Abstract
Delay to delivery of a reinforcer can decrease responding for that reinforcer and increase responding for smaller reinforcers that are available concurrently and delivered without delay; acute administration of drugs can alter responding for large, delayed reinforcers, although the impact of chronic treatment on delay discounting is not well understood. In this experiment, the effects of repeated administration of the benzodiazepine flunitrazepam were studied in 6 pigeons responding on one key to receive food that was delivered immediately and on a second key to receive a larger amount of food that was delivered following delays which increased across a single session. Pigeons responded predominantly for the large reinforcer when there were no delays and when delays were short; however, as delays increased, responding for the large reinforcer decreased. Acutely, flunitrazepam (0.32, 1.0 and 3.2 mg/kg) dose-dependently increased responding for the large reinforcer, shifting the discounting curve rightward and upward. Repeated administration of flunitrazepam (0.32, 1.0 and 3.2 mg/kg, each for six sessions, separated by one session during which vehicle was administered) did not markedly alter its effects on responding for the large reinforcer, indicating that the development of tolerance to this effect of flunitrazepam is modest under these conditions.
Keywords: delay discounting, fixed delays, flunitrazepam, tolerance, key peck, pigeons
Impulsivity involves a number of behavioral traits which, broadly defined, include premature actions, failure to inhibit responses, and acting without considering the possible consequences associated with a particular choice. It is frequently observed in drug abusers and might play a role in ongoing abuse and possibly relapse. Several behavioral tasks have been developed to study the effects of drugs of abuse on various aspects of impulsivity, including the delay-discounting procedure. In this task, subjects generally respond on one manipulandum to receive a reinforcer and on a second manipulandum to receive a different reinforcer; in many delay-discounting procedures, the two reinforcers are qualitatively the same and vary only in magnitude. When both reinforcers are available immediately, subjects respond predominantly for the large reinforcer, and as delay to its delivery increases, responding for the large reinforcer decreases. Delay discounting has been studied in pigeons (e.g. Mazur, 2000; Mazur & Biondi, 2009), rats (e.g. Evenden & Ryan, 1996; Kieres, Hausknecht, Farrar, Acheson, de Wit, & Richards, 2004), nonhuman primates (e.g. Hwang, Kim, & Lee, 2009; Woolverton, Myerson, & Green, 2007) and humans (see review by Reynolds, 2006) and, in all of these species, delayed reinforcers are less preferred, as compared to reinforcers that are delivered without delay. The curve generated by plotting responding for the large reinforcer as a function of delay is best described by a hyperbola (Mazur, 2000), and it can be shifted right/left and up/down by manipulating variables such as food or water deprivation and by administration of drugs. Increased discounting is evident by decreased responding for the large reinforcer when its delivery is delayed, resulting in a leftward or downward shift in the discounting curve, and decreased discounting is evident by increased responding for the large reinforcer in spite of its delayed delivery, resulting in a rightward or upward shift in the discounting curve.
Acute administration of drugs can alter discounting. For example, in rats responding for food, ethanol (Evenden & Ryan, 1999), morphine (Pattij, Schetters, Janssen, Wiskerke, & Schoffelmeer, 2009) and nicotine (Dallery & Locey, 2005) decrease responding for large reinforcers that are delivered after a delay, shifting the discounting curve leftward. However, drugs can produce differential effects on delay discounting, depending on the conditions under which they are studied. Amphetamine increases discounting slightly when a stimulus light is illuminated during the delay (Cardinal, Robbins, & Everitt, 2000), and decreases discounting when stimuli are not present during the delay (Cardinal et al., 2000; Evenden & Ryan, 1996). In addition, benzodiazepines can produce differential effects: modestly increasing (Cardinal et al., 2000) and decreasing (Evenden & Ryan, 1996) discounting in rats and increasing discounting in pigeons (Wolff & Leander, 2002) responding for food.
Repeated administration of drugs can also change discounting. For instance, rats treated chronically with methamphetamine discount more, as compared to discounting before treatment (Richards, Sabol, & de Wit, 1999). Similarly, nicotine increases discounting in rats, and this effect appears to be long-lasting. Rats were treated daily with 0.3 mg/kg of nicotine for 14 weeks; increased discounting was observed during chronic treatment and continued for one month following discontinuation of nicotine treatment (Dallery & Locey, 2005). Similarly, repeated cocaine treatment (30 mg/kg/day for 2 weeks) increased discounting in rats, and this effect also persisted after discontinuation of treatment (e.g. Roesch, Takahashi, Gugsa, Bissonette, & Schoenbaum, 2007; Simon, Mendez, & Setlow, 2007). Thus, chronic drug treatment can increase discounting during and after treatment and might contribute to both continued drug abuse and relapse long after treatment is discontinued.
Although the incidence of exclusive benzodiazepine abuse is low, they are often abused with other drugs, such as opioids (Gelkopf, Bleich, Hayward, Bodner, & Adelson, 1999; Lavie, Fatséas, Denis, & Auriacombe, 2009; Peles, Schreiber, & Adelson, 2006; San, Tato, Torrens, Castillo, Farré, & Camí, 1993). Use of benzodiazepines by patients in methadone-maintenance programs predicts poorer treatment outcomes and those patients are also more likely to engage in risky behaviors (Bleich, Gelkopf, Schmidt, Hayward, Bodner, & Adelson, 1999; Ghitza, Epstein, & Preston, 2008), perhaps indicating that these patients are more impulsive. In the current study, the effects of the benzodiazepine flunitrazepam were examined on one aspect of impulsivity. Six pigeons responded under conditions where delays to the large reinforcer were increased in fixed amounts within an experimental session (Evenden & Ryan, 1996). Once a stable delay-discounting curve was established, the acute and chronic effects of the benzodiazepine flunitrazepam were studied.
METHOD
Subjects
Six adult white Carneaux pigeons (435–600 g) were housed individually in a temperature- and humidity-controlled vivarium under a 14-hr light/10-hr dark cycle with unlimited access to water and grit in the home cage. Pigeons were maintained at 85–90% of their free-feeding weights with food (Purina Pigeon Checkers) received during experimental sessions and, if necessary, provided in the home cage. All pigeons had a history of responding for food under various schedules of reinforcement and received morphine, diazepam and dizocilpine in previous experiments; they were drug-free for at least 3 months prior to the current experiment. All animal care and experimental protocols were in accordance with the National Institutes of Health Guide to the Care and Use of Laboratory Animals (National Academy of Sciences, 1996) and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Apparatus
Two different models of operant conditioning chambers were used in this experiment. Two self-contained chambers (BRS/LVE, Laurel, MD, USA) and two chambers (Med Associates, Inc., St. Albans, VT, USA) contained in sound-attenuating boxes (BRS/LVE, Laurel, MD, USA) were used; each pigeon was assigned to a specific experimental chamber for the duration of the study. Each box had a response panel consisting of three translucent response keys that were arranged horizontally and could be illuminated with red lights. Centered below the response keys was an opening which allowed access to a food hopper. During presentation of the food hopper, a white light illuminated the opening. A white house light was located on the opposite wall of the chamber and was illuminated during delay periods. An interface connected chambers to a computer which controlled experimental events and collected data using MED-PC/Medstate Notation software (MED Associates Inc., East Fairfield, VT).
Procedure
The fixed-delays procedure used in this experiment was based on a previous study in rats (Evenden and Ryan, 1996). Sessions began with a 15-min timeout during which time the chamber was dark and responses had no programmed consequence. The remainder of the session was divided into five discrete, 16-min cycles for a total session duration of 95 min. Cycles began with two forced trials followed by 10 choice trials. The center key was illuminated red signaling the beginning of a trial. On forced trials, a single response on the center key extinguished the center key and illuminated one of the outer keys. A response on the illuminated key extinguished that key and resulted in the immediate presentation of the food hopper or initiation of the delay during which the house light was illuminated; the delay was followed by presentation of the food hopper. The outer response key that was associated with delivery of the large reinforcer was counterbalanced across pigeons and was maintained for each individual pigeon throughout the experiment. During the second forced trial, the opposite outer key was illuminated after the response on the center key; the order in which the forced trials were presented varied randomly across sessions. There was a 10-s limited hold on each forced trial; if a pigeon did not complete both forced trials, the cycle ended and the chamber remained dark until the beginning of the next cycle. After completion of the two forced trials, the 10 choice trials began with illumination of the center key. A response on the center key turned off the center key and illuminated both outer keys; the pigeon could respond on either key to receive food. A single response on the left or right key turned off both keys and was followed by immediate presentation of the food hopper or illumination of the house light during a delay followed by presentation of the food hopper. Pecking one key resulted in 1.5-s access to the food hopper and pecking the other key resulted in 4-s access to the food hopper; these times were selected based on a previous study which found that similar reinforcer magnitudes (2-, 4- and 8-s access to the food hopper) maintained high levels of responding in pigeons (Lamb & Ginsburg, 2005). The next trial began 60 s after the completion of the response requirement; if no response was made within 10 s of the beginning of a choice trial, that trial ended and a 60-s timeout began. During the first cycle of the session, there was no delay between a response and delivery of the reinforcer. Beginning on the second cycle, there was a delay to the large reinforcer that was doubled from one cycle to the next; delays to the large reinforcer were 2, 4, 8 and 16 s for 5 pigeons and 6, 12, 24 and 48 s for the other pigeon.
Testing began when the following criteria were satisfied for three consecutive sessions: ≥ 90% responding during the first cycle on the key associated with the large reinforcer, ≤ 20% responding during the last cycle on the key associated with the large reinforcer, and at least 5 out of 10 choice trials completed during each cycle. To determine whether delays to the large reinforcer accounted for the switch in responding to the key associated with the small reinforcer across the session, delays were removed for consecutive sessions and a response on either key resulted in immediate access to the food hopper. Sessions without delays were conducted until the percentage of responding on the key associated with the large reinforcer was ≥ 80% on the last cycle for all pigeons. Thereafter, sessions with delays were conducted until the testing criteria were satisfied for one session.
Before drug administration began, the testing criteria were again satisfied for three consecutive sessions during which saline or sham injections were administered. On separate occasions, vehicle or flunitrazepam (0.1–3.2 mg/kg) was acutely administered immediately prior to the session. Sessions during which vehicle or flunitrazepam were administered were separated by at least three sessions during which saline or sham injections were administered. Next, the effects of daily flunitrazepam treatment on delay discounting were studied for 20 days. For 6 consecutive days, pigeons were treated with 0.32 mg/kg flunitrazepam immediately prior to the session. On Day 7, a vehicle injection replaced the flunitrazepam injection. Because there was no change in discounting over this period of treatment, as compared to the first day of treatment, the daily dose of flunitrazepam was increased to 1.0 mg/kg for the next 6 days; on Day 14, a vehicle injection was given instead of the flunitrazepam injection. The daily treatment dose was then increased a third time to 3.2 mg/kg/day of flunitrazepam and administered for an additional 6 days. Daily flunitrazepam treatment was terminated on Day 21 with injections of vehicle administered immediately before the session for the next 3 days.
Drugs
Flunitrazepam (Sigma-Aldrich Co., St. Louis MO, USA) was dissolved in a vehicle containing 20% emulphor, 10% ethanol and 70% saline. All injections were administered intramuscularly in a volume of 1.0 ml/kg body weight.
Statistical analyses
Data were included in the analyses of the percentage of responding on the key associated with the large reinforcer as long as 5 of the 10 choice trials were completed during a cycle. The percentage of responding for the large reinforcer was determined by dividing the number of responses emitted on that key by the total number of responses emitted on the left and right keys and multiplying by 100. The total number of trials completed and response latencies for each cycle were also recorded. Data obtained during sessions without delays were analyzed by two-way repeated measures analysis of variance (ANOVA) with session and cycle as within-subjects factors. When flunitrazepam was administered acutely, data were analyzed by two-way repeated measures ANOVA with treatment dose and cycle as within-subjects factors. Where appropriate, Dunnett's Multiple Comparison test was used to conduct post-hoc analyses. Chronic flunitrazepam studies were analyzed by first calculating area under the curve (AUC) for each segment of the individual delay-discounting curves using the trapezoid rule (ΔX * (Y1 + Y2/2)). An AUC score and 95% confidence interval were determined for the session that immediately preceded chronic treatment; AUC scores obtained during chronic treatment were considered significantly different when they fell outside of the confidence interval obtained before chronic treatment. Data were analyzed using SPSS 14.0 for Windows (Chicago, IL, USA, www.spss.com) or GraphPad 5.01 Software (San Diego, CA, USA, www.graphpad.com). Statistical tests were deemed significant when p < 0.05.
RESULTS
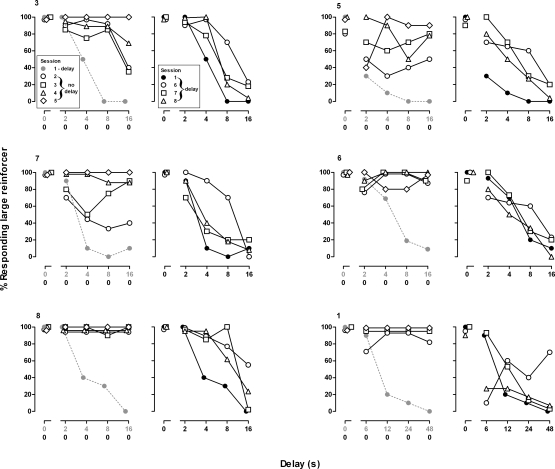
During the first cycle of sessions, there was no delay to the delivery of the large reinforcer, and when saline was administered immediately before the session, pigeons responded exclusively for the large reinforcer (Figure 1, leftmost gray circles). On the second cycle, there was a short delay to delivery of the large reinforcer (2 s for 5 pigeons, 6 s for 1 pigeon), and 5 of the 6 pigeons, including the one with the 6-s delay, responded predominantly on the key associated with the large reinforcer. As the delays increased over subsequent cycles, pigeons switched responding from the key associated with the large reinforcer to the key associated with the small reinforcer; for 3 of the 6 pigeons, responding for the large reinforcer was decreased to < 20% on the third cycle (Figure 1, filled circles). By the last cycle, an average of 4% responding occurred on the key associated with the large reinforcer (Figure 2, filled circles). Pigeons completed nearly all of the trials (mean number of trials completed/cycle ± S.E.M.: 9.8 ± 0.2), and response latencies were less than two seconds (mean range ± S.E.M.: 1.1 ± 0.2 to 1.4 ± 0.3) for each cycle across the entire session (data not shown).
Fig 1.
Responding on the key associated with the large reinforcer in 6 individual pigeons (3, 5, 7, 6, 8 and 1). Filled circles represent the percentage of responding for the large reinforcer during the session with delays that immediately preceded the first session without delays. Results obtained during four sessions without delays are shown in the left panel and results obtained during the three subsequent sessions with delays are shown in the right panels. Abscissa: delay in seconds to the large reinforcer. Ordinate: percentage of responding for the large reinforcer.
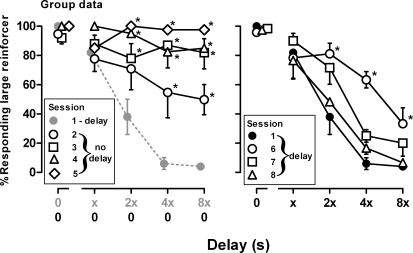
Fig 2.
Responding on the key associated with the large reinforcer averaged across 6 pigeons. Filled circles represent the percentage of responding for the large reinforcer during the session with delays that immediately preceded the first session without delays. Results obtained during four sessions without delays are shown in the left panel and results obtained during the three subsequent sessions with delays are shown in the right panel. Abscissa: delay to the large reinforcer. Ordinate: mean percentage of responding for the large reinforcer (± 1 SEM). * p < .05, as compared to filled circles.
During sessions without delays, there was an increase in responding for the large reinforcer both within and across sessions (Figure 1, open symbols, left columns). This effect was evident during the first session with no delays when 3 of the 6 pigeons (6, 8 and 1) emitted at least 70% of their responses on the key associated with the large reinforcer on all cycles (Figure 1, open circles, left panels). A total of four sessions without delays were needed before all 6 pigeons responded ≥ 80% on the key associated with the large reinforcer on the last cycle of the session (Figure 1, diamonds, left panels). A two-way ANOVA of the group data revealed that there was more responding for the large reinforcer late in the session, F (4, 144) = 61.78, p < .05, with responding for the large reinforcer significantly greater on the last two (Figure 2, open circles) or three cycles (Figure 2, diamonds, squares and triangles), as compared to sessions with delays (Figure 2, filled circles). Analysis of the group data also revealed a significant change in responding for the large reinforcer across sessions without delays, F (4, 144) = 9.82, p < .05. There were no differences in the mean number of trials completed, F (28, 152) = 0.74, ns, or in mean response latencies, F (28, 152) = 0.48, ns, during sessions without delays, as compared to sessions with delays (data not shown).
After four sessions during which there were no delays to delivery of the large reinforcer, sessions with delays were conducted. Beginning on the first session with delays, responding for the large reinforcer decreased across the session for all pigeons except pigeon 1 (Figure 1, open symbols, right columns); all pigeons satisfied the testing criteria within three sessions. Analysis of the group data revealed a significant decrease in responding for the large reinforcer across delays within a session, F (4, 100) = 67.91, p < .05, and across the three sessions, F (3, 100) = 7.13, p < .05. On the first session with delays, the mean percentage of responding for the large reinforcer remained elevated late in the session, as compared to responding during the session that immediately preceded sessions without delays (Figure 2, compare open and filled circles) and was not different during the subsequent session (Figure 2, compare open triangles and filled circles).
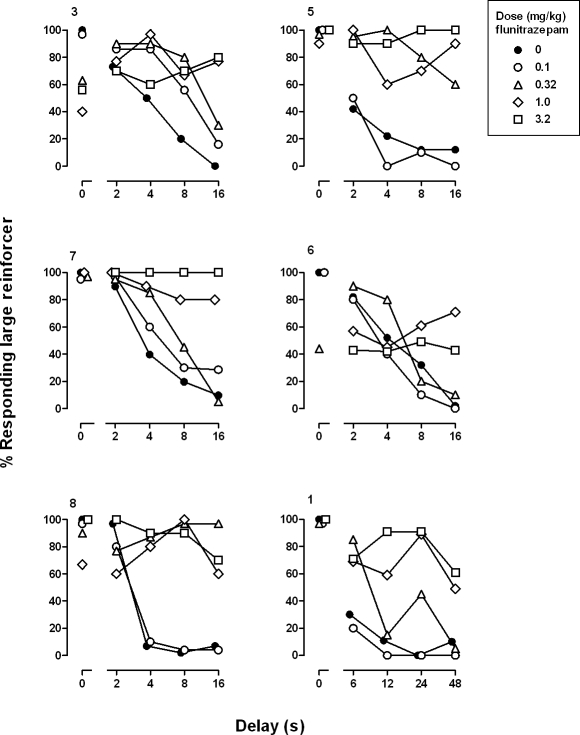
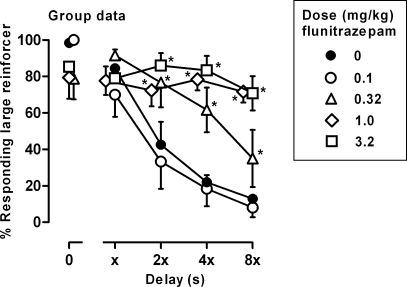
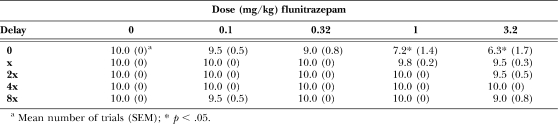
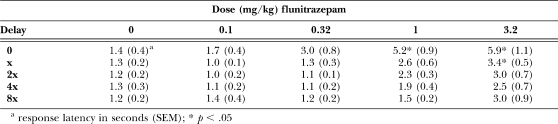
In 5 of the 6 pigeons, an acute dose of 0.1 mg/kg of flunitrazepam did not alter the distribution of responding on the two outer keys, and in Pigeon 3, there was an increase in responding for the large reinforcer, as compared to responding during sessions when vehicle was administered (Figure 3, compare open and filled circles). In 3 pigeons (3, 6 and 8), larger doses of flunitrazepam decreased responding for the large reinforcer during the first cycle when there was no delay to delivery of the large reinforcer; in the other 3 pigeons, there was no change in responding for the large reinforcer during the first cycle. In pigeons 6 and 8, decreased responding on the key associated with the large reinforcer was still evident during the second cycle when there was a short delay to delivery of the large reinforcer. As the delay increased on subsequent cycles, responding for the large reinforcer did not change across the session, shifting the discounting curve to the right and upward. For all pigeons except pigeon 6, doses of 1.0 and 3.2 mg/kg of flunitrazepam produced > 50% responding on the key associated with the large reinforcer during the two cycles with the longest delays (Figure 3, diamonds and squares). Analysis of the group data by two-way ANOVA revealed a significant dose × cycle interaction, F (16, 100) = 7.09, p < .05. Doses of flunitrazepam larger than 0.1 mg/kg increased responding for the larger reinforcer during the last three cycles of the session, as compared to responding following administration of vehicle (Figure 4, triangles, diamonds and squares). There was a significant dose × cycle interaction on the number of trials completed, F (16, 100) = 1.77, p < .05 (see Table 1), with a significant decrease in the number of trials completed on the first cycle of the session following administration of 1.0 and 3.2 mg/kg of flunitrazepam, as compared to the number of trials completed following administration of vehicle. Likewise, there was a significant dose × cycle interaction on response latencies, F (16, 100) = 2.40, p < .05 (Table 2). Latency to respond increased significantly on the first cycle when 1.0 mg/kg of flunitrazepam was administered and on the first and second cycles when 3.2 mg/kg of flunitrazepam was administered, as compared to latencies during sessions when vehicle was administered.
Fig 3.
Effects of flunitrazepam on responding for the large reinforcer in 6 individual pigeons (3, 5, 7, 6, 8 and 1). Filled circles represent the effects of vehicle. Pigeon 7 did not respond during the first cycle following administration of 3.2 mg/kg of flunitrazepam, and Pigeon 1 did not respond during the first cycle following administration of 1.0 mg/kg of flunitrazepam. Abscissa: delay in seconds to the large reinforcer. Ordinate: percentage of responding for the large reinforcer.
Fig 4.
Effects of flunitrazepam on responding averaged across 6 pigeons. Filled circles represent the effects of vehicle. Abscissa: delay to the large reinforcer. Ordinate: mean percentage of responding for the large reinforcer (± 1 SEM). * p < .05, as compared to filled circles.
Table 1.
Number of trials completed following acute administration of flunitrazepam.
Table 2.
Initial response latencies (s) following acute administration of flunitrazepam.
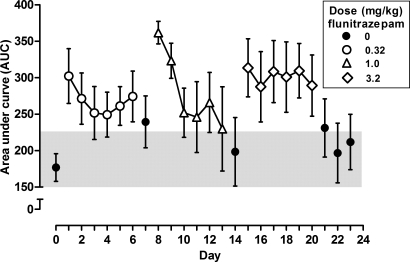
Daily treatment with flunitrazepam began with a dose of 0.32 mg/kg/day, which was administered immediately before sessions. On the first day of chronic treatment, responding for the large reinforcer increased, as compared to responding during the previous session when saline was administered, resulting in a larger AUC score (Figure 5, compare filled circle above 0 to open circle above 1). A two-way repeated measures ANOVA of AUC scores revealed a significant treatment day x dose interaction, F (15, 75) = 2.60, p < .05. During repeated daily treatment with 0.32 (open circles) and 1.0 mg/kg of flunitrazepam (triangles), AUC scores decreased slightly across days; in contrast, when 3.2 mg/kg of flunitrazepam (diamonds) was administered for 6 days, there was little change across days. When flunitrazepam treatment was temporarily suspended (days 7 and 14) or discontinued (days 21, 22 and 23), AUC scores decreased and were not different from scores obtained before chronic treatment on days 14, 22 and 23 (Figure 5, filled circles).
Fig 5.
Area under the curve (AUC) scores calculated for the percentage of responding for the large reinforcer before, during and after chronic flunitrazepam treatment. The shaded area indicates the 95% confidence intervals of the AUC scores for the session that preceded daily flunitrazepam treatment; points falling outside of the 95% confidence interval are considered significantly different from before treatment. Abscissa: day of treatment. Ordinate: AUC scores for percentage of responses for the large reinforcer (± 1 SEM).
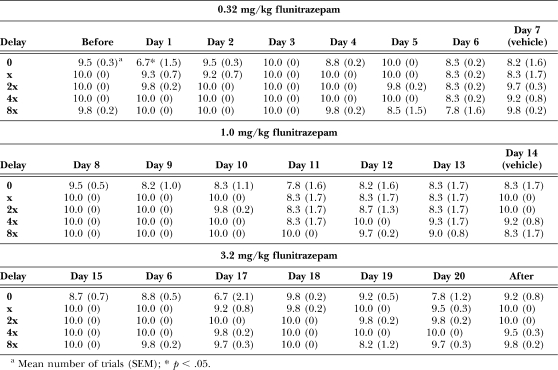
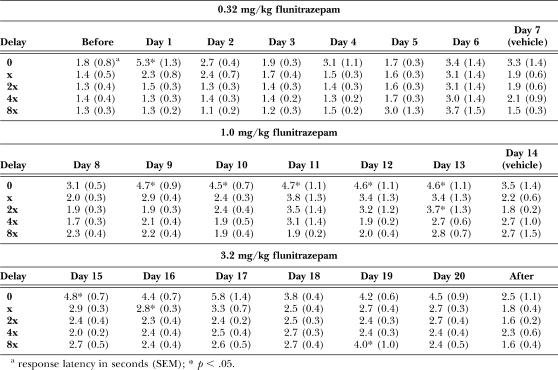
Daily treatment with 0.32 mg/kg of flunitrazepam significantly decreased the number of trials completed, F (24, 140) = 2.16, p < .05 (see Table 3), with significantly fewer trials completed on the first cycle of the first day of treatment. Increasing the treatment dose to 1.0, F (24, 140) = 0.61, ns, and 3.2 mg/kg of flunitrazepam, F (24, 140) = 1.12, ns, did not alter the number of trials completed. There was an overall significant main effect of dose on response latency during chronic treatment, F (22, 550) = 4.97, p < .05 (see Table 4). On the first cycle of the first day of treatment, 0.32 mg/kg of flunitrazepam increased response latencies, as compared to latency during the session that preceded chronic treatment. When the daily treatment dose was increased to 1.0 mg/kg of flunitrazepam, response latencies were increased on the first cycle of days 9–13. At a treatment dose of 3.2 mg/kg of flunitrazepam, there was a significant increase in latency on the first cycle of days 15–20, and on the second cycle of day 16 and the fifth cycle of day 19.
Table 3.
Number of trials completed before, during and after chronic administration of flunitrazepam. Vehicle injections were given in place of flunitrazepam on days 7 and 14 of chronic treatment.
Table 4.
Response latencies (s) before, during and after chronic administration of flunitrazepam. Vehicle injections were given in place of flunitrazepam on days 7 and 14 of chronic treatment.
DISCUSSION
In this study, pigeons responded under a delay-discounting procedure for a small reinforcer that was always delivered immediately and a large reinforcer that was delivered after a delay; these delays increased across cycles within experimental sessions. When there were no delays or when delays were short, pigeons responded predominantly on the key associated with delivery of the large reinforcer; as delays increased, responding for the large reinforcer decreased. By the last cycle of the session, pigeons responded predominantly on the key associated with the small reinforcer. When delays were removed for all cycles of the session, responding on the key associated with the large reinforcer increased. For 3 of the 6 pigeons, this change in responding was evident during the first session without delays, although 4 sessions were needed before all pigeons responded predominantly on the key associated with the large reinforcer across the entire session. In sessions with delays, responding on the key associated with the large reinforcer decreased over the session. Taken together, these results suggest that responding for the large reinforcer was controlled by the delay to its delivery.
When flunitrazepam was administered acutely, the discounting curve shifted rightward and upward in a dose-dependent manner with doses of 1.0 and 3.2 mg/kg of flunitrazepam also decreasing the number of trials completed and increasing response latencies. Under other conditions, benzodiazepines also alter discounting, although the effects are somewhat inconsistent across studies. For example, in rats running a T-maze, several benzodiazepines, including diazepam, nitrazepam, chlordiazepoxide and clobazam, decreased responding for the large reinforcer (Thiébot, Le Bihan, Soubrié, & Simon, 1985). In procedures involving operant conditioning, diazepam decreased discounting in rats (Evenden & Ryan, 1996) while alprazolam and chlordiazepoxide increased discounting in pigeons (Wolff & Leander, 2002). One possible explanation for these differences among studies is the use of different species; however, results from the current study with pigeons indicate that flunitrazepam decreased discounting whereas other benzodiazepines increased discounting in pigeons responding under an adjusting-delay procedure (Wolff & Leander, 2002). Other effects of benzodiazepines could impact results of delay-discounting procedures, independent of their effects on discounting. For example, benzodiazepines can impair working memory (Dåderman, Frederiksson, Kristiansson, Nilsson, & Lidberg, 2002; Lane, Cherek, & Nouvion, 2008), although an impairment in working memory might be expected to result in an equal distribution of responses across the two keys rather than responding predominantly on one key throughout the entire session. Another effect of benzodiazepines that might impact results in the delay-discounting procedure is their effect on punished responding. Benzodiazepines can increase responding suppressed by punishment in rats (Witkin, 2002) and pigeons (Kleven & Koek, 1999); to the extent that delay to delivery of the reinforcer is a punisher, then benzodiazepines would be expected to increase responding for the large reinforcer.
Although AUC scores decreased during repeated treatment with 0.32 and 1 mg/kg of flunitrazepam, they remained significantly greater than the AUC score obtained before treatment began, suggesting that the development of tolerance was modest. In addition, tolerance did not develop to the effects of flunitrazepam on response latency, which was increased during chronic treatment, particularly on the first cycle. Under other conditions, tolerance develops rapidly to the rate-decreasing effects of flunitrazepam in rats responding under a fixed-ratio schedule of food presentation (Gerak, 2009) and to the rate-decreasing effects of diazepam in pigeons responding under fixed-interval schedules of food presentation (McMillan, 1992). In addition, the effects of chronic flunitrazepam administration on delay discounting were short-lived; when treatment was terminated, responding for the large reinforcer decreased and was not different from the responding obtained on the day before chronic treatment. In contrast, other drugs that alter discounting during chronic treatment, including nicotine (Dallery & Locey, 2005) and cocaine (Roesch et al., 2007; Simon et al., 2007), have effects that persist long after treatment is discontinued. Results from the present study suggest that, while the effects of flunitrazepam on discounting are robust during treatment, these effects dissipate rapidly once treatment is discontinued.
Delay discounting measures just one aspect of impulsivity, and benzodiazepines might have different effects when other aspects of impulsivity are measured. For example, benzodiazepines have been shown to increase prepotent responding and disrupt the inhibition of premature responses in a five-choice serial reaction time task (Oliver, Ripley, & Stephens, 2009), a go/no-go procedure (Sokolic & McGregor, 2007) and a stop signal reaction time test (Fillmore, Rush, Kelly, & Hays, 2001). Case studies suggest that flunitrazepam can increase impulsive behavior in humans, particularly aggressive behavior (Dåderman et al., 2002). In addition, laboratory tasks measuring risky decision making have shown that acute administration of flunitrazepam dose-dependently increases choice of more risky options (Lane et al., 2008), suggesting that benzodiazepines decrease risk aversion.
In summary, flunitrazepam increased responding on the key associated with the large reinforcer in a dose-dependent manner under both acute and chronic treatment conditions. Miminal tolerance developed to the effects of flunitrazepam on discounting and the effects on discounting were no longer evident 24 hr after discontinuation of treatment. Additional studies are needed to determine how these findings might extend to other species, particularly humans, as well as the importance of the differential effects of benzodiazepines on various measures of impulsivity; such studies appear to be warranted given that repeated benzodiazepine treatment and its discontinuation did not markedly alter discounting in the current study.
Acknowledgments
Amy K. Eppolito and Lisa R. Gerak are in the Department of Pharmacology, University of Texas Health Science Center at San Antonio. Charles P. France is in the Departments of Pharmacology and Psychiatry, University of Texas Health Science Center at San Antonio. Charles P. France is supported by a Senior Scientist Award DA 17918.
Portions of these data were presented at Behavior, Biology and Chemistry: Translational Research in Addiction Conference, held March 6–7, 2010 in San Antonio, Texas.
REFERENCES
- Bleich A, Gelkopf M, Schmidt V, Hayward R, Bodner G, Adelson M. Correlates of benzodiazepine abuse in methadone maintenance treatment. A 1 year prospective study in an Israeli clinic. Addiction. 1999;94:1533–1540. doi: 10.1046/j.1360-0443.1999.941015339.x. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N, Robbins T.W, Everitt B.J. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Dåderman A.M, Fredriksson B, Kristiansson M, Nilsson L.H, Lidberg L. Violent behavior, impulsive decision-making, and anterograde amnesia while intoxicated with flunitrazepam and alcohol or other drugs: a case study in forensic psychiatric patients. The Journal of the American Academy of Psychiatry and the Law. 2002;30:238–251. [PubMed] [Google Scholar]
- Dallery J, Locey M.L. Effects of acute and chronic nicotine on impulsive choice in rats. Behavioural Pharmacology. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Evenden J.L, Ryan C.N. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Evenden J.L, Ryan C.N. The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1999;146:413–421. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- Fillmore M.T, Rush C.R, Kelly T.H, Hays L. Triazolam impairs inhibitory control of behavior in humans. Experimental and Clinical Psychopharmacology. 2001;9:363–371. doi: 10.1037//1064-1297.9.4.363. [DOI] [PubMed] [Google Scholar]
- Gelkopf M, Bleich A, Hayward R, Bodner G, Adelson M. Characteristics of benzodiazepine abuse in methadone maintenance treatment patients: a 1 year prospective study in an Israeli clinic. Drug and Alcohol Dependence. 1999;55:63–68. doi: 10.1016/s0376-8716(98)00175-6. [DOI] [PubMed] [Google Scholar]
- Gerak L.R. Selective changes in sensitivity to benzodiazepines, and not to other positive GABAA modulators, in rats receiving flunitrazepam chronically. Psychopharmacology. 2009;204:667–677. doi: 10.1007/s00213-009-1497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza U.E, Epstein D.H, Preston K.L. Self-report of illicit benzodiazepine use on the Addiction Severity Index predicts treatment outcome. Drug and Alcohol Dependence. 2008;97:150–157. doi: 10.1016/j.drugalcdep.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Kim S, Lee D. Temporal discounting and inter-temporal choice in rhesus monkeys. Frontiers in Behavioral Neuroscience. 2009;3:9. doi: 10.3389/neuro.08.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieres A.K, Hausknecht K.A, Farrar A.M, Acheson A, de Wit H, Richards J.B. Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology. 2004;173:167–174. doi: 10.1007/s00213-003-1697-2. [DOI] [PubMed] [Google Scholar]
- Kleven M.S, Koek W. Effects of benzodiazepine agonists on punished responding in pigeons and their relationship with clinical doses in humans. Psychopharmacology. 1999;141:206–212. doi: 10.1007/s002130050826. [DOI] [PubMed] [Google Scholar]
- Lamb R.J, Ginsburg B.C. Fluvoxamine and desipramine on fixed-ratio responding: effects of reinforcement magnitude. Behavioural Pharmacology. 2005;16:573–578. doi: 10.1097/01.fbp.0000181594.01244.a2. [DOI] [PubMed] [Google Scholar]
- Lane S.D, Cherek D.R, Nouvion S.O. Modulation of human risky decision making by flunitrazepam. Psychopharmacology. 2008;196:177–188. doi: 10.1007/s00213-007-0951-4. [DOI] [PubMed] [Google Scholar]
- Lavie E, Fatséas M, Denis C, Auriacombe M. Benzodiazepine use among opiate-dependent subjects in buprenorphine maintenance treatment: correlates of use, abuse and dependence. Drug and Alcohol Dependence. 2009;99:338–344. doi: 10.1016/j.drugalcdep.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Mazur J.E. Tradeoffs among delay, rate, and amount of reinforcement. Behavioural Processes. 2000;49:1–10. doi: 10.1016/s0376-6357(00)00070-x. [DOI] [PubMed] [Google Scholar]
- Mazur J.E, Biondi D.R. Delay–amount tradeoffs in choices by pigeons and rats: hyperbolic versus exponential discounting. Journal of the Experimental Analysis of Behavior. 2009;91:197–211. doi: 10.1901/jeab.2009.91-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan D.E. Effects of drugs on behavior before and during chronic diazepam administration. European Journal of Pharmacology. 1992;215:145–152. doi: 10.1016/0014-2999(92)90022-v. [DOI] [PubMed] [Google Scholar]
- Oliver Y.P, Ripley T.L, Stephens D.N. Ethanol effects on impulsivity in two mouse strains: similarities to diazepam and ketamine. Psychopharmacology. 2009;204:679–692. doi: 10.1007/s00213-009-1500-0. [DOI] [PubMed] [Google Scholar]
- Pattij T, Schetters D, Janssen M.C, Wiskerke J, Schoffelmeer A.N. Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology. 2009;205:489–502. doi: 10.1007/s00213-009-1558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Adelson M. Factors predicting retention in treatment: 10-year experience of a methadone maintenance treatment (MMT) clinic in Israel. Drug and Alcohol Dependence. 2006;82:211–217. doi: 10.1016/j.drugalcdep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behavioural Pharmacology. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Richards J.B, Sabol K.E, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology. 1999;146:432–439. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- Roesch M.R, Takahashi Y, Gugsa N, Bissonette G.B, Schoenbaum G. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. Journal of Neuroscience. 2007;27:245–250. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San L, Tato J, Torrens M, Castillo C, Farré M, Camí J. Flunitrazepam consumption among heroin addicts admitted for in-patient detoxification. Drug and Alcohol Dependence. 1993;32:281–286. doi: 10.1016/0376-8716(93)90093-6. [DOI] [PubMed] [Google Scholar]
- Simon N.W, Mendez I.A, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behavioural Neuroscience. 2007;121:543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolic L, McGregor I.S. Benzodiazepines impair the acquisition and reversal of olfactory go/no-go discriminations in rats. Behavioural Neuroscience. 2007;121:527–534. doi: 10.1037/0735-7044.121.3.527. [DOI] [PubMed] [Google Scholar]
- Thiébot M.H, Le Bihan C, Soubrie P, Simon P. Benzodiazepines reduce the tolerance to reward delay in rats. Psychopharmacology. 1985;86:147–152. doi: 10.1007/BF00431700. [DOI] [PubMed] [Google Scholar]
- Witkin J.M. Some contextual and historical determinants of the effects of chlordiazepoxide on punished responding of rats. Psychopharmacology. 2002;163:488–494. doi: 10.1007/s00213-002-1135-x. [DOI] [PubMed] [Google Scholar]
- Wolff M.C, Leander J.D. Selective serotonin reuptake inhibitors decrease impulsive behavior as measured by an adjusting delay procedure in the pigeon. Neuropsychopharmacology. 2002;27:421–429. doi: 10.1016/S0893-133X(02)00307-X. [DOI] [PubMed] [Google Scholar]
- Woolverton W.L, Myerson J, Green L. Delay discounting of cocaine by rhesus monkeys. Experimental and Clinical Psychopharmacology. 2007;15:238–244. doi: 10.1037/1064-1297.15.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]