Blocking autoantibodies against the calcium-sensing receptor were detected in a patient with autoimmune hypocalciuric hypercalcemia whose hypercalcemia failed to respond to glucocorticoid therapy.
Abstract
Context:
Autoantibodies directed against the calcium-sensing receptor (CaSR) have been reported in several individuals with various autoimmune disorders and PTH-mediated hypercalcemia. Previously, glucocorticoid treatment has been shown to decrease the CaSR autoantibody titers and normalize the hypercalcemia in a patient with autoimmune hypocalciuric hypercalcemia (AHH).
Objective:
The objective of the study was to evaluate a patient with AHH for the presence of blocking autoantibodies against the CaSR and to monitor her biochemical and serological responses to a trial of glucocorticoid therapy.
Results:
Glucocorticoid treatment had no effect on serum total or ionized calcium concentration or serum PTH levels, all of which remained at higher than normal levels. In contrast, on prednisone, urinary calcium excretion increased from overtly hypocalciuric levels to normal values. Anti-CaSR autoantibodies were detected at similar levels in the patient's serum before, during, and after glucocorticoid treatment. Functional testing of these antibodies showed that they inhibited the stimulatory effect of extracellular Ca2+ on ERK1/2 but did not suppress the calcium-induced accumulation of inositol-1-phosphate.
Conclusions:
We report a patient with AHH with frankly elevated PTH levels who was found to have autoantibodies against the CaSR. The hypercalcemia and CaSR autoantibody titers failed to respond to glucocorticoid therapy, unlike a previously reported patient with similar clinical and biochemical features. The anti-CaSR antibody-mediated inhibition of CaSR-stimulated ERK1/2 activity, but not of inositol-1-phosphate accumulation, suggests that ERK1/2 may mediate, at least in part, the regulation of PTH secretion and urinary calcium excretion by the CaSR.
Autoantibodies directed against the calcium-sensing receptor (CaSR) have been described in a substantial percentage of patients with hypoparathyroidism occurring either as an isolated entity or as a component of autoimmune polyendocrine syndrome type 1 (APS1) (1–6). In contrast, anti-CaSR autoantibodies have been found in only a handful of subjects with PTH-dependent hypercalcemia (7–10). Most of these patients also had hypocalciuria in the setting of various other autoimmune disorders. Familial hypocalciuric hypercalcemia (FHH) was excluded in these patients by either directly sequencing the CASR gene or demonstrating previously normal serum calcium levels. In vitro functional assays demonstrated that anti-CaSR autoantibodies isolated from the sera of patients with autoimmune hypocalciuric hypercalcemia (AHH) inhibited extracellular Ca2+-stimulated phosphorylation of ERK1/2 (7, 9). By interfering with the CaSR's capacity to sense Ca2+, anti-CaSR autoantibodies mimicked the biochemical phenotype caused by loss-of-function mutations in the CASR gene that cause FHH.
We had previously described a 66-yr-old woman with AHH and anti-CaSR autoantibodies whose hypercalcemia had failed to respond to subtotal parathyroidectomy but resolved with glucocorticoid treatment (8). In addition to AHH, the patient had other manifestations of immune dysfunction including eosinophilia, elevated IgE and IgG4 levels, autoimmune pancreatitis, bullous pemphigoid, and a history of several other autoimmune disorders. Glucocorticoid therapy, initially given for treatment of bullous pemphigoid and later to normalize calcium levels specifically, decreased anti-CaSR autoantibody titers and resulted in near normalization of serum PTH levels. AHH was directly correlated with anti-CaSR autoantibody titers and the maximal peak prednisone dose required to normalize her PTH-dependent hypercalcemia was in the range of 40–70 mg.
Here we report an additional patient with elevated IgE levels, high antinuclear antibody (ANA) titers and AHH, who was found to have anti-CaSR autoantibodies but whose hypercalcemia failed to respond to a trial of glucocorticoids. Furthermore, functional studies showed that her anti-CaSR autoantibodies blunted high Ca2+-stimulated ERK1/2 activity but not inositol-1-phosphate (IP1) accumulation, suggesting that ERK1/2, rather than phospholipase C, may be a key mediator of CaSR-regulated PTH secretion and urinary calcium excretion.
Case Reports
Patient clinical history
A 74-yr-old African-American woman was referred for evaluation of asymptomatic hypercalcemia. The patient had a history of hypertension, depression, chronic pruritus, osteoarthritis, chronic gastritis, and colon cancer for which she had undergone partial colectomy. She had a history of alopecia and was being evaluated by rheumatology for possible Raynaud's syndrome and elevated ANA (1:2560 with a centromeric pattern) and antiribonuclear protein (RNP)-antibody (Ab) titers. She had undergone bilateral knee replacements and had a prior left distal phalangeal fracture, osteopenia, and Paget's disease involving the lumbar spine and right ulna. A neck ultrasound had revealed a left lower pole thyroid nodule with cytology from fine-needle aspiration suggesting a macrofollicular lesion. Her examination was otherwise unremarkable.
The study of this patient was approved by the Institutional Review Board of Partners HealthCare (Boston, Massachusetts), and serum samples were collected from the patient after obtaining written informed consent.
Biochemical analyses
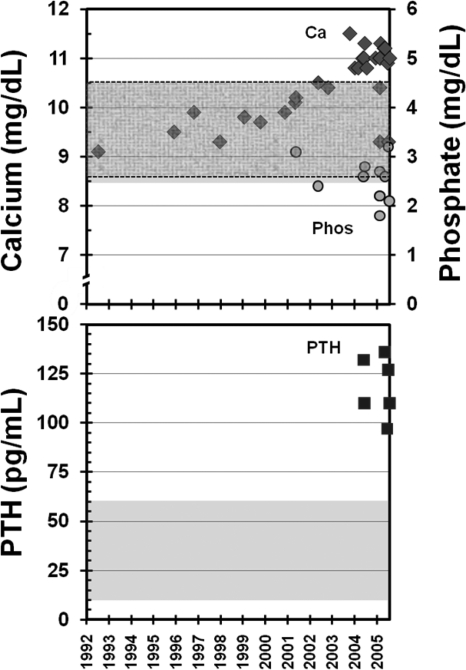
Biochemical analyses revealed normal electrolyte levels and renal function. She was hypercalcemic with total serum calcium of 11.3 mg/dl (normal, 8.5–10.5 mg/dl) (Fig. 1) and ionized calcium of 1.46 mmol/liter (normal, 1.14–1.30 mmol/liter). Serum albumin was normal at 4.0 g/dl. In the previous year, her peak calcium level had been recorded as 11.5 mg/dl. Phosphorus was in the lower normal range at 2.8 mg/dl (normal, 2.6–4.5 mg/dl). Alkaline phosphatase at 226 U/liter (normal, 30–100 U/liter) and PTH at 110 pg/ml (normal, 10–60 pg/ml) were elevated (Fig. 1). 25-Hydroxyvitamin D concentration was low at 12 ng/ml (normal, >32 ng/ml). Subsequent repletion and normalization of 25-hydroxyvitamin D levels to 42 ng/ml did not result in further elevation of serum calcium levels (11.1 mg/dl). 1,25-Dihydroxyvitamin D level was 28 pg/ml (normal, 6–62 pg/ml). Repeat measurements of 24-h urinary calcium excretion showed marked hypocalciuria with total daily calcium excretion of 9, 39, and 24 mg on three separate occasions. These values corresponded to calcium to creatinine clearance ratios of 0.0024, 0.0034, and 0.0027, respectively.
Fig. 1.
Temporal profile of the patient's calcium and PTH levels. The patient's serum calcium (diamonds in top panel), phosphate (circles in top panel), and PTH levels are shown over time, with shaded areas (and dashed areas for phosphate) indicating the range of normal values. In the decade before developing PTH-mediated hypercalcemia, the patient had multiple normal serum calcium values.
Immunological analyses
Given her elevated ANA and anti-RNP Ab titers, we performed additional immunological analyses. Serum protein electrophoresis showed mild diffuse increases in IgG at 1660 mg/dl (normal, 614–1295 mg/dl) and in IgA at 673 mg/dl (normal, 69–309 mg/dl) with a very low concentration (12.5–25 mg/dl) of an IgG κ-M-component. IgM and complement levels were within normal limits, but IgE levels were found to be elevated at 635 IU/ml (normal, 0–100 IU/ml). IgG4 levels were normal at 52 mg/dl (normal, 8–140 mg/dl). There was no eosinophilia with an absolute eosinophil count of 250/mm3 (normal, 100–300/ mm3). The erythrocyte sedimentation rate was elevated at 51 mm/h (normal, <25 mm/h). With the exception of ANA, anti-RNP, and anti-Ro antibodies, an extended rheumatological screening panel was negative. She had normal TSH levels.
Anti-CaSR autoantibody assays
The patient had no family history of abnormalities in calcium metabolism, and previous serum calcium levels had been normal until the age of 73 yr (Fig. 1), ruling out FHH as the cause of her hypocalciuric hypercalcemia. In addition, no evidence of a parathyroid adenoma was seen in a neck ultrasound. The pattern of PTH-dependent hypercalcemia with marked hypocalciuria pointed to possible dysfunction of the CaSR. The patient was therefore evaluated for the presence of anti-CaSR autoantibodies.
Immunoprecipitation (IP) assays
IP assays for anti-CaSR autoantibodies were carried out as previously detailed (4). The patient's serum samples (n = 8) and healthy control sera (n = 20), which are detailed elsewhere (4), were stored at −80 C before use. Human embryonic kidney 293 (HEK293) cells were grown, transiently transfected with plasmid pcCaSR-FLAG (4), and cell extracts containing expressed CaSR-FLAG protein prepared as previously described (4). Aliquots (50 μl) of GammaBind Sepharose beads (Amersham Biosciences, Little Chalfont, UK) were mixed with sera (1:50 dilution) in duplicate in 1 ml of IP buffer and incubated for 1 h at 4 C. The resulting bead/IgG complexes were collected by centrifugation and then mixed with the cell lysate and incubated at 4 C for 16 h. Subsequently the bead/IgG/protein complexes were collected by centrifugation, washed, denatured, and subjected to SDS-PAGE in 7.5% polyacrylamide gels as detailed elsewhere (4). The separated proteins were transferred onto Trans-Blot transfer membrane (Bio-Rad Laboratories Ltd., Hemel Hempstead, UK) using standard Western blotting protocols. Immunoprecipitated CaSR-FLAG protein was detected using anti-FLAG M2-peroxidase conjugate (Sigma, Poole, UK) and an enhanced chemiluminescence Western blotting analysis system (Amersham Biosciences) with final exposure to preflashed x-ray film for 5-min periods. Densitometry of the bands on exposed films was carried out in a Bio-Rad GS 690 scanning densitometer with Multi-Analyst version 1.1 software (Bio-Rad Laboratories), which produced a densitometry value for each individual band.
A CaSR Ab index for each serum tested in the IP assay was calculated as the densitometry value of tested serum/mean densitometry value of 20 healthy control sera. Each serum was tested in duplicate in three experiments, and the mean CaSR Ab index was calculated. The upper level of normal for the assay was calculated using the mean CaSR Ab index + 3 sd of the population of 20 healthy individuals. Any serum with a CaSR Ab index above the upper level of normal was designated as positive for anti-CaSR autoantibody reactivity.
Measurement of CaSR-stimulated IP1 accumulation
HEK293 cells stably expressing the CaSR (HEK293-CaSR) were cultured as previously described (11). The response of HEK293 cells expressing the CaSR (HEK293-CaSR cells) to Ca2+ was assessed by measuring intracellular IP1 accumulation using a specific IP1-ELISA, as detailed elsewhere (11). The IP-1 ELISA is highly specific, with no cross-reactivity to myo-inositol, inositol bisphosphate, inositol trisphosphate, or inositol tetrakisphosphate, and is a comparable method to measuring inositol phosphate by the tritium-labeling technique (12).
Briefly, monolayers of HEK293-CaSR cells were washed first with serum-free medium and then with Ca2+-free assay buffer containing 10 mm LiCl2 (11). Cells were subsequently incubated for 60 min at 37 C in 200 μl of buffer alone or buffer containing varying concentrations of Ca2+ (as CaCl2). After treatment, cells were lysed for 30 min at 37 C with 50 μl of 2.5% IP-One ELISA kit lysis reagent (CIS BioInternational, Gif-sur-Yvette, France). The accumulation of intracellular IP1 was measured according to an IP-One ELISA kit (CIS BioInternational), an immunoassay based on competition between free IP1 and IP1-horseradish peroxidase (HRP) conjugate for binding to anti-IP1 monoclonal Ab. The results for IP1 accumulation were expressed as: percentage inhibition of IP1-HRP binding = [1 − IP1-HRP binding in stimulated cells/IP1-HRP binding in unstimulated cells] × 100. An increase in IP1 accumulation in the HEK293-CaSR cells is reflected by an increase in the percentage inhibition of IP1-HRP binding.
For measuring the effects of IgG from the AHH patient and from healthy controls on CaSR-stimulated IP1 accumulation, IgG was prepared from the patient's serum samples (n = 8) and control sera (n = 10) as described previously (11). HEK293-CaSR cell monolayers were incubated with IgG at a 1:50 dilution in 100 μl of buffer for 10 min at 37 C. Cells were then incubated for 60 min at 37 C with a further 100 μl of buffer alone or buffer containing varying concentrations of Ca2+ before measuring IP1 accumulation. The 60-min time point was chosen based on previous experience with this assay and on other anti-CaSR autoantibody studies showing significant changes in accumulation of inositol phosphates in this time frame in response to Ca2+ stimulation (9, 11). Each experiment included HEK293-CaSR cells stimulated with Ca2+ alone and HEK293-CaSR cells left untreated.
Measurement of CaSR-stimulated ERK1/2 phosphorylation
The response of HEK293-CaSR cells to Ca2+ was assessed by measuring the phosphorylation of ERK1/2 as detailed elsewhere (11). Briefly, HEK293-CaSR cell monolayers were washed first with serum-free medium and then with assay buffer (11). Cells were subsequently incubated for 60 min at 37 C in 100 μl of buffer alone or buffer containing varying concentrations of Ca2+. After treatment, cells were fixed for 20 min at room temperature with 100 μl of 4% cell-fixing buffer according to a Cellular Activation of Signaling ELISA (CASE) kit (SuperArray Bioscience Corp., Frederick, MD). The phosphorylation of ERK1/2 was then measured according to a CASE kit (SuperArray Bioscience) and the results expressed as the ratio of phosphorylated ERK1/2 to total ERK1/2. For measuring the effects of IgG from the AHH patient and controls on CaSR-stimulated ERK1/2 phosphorylation, HEK293-CaSR cell monolayers were incubated with IgG at a 1:50 dilution in 100 μl of buffer for 10 min at 37 C. Cells were then incubated for 60 min at 37 C with a further 100 μl of buffer alone or buffer containing varying concentrations of Ca2+ before measuring ERK1/2 phosphorylation. Each experiment included HEK293-CaSR cells stimulated with Ca2+ alone and HEK293-CaSR cells left untreated.
Statistical analyses
Statistical analyses were performed using Student's unpaired t tests. P < 0.05 (two tailed) was regarded as statistically significant.
Glucocorticoid therapy
The patient was started on an infusion of 30 mg of pamidronate every 6 months for the management of her osteopenia, hypercalcemia, and Paget's disease. After the patient was found to have circulating anti-CaSR autoantibodies, a 2-month trial of glucocorticoids was undertaken while she was being treated with iv bisphosphonate in an attempt to lower her serum calcium levels. Maintenance prednisone doses of 30 and 60 mg were selected and were based on the treatment response from our previous patient with AHH due to confirmed anti-CaSR autoantibodies (8). Pamidronate infusion was given 1 month before the start of the glucocorticoid trial for skeletal protection. Serum and spot urine calcium and creatinine levels, along with serum ionized calcium, phosphate, and PTH levels, were measured at baseline and every 1–2 wk during the trial. Several 24-h urine samples were collected at baseline and while the patient was on glucocorticoids.
Results
Biochemical analyses before, during, and after glucocorticoid treatment
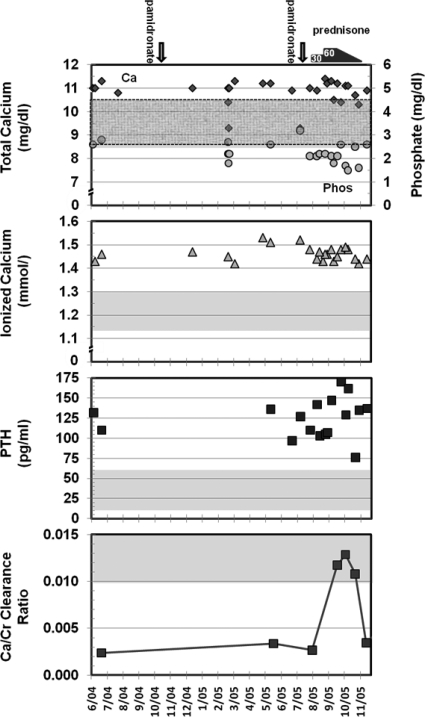
Compared with baseline values, serum total and ionized calcium levels remained unchanged after an infusion of 30 mg of pamidronate and after treatment with 30 or 60 mg of prednisone per day for 2 months (Fig. 2). The average total (and ionized) calcium levels ± sd were: baseline, 11.0 ± 0.28 mg/dl (1.45 ± 0.02 mmol/liter); after pamidronate, 10.7 ± 0.73 mg/dl (1.48 ± 0.04 mmol/liter); on 30 mg prednisone, 11.0 ± 0.40 mg/dl (1.45 ± 0.02 mmol/liter); on 60 mg prednisone, 10.9 ± 0.38 mg/dl (1.47 ± 0.03 mmol/liter); P values were greater than 0.05 for all treatment groups compared with baseline levels. Although several serum calcium measurements were within the normal range both before and after the initiation of glucocorticoids, most of these values were in the hypercalcemic range when corrected for albumin, which ranged from 3.2 to 4.2 g/dl. The patient's serum ionized calcium levels remained uniformly elevated (Fig. 2). Pamidronate infusions normalized alkaline phosphatase levels from pretreatment levels of 226 U/liter and maintained them at less than 110 U/liter. Pamidronate and glucocorticoid therapy had little effect on PTH levels, which remained elevated throughout the trial period (Fig. 2). The average PTH levels ± sd were: baseline, 122.0 ± 15.6 pg/ml; after pamidronate, 117.5 ± 17.4 pg/ml; on 30 mg prednisone, 116.7 ± 22.0 pg/ml; on 60 mg prednisone, 148.4 ± 32.4 pg/ml; P > 0.05 for all treatment groups compared with baseline levels. Urinary calcium excretion, as measured by 24-h urine calcium measurements, and calcium to creatinine clearance ratios were similar before and after pamidronate infusion: 24-h urine calcium excretion was 39 mg before and 24 mg after the infusion, with corresponding calcium to creatinine clearance ratios of 0.003 and 0.003 (Fig. 2). In contrast, urinary calcium excretion increased with glucocorticoid treatment, with a peak 24-h urine calcium excretion of 102 mg and a peak calcium to creatinine clearance ratio of 0.013 while the patient was receiving prednisone (Fig. 2). As the prednisone was tapered, hypocalciuria recurred, with the calcium to creatinine clearance ratio returning to baseline levels (Fig. 2). The preprednisone average ± sd was 0.003 ± 0.0005. Values during treatment with prednisone were: on 60 mg prednisone, 0.012; on 40 mg prednisone, 0.013; on 20 mg prednisone, 0.011; and on 2.5 mg prednisone, 0.004; P values were less than 0.05 for comparison between pretreatment and on-treatment values. Weekly spot urine measurements of calcium to creatinine ratios showed the same pattern seen with the 24-h urine collections. Vitamin D levels were unchanged during this period. On glucocorticoid treatment, the patient's erythrocyte sedimentation rate normalized and her IgE levels dropped from a baseline value of 510 IU/ml to 154 IU/ml (normal, <100 IU/ml) suggestive of adequate compliance.
Fig. 2.
Biochemical response to bisphosphonate and glucocorticoid therapy. The patient's serum calcium (diamonds in top panel), phosphate (circles in top panel), serum ionized calcium, and PTH values and ratios of calcium to creatinine clearance, with shaded areas (and dashed areas for phosphate) indicating normal ranges, are shown over an 18-month period after her diagnosis with probable AHH. Pamidronate infusions (indicated by the arrows) and prednisone treatment (indicated by the bars) had no effect on serum calcium and PTH levels. In contrast, urinary calcium excretion increased from a calcium to creatinine clearance ratio of 0.003 at baseline to 0.013 on glucocorticoid therapy. Prednisone treatment resulted in only transient normalization of the hypocalciuria because the ratio of calcium clearance to creatinine clearance returned to baseline levels (0.004) when glucocorticoids were tapered.
Evaluation of anti-CaSR autoantibodies before, during, and after glucocorticoid treatment
Detection of anti-CaSR autoantibodies by IP assays
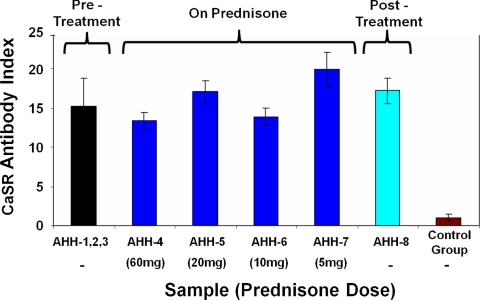
IP assays for anti-CaSR autoantibodies showed that all eight serum samples from the AHH patient tested positive for anti-CaSR autoantibodies: the CaSR Ab index for each serum sample in the IP assay was greater than the upper level of normal (a CaSR Ab index of 2.18) calculated from a population of 20 healthy control sera (Fig. 3). Samples were collected from the AHH patient before (samples 1–3), during (sample 4 at 60 mg prednisone; sample 5 at 20 mg prednisone; sample 6 at 10 mg prednisone; sample 7 at 5 mg prednisone), and after (sample 8) glucocorticoid treatment. The CaSR Ab index of each sample at each dose of prednisone did not differ significantly from the average CaSR Ab index from the pretreatment samples (samples 1–3): P values were greater than 0.05 (Fig. 3). This suggests that treatment with prednisone did not reduce autoantibody levels.
Fig. 3.
Detection of anti-CaSR autoantibodies in the patient's serum samples using IP assays. Serum samples from the patient (n = 8), and healthy control sera (n = 20) were analyzed for anti-CaSR autoantibodies in IP assays at a 1:50 dilution, exactly as previously detailed (4). The patient's serum samples were taken before (samples 1–3), during (sample 4 at 60 mg prednisone; sample 5 at 20 mg prednisone; sample 6 at 10 mg prednisone; sample 7 at 5 mg prednisone), and after (sample 8) glucocorticoid treatment. The average CaSR Ab index (±sd) of the pretreatment samples is shown next to the CaSR Ab index values (±sd) for each of the patient's treatment and posttreatment serum samples, each representing the mean of three experiments. The mean CaSR Ab index (±sd) of the group of 20 healthy control sera is also shown. The upper level of normal for the IP assay was a CaSR Ab index of 2.18, which was significantly lower than the values for each of the AHH samples (P < 0.05). P values were greater than 0.05 when comparing the mean CaSR Ab index of all pretreatment samples (1–3) with the CaSR Ab index of each sample at each dose of prednisone and with the posttreatment sample.
CaSR-modulating effects of the AHH patient's IgG
To determine the effects of the patient's anti-CaSR autoantibodies on CaSR function, HEK293-CaSR cells were incubated with IgG (1:50 dilution) before measurement of Ca2+-induced IP1 accumulation and ERK1/2 phosphorylation.
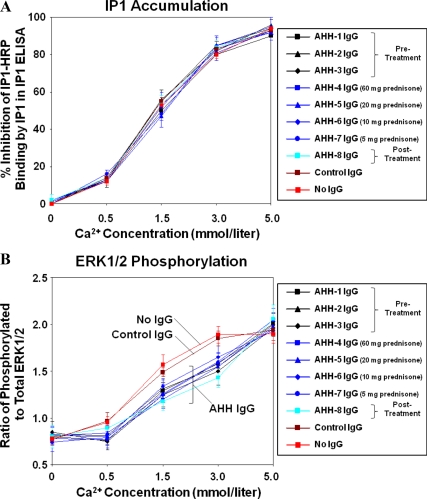
The results indicated that neither the patient's IgG samples (n = 8) nor the control IgG samples (n = 10) significantly affected the levels of IP1 accumulation when compared with the Ca2+ stimulation of HEK293-CaSR cells that were not preincubated with IgG (Fig. 4A): P values were greater than 0.05 when comparing Ca2+-stimulated IP1 accumulation with and without preincubation with IgG samples at all the Ca2+ concentrations used. In contrast, the patient's IgG samples (n = 8) but not the control IgG samples (n = 10) significantly decreased the levels of ERK1/2 phosphorylation when compared with stimulation of HEK293-CaSR cells by Ca2+ alone: P values were less than 0.05 when comparing Ca2+-stimulated ERK1/2 phosphorylation with and without preincubation with patient's IgG samples at Ca2+ concentrations of 0.5, 1.5, and 3 mmol/liter (Fig. 4B).
Fig. 4.
Effect of the patient's IgG on the response of the CaSR to Ca2+ stimulation in HEK293-CaSR cells. Changes in IP1 accumulation and ERK1/2 phosphorylation were measured in response to Ca2+ at 0, 0.5, 1.5, 3.0, and 5.0 mmol/liter in HEK293-CaSR cells preincubated with the patient's IgG samples (n = 8) at a 1:50 dilution. The IgG samples were from sera taken before (samples 1–3), during (sample 4 at 60 mg prednisone; sample 5 at 20 mg prednisone; sample 6 at 10 mg prednisone; sample 7 at 5 mg prednisone), and after (sample 8) glucocorticoid treatment. IgG samples from healthy controls (n = 10) were also tested. HEK293-CaSR cells without preincubation with IgG were also included. A, The results show IP1 accumulation (mean ± sd of three experiments) in Ca2+-stimulated HEK293-CaSR cells that were preincubated with either IgG from the patient or IgG from a single control or that were not preincubated with IgG. The patient's IgG samples and the control IgG samples did not significantly affect the levels of IP1 accumulation at any Ca2+ concentration when compared with HEK293-CaSR cells that were not preincubated with IgG: P values were greater than 0.05 when comparing Ca2+-stimulated IP1 accumulation with and without preincubation with IgG samples at all the Ca2+ concentrations used. B, The results show the phosphorylation of ERK1/2 (mean ± sd of three experiments) in Ca2+-stimulated HEK293-CaSR cells that were preincubated with either IgG from the patient or IgG from a single control or that were not preincubated with IgG. The patient's IgG samples, but not the control IgG samples, significantly decreased ERK1/2 phosphorylation when compared with stimulation of HEK293-CaSR cells by Ca2+ alone at concentrations of 0.5, 1.5, and 3.0 mmol/liter: P values were less than 0.05 when comparing Ca2+-stimulated ERK1/2 phosphorylation with and without preincubation with the patient's IgG samples at Ca2+ concentrations of 0.5, 1.5, and 3 mmol/liter.
The results indicate the presence of anti-CaSR autoantibodies that can downmodulate ERK1/2 phosphorylation, but not IP1 accumulation, mediated by the CaSR in response to Ca2+ stimulation. The results also demonstrate that the anti-CaSR autoantibodies suppressed receptor function to similar degrees in samples taken before, during, and after glucocorticoid therapy, suggesting that treatment with prednisone did not alter autoantibody-mediated receptor inhibition.
Discussion
Here we describe a patient with AHH diagnosed with hypercalcemia at 73 yr of age and her response to a trial of glucocorticoids. Impaired CaSR signaling in the parathyroid glands and kidneys was suggested by PTH-dependent hypercalcemia and marked hypocalciuria. The absence of calcium disorders in the family and the acquired nature of the hypercalcemia in the eighth decade of life ruled out FHH caused by a mutation in the CASR gene. Elevated ANA and anti-RNP titers raised suspicion for the presence of blocking autoantibodies against the CaSR. IP assays led to the discovery of autoantibodies directed against the CaSR in the patient and peptide phage-display studies had previously shown that the patient's anti-CaSR autoantibodies recognized at least one epitope, amino acid residues 41–69 in the N terminal of the extracellular domain of the receptor (5). Functional studies using HEK293 cells stably transfected with the CaSR showed that the anti-CaSR autoantibodies inhibited activation of the receptor by calcium, as assessed by changes in ERK1/2 phosphorylation, and confirmed the diagnosis of AHH.
Because AHH has been described in only a handful of cases (7–10), little information is available to guide the diagnosis of this condition or the treatment of the associated hypercalcemia. Previous reports showed that the hypercalcemia in AHH failed to respond to parathyroidectomy or bisphosphonate treatment (7, 8). We had previously reported an AHH patient's response to glucocorticoids, which raised the possibility that immune therapy could be used to both diagnose AHH and treat the associated hypercalcemia. Although neither bisphosphonates nor subtotal parathyroidectomy successfully controlled this patient's hypercalcemia, glucocorticoid treatment completely normalized her calcium levels and lowered her anti-CaSR autoantibody titers and PTH levels (8). Our current patient's hypercalcemia, with a peak total calcium levels of 11.5 mg/dl and ionized calcium of 1.53 mmol/liter did not respond to pamidronate infusions and was not expected to respond to parathyroidectomy, given the underlying autoantibody-mediated CaSR dysfunction. Prednisone doses that had normalized serum calcium levels in our previous AHH patient had little effect on serum calcium, phosphate, or PTH levels in the current patient. Because corticosteroids also had no effect on either anti-CaSR autoantibody levels or autoantibody-mediated receptor inactivation, the observed increase in urinary calcium excretion is unlikely to be caused by changes in anti-CaSR autoantibody activity. Instead, it is likely that glucocorticoid treatment had a direct effect in increasing urinary calcium excretion as has been previously described in non-AHH patients (13–15). As has been demonstrated previously, PTH levels did not significantly increase with glucocorticoid treatment despite the increased urinary calcium excretion and stable serum calcium levels, suggesting that other mechanisms such as increased bone resorption or subtle alterations in PTH secretion or responsiveness may have accounted for the stable hypercalcemia (16–19). Whether other immune modulators (e.g. rituximab), or specific calcimimetics targeting the CaSR, would have had an effect on this patient's hypercalcemia remains unknown.
It is unclear what determines glucocorticoid responsiveness in patients with AHH. One possible explanation is the nature of the autoreactive anti-CaSR autoantibodies. The previously described glucocorticoid-responsive patient had elevated IgG4 levels and other autoimmune disorders characterized by autoreactive IgG4 autoantibodies. Indeed, that patient's anti-CaSR autoantibodies were found to be exclusively of the IgG4 subtype (8). This feature may account for the responsiveness to steroids because other disorders classically associated with IgG4 autoantibodies, such as autoimmune pancreatitis and bullous pemphigoid, also readily respond to corticosteroids (20–22). In contrast, the current patient had normal IgG4 levels despite having other markers of a Th2 type immune response such as elevated IgE levels. Because of normal baseline IgG4 levels and a lack of response to glucocorticoids, IgG subclass analysis was not performed on the patient's anti-CaSR autoantibodies.
Another feature that remains unexplained is why some autoantibodies against the CaSR give rise to AHH and others to autoimmune hypoparathyroidism, either in isolation or as part of the APS1. Anti-CaSR autoantibodies are commonly found in patients with autoimmune hypoparathyroidism (1, 4, 23). These autoantibodies can cause hypoparathyroidism either through cytotoxic destruction of the parathyroid gland induced by complement activation (1, 24) or through inhibition of PTH release by CaSR activation (3, 11). In AHH, the mechanisms that prevent the destruction of the parathyroid glands in these patients are not well known. In at least one case, complement activation by the exclusively IgG4 anti-CaSR autoantibodies would not have been possible because this IgG subclass does not bind complement. In addition to not causing glandular destruction, the anti-CaSR autoantibodies in AHH patients can suppress receptor signaling in some cases, as was shown in this patient. CaSR signaling regulates both inositol phosphate accumulation and ERK1/2 phosphorylation (25), and several patients with autoantibody-induced CaSR dysfunction have been shown to have concordant alterations in both pathways (3, 7, 11). Interestingly, the current patient's anti-CaSR autoantibodies inhibited Ca2+-induced ERK1/2 phosphorylation but had no detectable impact on Ca2+-induced IP1 accumulation. The impaired CaSR signaling resulted in the rightward shift of the dose-response relationship between extracellular Ca2+ concentration and ERK1/2 phosphorylation. In parathyroid cells, ERK1/2 activation has been shown to inhibit PTH secretion (26, 27). Anti-CaSR autoantibodies in other AHH patients have been similarly shown to block Ca2+-induced ERK1/2 phosphorylation (7, 9), pointing to the importance of this signaling pathway in modulating PTH release and possibly urinary calcium reabsorption. In contrast to the ERK1/2 pathway, the Gq/11-coupled Ca2+-induced IP1 accumulation has been reported to be impaired (7), enhanced (9), or, as in this patient, unaffected by the anti-CaSR autoantibodies in AHH. It has been proposed that the blocking autoantibodies against the anti-CaSR in AHH patients induce a conformational change in the receptor that inhibits signaling through the Gi pathway and facilitates PTH release (9). Our data seem to suggest, but do not definitely prove, that inhibition of the Gq/11 pathway that gives rise to Ca2+-induced IP1 accumulation may not be as critical in the regulation of PTH secretion. Although we cannot exclude that biologically relevant changes in IP1 accumulation may have occurred at earlier time points, the discordant results of enhanced inositol phosphate accumulation and depressed ERK1/2 phosphorylation in response to Ca2+ stimulation in a previous AHH patient (9) support our findings. Although differences in epitopes recognized by the CaSR autoantibodies among the AHH patients (5, 7–9) may contribute to differential receptor responses, the fact that autoantibodies from both AHH and APS1 patients map to the same region of the CaSR (5) makes it unlikely that this is the only contributing factor.
In conclusion, we have described a patient with AHH caused by anti-CaSR autoantibodies that impair receptor-mediated ERK1/2 phosphorylation but seem to have little effect on IP1 accumulation. Many questions about AHH remain unanswered, including how common it is in patients with PTH-dependent hypercalcemia. In addition to the case reports showing strong evidence that the autoantibodies are playing a causative role in AHH (7–9), anti-CaSR autoantibodies have been found in a small subset of patients with primary hyperparathyroidism (10, 28). However, it is unclear whether the hyperparathyroidism was caused by the anti-CaSR autoantibodies because the functional blockade of the receptor was not shown, and there was no correlation between autoantibody titers and hypercalcemia. Based on the current patient's results, it is unlikely that the clinical diagnosis of AHH could be made in a patient suspected of having the disease by simply evaluating the serum calcium and PTH responses to a trial of glucocorticoids. It remains to be seen whether other immune modulators could have either a diagnostic or therapeutic role in this disease.
Acknowledgments
E.M.B. is supported by Grant DK078331 from the National Institutes of Health.
Disclosure Summary: J.C.P., E.H.K., C.B., L.K., D.M.S., and A.P.W. have nothing to declare. E.M.B. has received lecture fees from Athena Diagnostics, Inc. and has a financial interest in the calcimimetic, cinacalcet, through NPS Pharmaceuticals, Inc.
Footnotes
- Ab
- Antibody
- AHH
- autoimmune hypocalciuric hypercalcemia
- ANA
- antinuclear antibody
- APS1
- autoimmune polyendocrine syndrome type 1
- CaSR
- calcium-sensing receptor
- FHH
- familial hypocalciuric hypercalcemia
- HEK293 cells
- human embryonic kidney 293 cells
- HEK293-CaSR cells
- HEK293 cells expressing the CaSR
- HRP
- horseradish peroxidase
- IP
- immunoprecipitation
- IP1
- inositol-1-phosphate
- RNP
- ribonuclear protein.
References
- 1. Li Y, Song YH, Rais N, Connor E, Schatz D, Muir A, Maclaren N. 1996. Autoantibodies to the extracellular domain of the calcium sensing receptor in patients with acquired hypoparathyroidism. J Clin Invest 97:910–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goswami R, Brown EM, Kochupillai N, Gupta N, Rani R, Kifor O, Chattopadhyay N. 2004. Prevalence of calcium sensing receptor autoantibodies in patients with sporadic idiopathic hypoparathyroidism. Eur J Endocrinol 150:9–18 [DOI] [PubMed] [Google Scholar]
- 3. Kifor O, McElduff A, Leboff MS, Moore FD, Jr, Butters R, Gao P, Cantor TL, Kifor I, Brown EM. 2004. Activating antibodies to the calcium-sensing receptor in two patients with autoimmune hypoparathyroidism. J Clin Endocrinol Metab 89:548–556 [DOI] [PubMed] [Google Scholar]
- 4. Gavalas NG, Kemp EH, Krohn KJ, Brown EM, Watson PF, Weetman AP. 2007. The calcium-sensing receptor is a target of autoantibodies in patients with autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab 92:2107–2114 [DOI] [PubMed] [Google Scholar]
- 5. Kemp EH, Gavalas NG, Akhtar S, Krohn KJ, Pallais JC, Brown EM, Watson PF, Weetman AP. 2010. Mapping of human autoantibody binding sites on the calcium-sensing receptor. J Bone Miner Res 25:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown EM. 2009. Anti-parathyroid and anti-calcium sensing receptor antibodies in autoimmune hypoparathyroidism. Endocrinol Metab Clin North Am 38:437–445, x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kifor O, Moore FD, Jr, Delaney M, Garber J, Hendy GN, Butters R, Gao P, Cantor TL, Kifor I, Brown EM, Wysolmerski J. 2003. A syndrome of hypocalciuric hypercalcemia caused by autoantibodies directed at the calcium-sensing receptor. J Clin Endocrinol Metab 88:60–72 [DOI] [PubMed] [Google Scholar]
- 8. Pallais JC, Kifor O, Chen YB, Slovik D, Brown EM. 2004. Acquired hypocalciuric hypercalcemia due to autoantibodies against the calcium-sensing receptor. N Engl J Med 351:362–369 [DOI] [PubMed] [Google Scholar]
- 9. Makita N, Sato J, Manaka K, Shoji Y, Oishi A, Hashimoto M, Fujita T, Iiri T. 2007. An acquired hypocalciuric hypercalcemia autoantibody induces allosteric transition among active human Ca-sensing receptor conformations. Proc Natl Acad Sci USA 104:5443–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pelletier-Morel L, Fabien N, Mouhoub Y, Boitard C, Larger E. 2008. Hyperparathyroidism in a patient with autoimmune polyglandular syndrome. Intern Med (Tokyo, Japan) 47:1911–1915 [DOI] [PubMed] [Google Scholar]
- 11. Kemp EH, Gavalas NG, Krohn KJ, Brown EM, Watson PF, Weetman AP. 2009. Activating autoantibodies against the calcium-sensing receptor in two patients with autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab 94:4749–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trinquet E, Fink M, Bazin H, Grillet F, Maurin F, Bourrier E, Ansanay H, Leroy C, Michaud A, Durroux T, Maurel D, Malhaire F, Goudet C, Pin JP, Naval M, Hernout O, Chrétien F, Chapleur Y, Mathis G. 2006. D-myo-inositol 1-phosphate as a surrogate of D-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation. Anal Biochem 358:126–135 [DOI] [PubMed] [Google Scholar]
- 13. Suzuki Y, Ichikawa Y, Saito E, Homma M. 1983. Importance of increased urinary calcium excretion in the development of secondary hyperparathyroidism of patients under glucocorticoid therapy. Metabolism 32:151–156 [DOI] [PubMed] [Google Scholar]
- 14. Reid IR, Ibbertson HK. 1987. Evidence for decreased tubular reabsorption of calcium in glucocorticoid-treated asthmatics. Horm Res 27:200–204 [DOI] [PubMed] [Google Scholar]
- 15. Reid IR. 1997. Glucocorticoid osteoporosis—mechanisms and management. Eur J Endocrinol 137:209–217 [DOI] [PubMed] [Google Scholar]
- 16. Slovik DM, Neer RM, Ohman JL, Lowell FC, Clark MB, Segre GV, Potts JT., Jr 1980. Parathyroid hormone and 25-hydroxyvitamin D levels in glucocorticoid-treated patients. Clin Endocrinol (Oxf) 12:243–248 [DOI] [PubMed] [Google Scholar]
- 17. Paz-Pacheco E, Fuleihan GE, LeBoff MS. 1995. Intact parathyroid hormone levels are not elevated in glucocorticoid-treated subjects. J Bone Miner Res 10:1713–1718 [DOI] [PubMed] [Google Scholar]
- 18. Hattersley AT, Meeran K, Burrin J, Hill P, Shiner R, Ibbertson HK. 1994. The effect of long- and short-term corticosteroids on plasma calcitonin and parathyroid hormone levels. Calcif Tissue Int 54:198–202 [DOI] [PubMed] [Google Scholar]
- 19. Bonadonna S, Burattin A, Nuzzo M, Bugari G, Rosei EA, Valle D, Iori N, Bilezikian JP, Vedhuis JD, Giustina A. 2005. Chronic glucocorticoid treatment alters spontaneous pulsatile parathyroid hormone secretory dynamics in human subjects. Eur J Endocrinol 152:199–205 [DOI] [PubMed] [Google Scholar]
- 20. Aalberse RC, Stapel SO, Schuurman J, Rispens T. 2009. Immunoglobulin G4: an odd antibody. Clin Exp Allergy 39:469–477 [DOI] [PubMed] [Google Scholar]
- 21. Finkelberg DL, Sahani D, Deshpande V, Brugge WR. 2006. Autoimmune pancreatitis. N Engl J Med 355:2670–2676 [DOI] [PubMed] [Google Scholar]
- 22. Joly P, Roujeau JC, Benichou J, Picard C, Dreno B, Delaporte E, Vaillant L, D'Incan M, Plantin P, Bedane C, Young P, Bernard P. 2002. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med 346:321–327 [DOI] [PubMed] [Google Scholar]
- 23. Mayer A, Ploix C, Orgiazzi J, Desbos A, Moreira A, Vidal H, Monier JC, Bienvenu J, Fabien N. 2004. Calcium-sensing receptor autoantibodies are relevant markers of acquired hypoparathyroidism. J Clin Endocrinol Metab 89:4484–4488 [DOI] [PubMed] [Google Scholar]
- 24. Brandi ML, Aurbach GD, Fattorossi A, Quarto R, Marx SJ, Fitzpatrick LA. 1986. Antibodies cytotoxic to bovine parathyroid cells in autoimmune hypoparathyroidism. Proc Natl Acad Sci USA 83:8366–8369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magno AL, Ward BK, Ratajczak T. 20 August 2010. The calcium-sensing receptor: a molecular perspective. Endocr Rev 10.1210/er.2009-0043 [DOI] [PubMed] [Google Scholar]
- 26. Corbetta S, Lania A, Filopanti M, Vicentini L, Ballaré E, Spada A. 2002. Mitogen-activated protein kinase cascade in human normal and tumoral parathyroid cells. J Clin Endocrinol Metab 87:2201–2205 [DOI] [PubMed] [Google Scholar]
- 27. Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. 2007. The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117:4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Charrié A, Chikh K, Peix JL, Berger N, Decaussin M, Veber S, Bienvenu J, Lifante JC, Fabien N. 2009. Calcium-sensing receptor autoantibodies in primary hyperparathyroidism. Clin Chim Acta 406:94–97 [DOI] [PubMed] [Google Scholar]