from 55.08 to 18.40 pg/ml (Table 1). About a third of women with an observable spontaneous FMP were obese.
Table 1.
Baseline and follow-up nine characteristics of the study population with an observed natural FMP
| Variables | Observed FMP (n = 1215) |
|
|---|---|---|
| Baseline (n = 1212) | Follow-up 9 (n = 833) | |
| Median (IQR) | Median (IQR) | |
| Age (yr) | 47.16 (4.02) | 56.01 (3.96) |
| BMI (kg/m2) | 26.37 (9.62) | 27.32 (9.66) |
| FSH (IU/liter) | 19.25 (20.94) | 99.30 (53.80) |
| E2 (pg/ml) | 55.08 (59.30) | 18.40 (10.35) |
| FSH × race (IU/liter) | ||
| AA | 19.14 (23.20) | 84.40 (52.00) |
| CA | 18.00 (17.90) | 107.30 (54.55) |
| CH | 20.80 (21.00) | 103.85 (49.80) |
| HI | 22.60 (36.20) | NA |
| JP | 19.60 (19.30) | 106.55 (44.95) |
| E2 × race (pg/ml) | ||
| AA | 56.70 (56.05) | 22.50 (11.25) |
| CA | 58.60 (60.75) | 18.40 (9.15) |
| CH | 49.50 (58.00) | 15.85 (5.55) |
| HI | 39.95 (85.10) | NA |
| JP | 46.65 (54.10) | 13.25 (6.68) |
| Race/ethnicity [n (%)] | ||
| AA | 386 (31.85) | 283 (33.97) |
| CA | 511 (42.16) | 352 (42.26) |
| CH | 121 (9.98) | 98 (11.76) |
| HI | 71 (5.86) | NA |
| JP | 123 (10.15) | 100 (12) |
| Overall health status [n (%)] | ||
| Worse | 181 (14.93) | 135 (16.21) |
| Same | 762 (62.87) | 287 (34.45) |
| Better | 260 (21.45) | 398 (47.78) |
| Missing | 9 (0.74) | 13 (1.56) |
| BMI at baseline [n (%)] | ||
| Nonobese (<30) | 813 (67.08) | 565 (67.83) |
| Obese (≥30) | 398 (32.84) | 252 (30.25) |
| Missing | 27 (2.23) | 16 (1.92) |
| Smoking at baseline [n (%)] | ||
| Never | 691 (57.01) | 497 (59.66) |
| Past only | 285 (23.51) | 195 (23.41) |
| Present | 209 (17.24) | 125 (15.01) |
| Missing | 27 (2.23) | 16 (1.92) |
AA, African-American; CA, Caucasian; CH, Chinese; HI, Hispanic; IQR, interquartile range; JP, Japanese; NA, not available.
Segments of FSH and E2 change by FMP (Fig. 1 and Table 2)
Fig. 1.
Adjusted population means (95% CI) for natural logarithm-transformed FSH and E2 (A), back-transformed FSH and E2 (B), rate of change for natural logarithm-transformed FSH (C) and E2 (D), and segmented mean profiles of log(FSH) and log(E2) (E), and FSH and E2 (F) across the FMP (total n = 1215). *, The y-axis is unitless for A, B, E, and F. The units of hormone are marked in the corresponding curves.
Table 2.
Adjusted population means of FSH and E2 at selected time points around the FMP and corresponding annualized rates of change during each time window (n = 1215)
| Number of years around FMP | Mean (se) |
Annualized rate of change (se) |
||
|---|---|---|---|---|
| FSH (IU/liter) | E2 (pg/ml) | logFSH | logE2 | |
| −8 | 15.15 (1.03) | 60.26 (4.40) | −8 to −7: −0.0553 (0.0696); P = 0.4266 | −8 to −2: −0.0069 (0.0087); P = 0.4251 |
| −7 | 15.39 (0.64) | 60.12 (2.57) | ||
| −2 | 27.57 (0.71) | 54.08 (1.48) | −7 to −2: 0.1189 (0.0073); P < 0.0001 | |
| 0 | 66.50 (1.60) | 30.01 (0.75) | −2 to 0: 0.4654 (0.0121); P < 0.0001 | −2 to 0: −0.2924 (0.0159); P < 0.0001 |
| +2 | 91.58 (2.14) | 18.35 (0.44) | 0 to +2: 0.1583 (0.0119); P < 0.0001 | 0 to +2: −0.3024 (0.0159); P < 0.0001 |
| +8 | 98.21 (4.01) | 19.12 (0.78) | +2 to +8: −0.0031 (0.0068); P = 0.6484 | +2 to +8: 0.0143 (0.0087); P = 0.0986 |
The trajectory of log(FSH) increased across the FMP from 8 yr before (−8) to 8 yr after (+8) the FMP with five major segments: no change (−8 to −7), a gradual increase (−7 to −2), a marked increase (−2 to FMP), a slower increase (FMP to +2), and stabilization (+2 to +8). The time span over which log(FSH) change occurred was thus 9 yr, including 7 yr before the FMP and 2 yr after the FMP. The segment with the steepest FSH change was −2 yr to the FMP. The mean FSH values at selected points relative to the FMP are shown in Table 2.
The trajectory of log(E2) decreased across the FMP from 8 yr before (−8) to 8 yr after (+8) the FMP and was characterized by a three-segment pattern: no change from 8 to 2 yr before the FMP (−8 to −2), a decline (−2 to +2), and stabilization (+2 to +8). The decrement in log(E2) spanned 4 yr, from −2 to +2. The mean E2 values at selected points relative to the FMP are shown in Table 2.
FSH and E2 by age at FMP (Tables 3 and 4)
Table 3.
Change points and lengths of time windows defined by adjacent change points for logFSH overall and stratified by BMI and by age at FMP
| Models | Change point 1 [yr (95% CI)] | Change point 2 [yr (95% CI)] | Change point 3 [yr (95% CI)] | Change point 4 [yr (95% CI)] | First window [yr (95% CI)] | Second window [yr (95% CI)] |
|---|---|---|---|---|---|---|
| Overall | −6.10 (−6.26, −5.94) | −2.05 (−2.07, −2.03) | −0.20 (−0.22, −0.18) | 2.00 (1.99, 2.01) | 4.05 (3.89, 4.21) | 4.06 (4.04, 4.08) |
| BMI (kg/m2) | ||||||
| <30 | −6.36 (−6.56, −6.16) | −2.00 (−2.01, −1.99) | −0.40 (−0.39, −0.41) | 2.00 (1.99, 2.01) | 4.36 (4.16, 4.56) | 4.00 (3.98, 4.02) |
| ≥30 | −5.45 (−5.63, −5.27) | −2.33 (−2.37, −2.29) | −0.41 (−0.40, −0.42) | 2.17 (2.15, 2.19) | 3.12 (2.94, 3.30) | 4.50 (4.46, 4.54) |
| Age at FMP (yr) | ||||||
| <49.5 | −6.01 (−6.09, −5.93) | −1.98 (−2.00, −1.96) | −0.24 (−0.26, −0.22) | 1.95 (1.93, 1.97) | 4.03 (3.95, 4.11) | 3.93 (3.89, 3.97) |
| 49.5–51 | −6.12 (−6.20, −6.04) | −2.05 (−2.09, −2.01) | −0.32 (−0.34, −0.30) | 1.93 (1.91, 1.95) | 4.07 (3.99, 4.15) | 3.98 (3.94, 4.02) |
| 51–53 | −6.13 (−6.17, −6.09) | −1.89 (−1.91, −1.87) | −0.20 (−0.19, −0.21) | 1.98 (1.96, 2.00) | 4.24 (4.18, 4.30) | 3.87 (3.85, 3.89) |
| ≥53 | −6.19 (−6.27, −6.11) | −1.90 (−1.92, −1.88) | −0.23 (−0.24, −0.22) | 2.08 (2.06, 2.10) | 4.29 (4.21, 4.37) | 3.98 (3.96, 4.00) |
Change point 1 is the first change point before FMP; change point 2 is the second change point before FMP; change point 3 is the third change point near/at FMP; and change point 4 is the fourth change point after FMP. First window is the time window with slower logFSH change before FMP, which is number of years elapsed from change point 1 to change point 2. Second window is the time window with faster logFSH change around FMP, which is number of years elapsed from change point 2 to change point 4.
Table 4.
Change points and lengths of time windows defined by adjacent change points for logE2 overall and stratified by BMI and by age at FMP
| Models | Change point 1 [yr (95% CI)] | Change point 2 [yr (95% CI)] | Change point 3 [yr (95% CI)] | Window [yr (95% CI)] |
|---|---|---|---|---|
| Overall | −2.03 (−2.07, −1.99) | −0.01 (−0.03, 0.01) | 2.17 (2.15, 2.19) | 4.19 (4.15, 4.23) |
| BMI (kg/m2) | ||||
| <30 | −2.03 (−2.05, −2.01) | −0.17 (−0.25, −0.09) | 2.15 (2.11, 2.19) | 4.17 (4.13, 4.21) |
| ≥30 | −1.97 (−2.01, −1.93) | −0.18 (−0.22, −0.14) | 2.20 (2.16, 2.24) | 4.17 (4.11, 4.23) |
| Age at FMP (yr) | ||||
| <49.5 | −1.87 (−1.95, −1.79) | −0.05 (−0.11, 0.01) | 2.34 (2.28, 2.40) | 4.22 (4.16, 4.28) |
| 49.5–51 | −2.07 (−2.11, −2.03) | −0.07 (−0.11, −0.03) | 2.27 (2.21, 2.33) | 4.34 (4.30, 4.38) |
| 51–53 | −2.00 (−2.04, −1.96) | 0.06 (−0.01, 0.12) | 1.97 (1.93, 2.01) | 3.98 (3.94, 4.02) |
| ≥53 | −1.97 (−2.01, −1.93) | −0.04 (−0.08, 0.00) | 1.98 (1.96, 2.00) | 3.95 (3.91, 3.99) |
Change point 1 is the first change point before FMP; change point 2 is the second change point near/at FMP; and change point 3 is the third change point after FMP. Window is the time window of major logE2 change around FMP, which is number of years elapsed from change point 1 to change point 3.
FSH and E2 hormone patterns of change across the MT did not differ by age at FMP (Tables 3 and 4). The onset of the mean serum FSH initial acceleration occurred from 6.19–6.01 yr before the FMP in the four FMP age groups: less than 49.5 (n = 302), 49.5–51 (n = 271), 51–53 (n = 339), and more than 53 (n = 303) years. Maximal rate of change was initiated 2.05–1.89 yr before the FMP, whereas deceleration began 0.32–0.20 yr before the FMP. Mean serum FSH stabilization was observed 1.93–2.08 yr after the FMP.
The onset of mean serum E2 decrease occurred from 2.07–1.87 yr before the FMP according to the four FMP age groups. Maximal rate of change occurred from 0.07 yr before to 0.06 yr after the FMP. Mean serum E2 stabilization was observed 1.97–2.34 yr after the FMP.
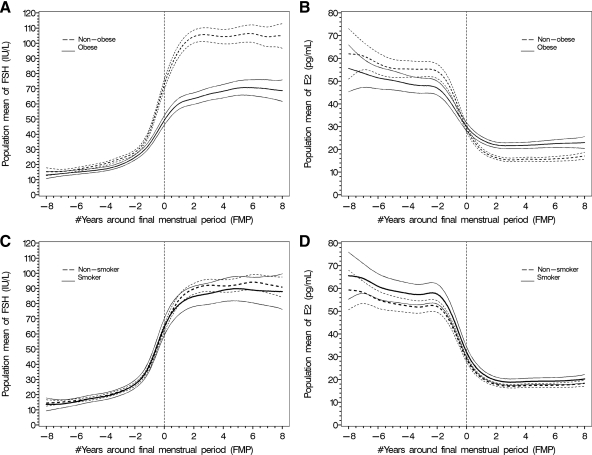
FSH and E2 by obesity (Fig. 2)
Fig. 2.
Population mean (95% CI) profiles for FSH and E2: obese vs. nonobese (A and B) and smoking vs. nonsmoking (C and D) women across the FMP. The vertical reference line is time at the FMP (total n = 1215). *, Natural logarithm-transformed FSH and E2 were used for modeling and back-transformed to original scales for data presentation.
The patterns of change in FSH and E2 in relation to the FMP were not statistically different when comparing obese to nonobese women, although significant differences in mean FSH and E2 levels were observed (Fig. 2, A and B). The FSH change was markedly less pronounced in obese women than in nonobese women and particularly notable commencing at the FMP. In nonobese women vs. obese women, the mean FSH levels relative to the FMP (−7, −2, 0, +2, and +8 yr) were 15 vs. 13, 27 vs. 23, 74 vs. 50, 105 vs. 65, and 105 vs. 73 IU/liter. The E2 change was less pronounced in obese women compared with nonobese women, because obese women had lower premenopausal mean E2 levels but higher postmenopausal mean E2 levels. In nonobese women vs. obese women, the mean E2 levels relative to the FMP (−8, −2, 0, +2, and +8 yr) were 58 vs. 52, 56 vs. 48, 30 vs. 30, 15 vs. 21, and 16 vs. 23 pg/ml.
FSH and E2 by smoking status (Fig. 2)
Smokers and nonsmokers had overlapping trajectories of mean FSH across the MT (Fig. 2C) and overlapping FSH rates of change within the four FMP age groups. The mean trajectory of E2 across the MT was higher among smokers compared with nonsmokers (Fig. 2D). However, there were overlapping E2 rates of change within the four FMP age groups. There was no interaction of BMI with smoking status.
FSH and E2 by race/ethnicity
The major change in FSH-related profiles was associated with obesity, not race/ethnicity. The mean acceleration in serum FSH levels commenced from 6.72–6.38 yr before the FMP in nonobese women of the four racial/ethnic groups, whereas the mean acceleration occurred at 5.04 and 4.30 yr before the FMP in the obese African-American and Caucasian women, respectively, where there was an adequate number of obese women to evaluate the question.
The decline in serum E2 occurred at 2.01 and 1.92 yr before the FMP in the African-American and Caucasian women, respectively, but at 1.33 and 1.56 yr before the FMP in Chinese and Japanese women, respectively. Mean serum E2 stabilized 1.95–2.03 yr after the FMP in all race/ethnic groups except the Japanese women in whom it stabilized at 1.66 yr.
Discussion
We have shown that the timing of the nonlinear changes in annual serum FSH and E2 levels with respect to the FMP is the same regardless of age at the FMP. Obese women did not have FSH pattern differences with respect to age at FMP but did have markedly lower serum FSH concentrations at stabilization in the postmenopause. Likewise, obese women did not have E2 pattern differences with respect to age at FMP but did have slightly lower premenopausal and higher postmenopausal serum E2 levels. Race/ethnicity was not associated with the duration of FSH change, but Chinese and Japanese women appeared to have a shorter span of E2 change and slightly lower serum E2 levels. Baseline smoking was not associated with the timing or magnitude of FSH change and with only slightly higher E2 levels but with no difference in timing. These data suggest that the time course and pattern of ovarian aging, as reflected by FSH change during the MT and E2 change occurring over the 4 yr centered on the FMP, is relatively constant and conserved across racial/ethnic groups, whereas circulating levels of FSH and E2 may vary by both BMI and race/ethnicity, and E2 levels vary minimally by smoking status.
The trajectories of both FSH and E2 anchored to the FMP in the entire cohort were remarkably similar to those reported by Sowers et al. (21, 22) in the Michigan Bone Health and Metabolism Study (MBHMS) with respect to duration and timing of change characteristics. Although the absolute levels of both hormones were somewhat different between the studies, this may reflect the greater heterogeneity and race/ethnic diversity in the SWAN population compared with the MBHMS cohort, which is Caucasian and, on average, less obese. Nonetheless, the patterns of change were quite consistent in segment lengths and occurred at very similar times with respect to the FMP. Such reproducibility argues for a biological process common across human populations.
The consistency of the total and incremental duration of the changes in both FSH and E2 across the MT, regardless of the timing of the FMP, suggests that the pattern of ovarian aging, particularly within the 4 yr around the FMP, is a relatively consistent process, regardless of variation in reproductive hormone levels. The FMP is associated with the loss of competent oocytes from a fixed pool of primordial follicles determined before birth and decaying in number at an undetermined rate (34). The wide variation in the timing of the normal FMP, from 44–58 yr old (35), has been associated with family history (36), socioeconomic status, race/ethnicity, smoking behavior, and other variables (4) and could be due to differences in the size of the initial oocyte complement, variation in oocyte decay rates, or both. The only study to date to examine change in reproductive hormones over the MT with respect to variation in the timing of the acceptably anchored marker of menopause, the FMP, was Burger et al. (7), who noted parallel age-stratified FSH and E2 curves across the FMP. Variation in the duration relative to variation in FMP has not been reported. A report of menstrual cycle length variability across the reproductive lifespan anchored to the FMP noted that variation was similar regardless of age at FMP, suggesting a relatively constant process as reported here (37). Although these data do not encompass ovarian secretion before the MT and do not directly measure oocyte number or quality, they do suggest that decay rates may be similar and that the wide variation in age at FMP may be a result of either variability in the initial size of the primordial follicle pool or in decay rates during premenopausal ovarian aging.
Obesity was not related to the timing of FSH or E2 change across the MT but had a distinct association with both FSH and E2 serum levels. Obesity markedly attenuated the rise in FSH, particularly in the period after the FMP, an effect seen in Caucasian and African-American women for whom we had sufficient numbers of obese women to provide robust data estimates. This is consistent with the cross-sectional report of premenopausal and early perimenopausal values reported from SWAN (38). Similarly, obesity was negatively associated with premenopausal E2 levels but then reversed with the FMP and became positively associated with serum E2, an effect also previously reported in SWAN (8) and the MBHMS population (22). This suggests a postmenopausal shift from ovarian E2 secretion to a compensatory source most likely in fat, a known site of the enzyme aromatase and peripheral production of estrogens from androgen precursors (39). It is likely that this was not observed in premenopausal women due to the much higher ovarian estrogen production then, which overshadows peripheral production. The obesity effects on both hormones were accompanied by resultant slower rates of change due to the smaller excursions over similar time spans.
The absence of racial/ethnic differences in the time span of FSH change but shorter, and different, time spans of E2 change in Chinese and Japanese women is intriguing in view of the reported lower E2 serum concentrations in those racial/ethnic groups (8). This is consistent with a constant rate of E2 change across race/ethnicities, leading to a shorter measurable span of change in groups with lower levels and smaller excursions. The overall FSH time span consistency suggests that the underlying physiological process of the MT is temporally similar in the race/ethnicities studied.
The absence of an association of smoking with FSH levels or change, or with E2 change, is surprising given the well documented association of smoking with an earlier menopause (40). Moreover, the slightly higher E2 levels in women who smoked do not agree with reports of lower estrogen levels in smokers. The absence of an interaction of smoking and body size suggests that it is not due to a BMI difference between smokers and nonsmokers.
The strengths of this report include a large sample of diverse women more representative of community-based population groups rather than a selected clinical convenience sample. The 9-yr observation period with annual assessments permitted the description of a 16-yr span centered around the FMP. However, the lower age limit for recruitment (42 yr) limited the observations of the earlier MT in women with an earlier FMP. The study design is prospective so that a spontaneous FMP was observed and not reliant upon recall. The same hormone assays were employed across the study time frame with no change in the antibodies, and blood was generally collected in the early follicular phase of the menstrual cycle to facilitate the comparison of E2 and FSH values in a standardized time frame. This protocol requirement, however, precluded evaluation of hormone variation during actual ovulatory events of the later follicular phase, or the luteal phase in which other ovarian peptides reflecting endocrine control, including inhibin-A, may be differentially expressed. Of note, the absolute concentrations of FSH are somewhat higher in this assay compared with values from many clinical laboratories, but consistent with the values in the MBHMS and Burger studies (1, 2, 7, 11, 12, 21, 22).
Taken together, these data suggest that the endocrinological patterns and time spans associated with the marked hormone changes of late ovarian aging are relatively consistent, regardless of the chronological age at which the FMP occurs. Moreover, although obesity, race/ethnicity, and smoking were associated with some differences in absolute serum concentrations of reproductive hormones, they were not associated with variation in the overall patterns of late ovarian aging.
Acknowledgments
We thank the study staff at each site and all the women who participated in SWAN.
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), Department of Health and Human Services, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women's Health (ORWH) (Grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Clinical Centers: University of Michigan, Ann Arbor, MI (MaryFran Sowers, PI); Massachusetts General Hospital, Boston, MA (Joel Finkelstein, PI 1999 to present; Robert Neer, PI 1994–1999); Rush University, Rush University Medical Center, Chicago, IL (Howard Kravitz, PI 2009 to present; Lynda Powell, PI 1994–2009); University of California, Davis/Kaiser (Ellen Gold, PI); University of California, Los Angeles (Gail Greendale, PI); Albert Einstein College of Medicine, Bronx, NY (Rachel Wildman, PI 2010; Nanette Santoro, PI 2004–2010); University of Medicine and Dentistry, New Jersey Medical School, Newark, NJ (Gerson Weiss, PI 1994–2004); and the University of Pittsburgh, Pittsburgh, PA (Karen Matthews, PI).
NIH Program Office: NIA, Bethesda, MD (Sherry Sherman 1994 to present; Marcia Ory 1994–2001); NINR, Bethesda, MD (Program Officers).
Central Laboratory: University of Michigan, Ann Arbor, MI (Daniel McConnell, Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA (Kim Sutton-Tyrrell, PI 2001 to present); New England Research Institutes, Watertown, MA (Sonja McKinlay, PI 1995–2001).
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- CI
- confidence interval
- E2
- estradiol
- FMP
- final menstrual period
- MBHMS
- Michigan Bone Health and Metabolism Study
- MT
- menopausal transition
- SWAN
- Study of Women's Health Across the Nation.
References
- 1. Robertson DM, Hale GE, Jolley D, Fraser IS, Hughes CL, Burger HG. 2009. Interrelationships between ovarian and pituitary hormones in ovulatory menstrual cycles across reproductive age. J Clin Endocrinol Metab 94:138–144 [DOI] [PubMed] [Google Scholar]
- 2. Hale GE, Burger HG. 2009. Hormonal changes and biomarkers in late reproductive age, menopausal transition and menopause. Best Pract Res Clin Obstet Gynaecol 23:7–23 [DOI] [PubMed] [Google Scholar]
- 3. McKinlay SM, Brambilla DJ, Posner JG. 1992. The normal menopause transition. Maturitas 14:103–115 [DOI] [PubMed] [Google Scholar]
- 4. Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. 2001. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 153:865–874 [DOI] [PubMed] [Google Scholar]
- 5. Metcalf MG, Donald RA, Livesey JH. 1982. Pituitary-ovarian function before, during and after the menopause: a longitudinal study. Clin Endocrinol (Oxf) 17:489–494 [DOI] [PubMed] [Google Scholar]
- 6. Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. 1995. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas 21:103–113 [DOI] [PubMed] [Google Scholar]
- 7. Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L. 1999. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab 84:4025–4030 [DOI] [PubMed] [Google Scholar]
- 8. Randolph JF, Jr, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ. 2004. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab 89:1555–1561 [DOI] [PubMed] [Google Scholar]
- 9. Landgren BM, Collins A, Csemiczky G, Burger HG, Baksheev L, Robertson DM. 2004. Menopause transition: annual changes in serum hormonal patterns over the menstrual cycle in women during a nine-year period prior to menopause. J Clin Endocrinol Metab 89:2763–2769 [DOI] [PubMed] [Google Scholar]
- 10. Dennerstein L, Lehert P, Burger HG, Guthrie JR. 2007. New findings from non-linear longitudinal modeling of menopausal hormone changes. Hum Reprod Update 13:551–557 [DOI] [PubMed] [Google Scholar]
- 11. Ebeling PR, Atley LM, Guthrie JR, Burger HG, Dennerstein L, Hopper JL, Wark JD. 1996. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab 81: 3366–3371 [DOI] [PubMed] [Google Scholar]
- 12. Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, Harlow S, Randolph JF., Jr 2008. Anti-Mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab 93:3478–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sherman BM, Korenman SG. 1975. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest 55:699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sherman BM, West JH, Korenman SG. 1976. The menopausal transition: analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab 42:629–636 [DOI] [PubMed] [Google Scholar]
- 15. Reyes FI, Winter JS, Faiman C. 1977. Pituitary-ovarian relationships preceding the menopause. I. A cross-sectional study of serum follice-stimulating hormone, luteinizing hormone, prolactin, estradiol, and progesterone levels. Am J Obstet Gynecol 129:557–564 [PubMed] [Google Scholar]
- 16. Metcalf MG, Donald RA, Livesey JH. 1981. Pituitary-ovarian function in normal women during the menopausal transition. Clin Endocrinol (Oxf) 14:245–255 [DOI] [PubMed] [Google Scholar]
- 17. Longcope C, Franz C, Morello C, Baker R, Johnston CC., Jr 1986. Steroid and gonadotropin levels in women during the peri-menopausal years. Maturitas 8:189–196 [DOI] [PubMed] [Google Scholar]
- 18. Lee SJ, Lenton EA, Sexton L, Cooke ID. 1988. The effect of age on the cyclical patterns of plasma LH, FSH, oestradiol and progesterone in women with regular menstrual cycles. Hum Reprod 3:851–855 [DOI] [PubMed] [Google Scholar]
- 19. Shideler SE, DeVane GW, Kalra PS, Benirschke K, Lasley BL. 1989. Ovarian-pituitary hormone interactions during the perimenopause. Maturitas 11:331–339 [DOI] [PubMed] [Google Scholar]
- 20. Burger HG. 1999. The endocrinology of the menopause. J Steroid Biochem Mol Biol 69:31–35 [DOI] [PubMed] [Google Scholar]
- 21. Sowers MR, Zheng H, McConnell D, Nan B, Harlow S, Randolph JF., Jr 2008. Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab 93:3958–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sowers MR, Zheng H, McConnell D, Nan B, Harlow SD, Randolph JF., Jr 2008. Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J Clin Endocrinol Metab 93:3847–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sowers MF, Crawford S, Morgenstein D. 2000. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R. eds. Menopause: biology and pathobiology. San Diego: Academic Press; 175–188 [Google Scholar]
- 24. Brambilla DJ, McKinlay SM, Johannes CB. 1994. Defining the perimenopause for application in epidemiologic investigations. Am J Epidemiol 140:1091–1095 [DOI] [PubMed] [Google Scholar]
- 25. Ferris BG. 1978. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis 118(6 Pt 2):1–120 [PubMed] [Google Scholar]
- 26. Zhang D, Lin X, Sowers M. 1998. Semiparametric stochastic mixed models for longitudinal data. J Am Stat Assoc 93:710–719 [Google Scholar]
- 27. Efron B, Tibshirani R. 1986. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci 1:54–77 [Google Scholar]
- 28. Claeskens G, Van Keilegom I. 2003. Bootstrap confidence bands for regression curves and their derivatives. Ann Stat 31:1852–1884 [Google Scholar]
- 29. Neter J, Wasserman W, Kutner M. 1985. Applied linear statistical models. 2nd ed. Homewood, IL: Irwin [Google Scholar]
- 30. Madigan D, Raftery AE. 1994. Model selection and accounting for model uncertainty in graphical models using Occam's window. J Am Stat Assoc 89:1535–1546 [Google Scholar]
- 31. Hoeting JA, Madigas D, Raftery AE, Volinsky C. 1999. Model averaging: a tutorial. Stat Sci 14382–14417 [Google Scholar]
- 32. Clyde M. 2003. Model averaging: subjective and objective Bayesian statistics. New York: Wiley; 320–333 [Google Scholar]
- 33. Santoro N, Brockwell S, Johnston J, Crawford SL, Gold EB, Harlow SD, Matthews KA, Sutton-Tyrrell K. 2007. Helping midlife women predict the onset of the final menses: SWAN, the Study of Women's Health Across the Nation. Menopause 14(3 Pt 1):415–424 [DOI] [PubMed] [Google Scholar]
- 34. Broekmans FJ, Soules MR, Fauser BC. 2009. Ovarian aging: mechanisms and clinical consequences. Endocr Rev 30:465–493 [DOI] [PubMed] [Google Scholar]
- 35. Morabia A, Costanza MC. 1998. International variability in ages at menarche, first livebirth, and menopause. World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives. Am J Epidemiol 148:1195–1205 [DOI] [PubMed] [Google Scholar]
- 36. de Bruin JP, Bovenhuis H, van Noord PA, Pearson PL, van Arendonk JA, te Velde ER, Kuurman WW, Dorland M. 2001. The role of genetic factors in age at natural menopause. Hum Reprod 16:2014–2018 [DOI] [PubMed] [Google Scholar]
- 37. Lisabeth L, Harlow S, Qaqish B. 2004. A new statistical approach demonstrated menstrual patterns during the menopausal transition did not vary by age at menopause. J Clin Epidemiol 57:484–496 [DOI] [PubMed] [Google Scholar]
- 38. Randolph JF, Jr, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, McConnell DS, Finkelstein JS, Korenman SG, Matthews KA, Sternfeld B, Lasley BL. 2003. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab 88:1516–1522 [DOI] [PubMed] [Google Scholar]
- 39. Simpson ER. 2004. Aromatase: biologic relevance of tissue-specific expression. Semin Reprod Med 22:11–23 [DOI] [PubMed] [Google Scholar]
- 40. Soares SR, Melo MA. 2008. Cigarette smoking and reproductive function. Curr Opin Obstet Gynecol 20:281–291 [DOI] [PubMed] [Google Scholar]