Abstract
Acid-sensing ion channels (ASICs) are H+-gated Na+ channels, which are present in most, if not all, neurons. The typical ASIC current is transient and is elicited by a rapid drop in the extracellular pH. In the human genome, four genes for ASICs are present: asic1 – 4. In this review, we will focus on ASIC1a, one of the key subunits in the central nervous system. We will describe the structure of this channel, a topic that has enormously profited from the recent elucidation of the first crystal structure of an ASIC. We will then relate the ASIC1 structure to current models of the gating mechanism of ASICs. Finally, we will review the pharmacology of ASIC1a. Advances in the pharmacological inhibition of individual ASIC currents have greatly contributed to our current knowledge of the functional roles of this channel in physiology, including learning, memory, and fear conditioning, and in pathophysiological states, including the neurodegeneration accompanying stroke, and axonal degeneration in autoimmune inflammation.
Keywords: Acidosis, desensitization, excitatory postsynaptic current, fear conditioning, ischemia, psalmotoxin
Introduction
In 1981, Krishtal and Pidoplichko described for the first time a receptor for H+ that carried inward Na+ currents in mammalian sensory neurons [1, 2]. It was not until 1997 that the group of Lazdunski cloned a channel from rat brain that was directly activated by H+and carried an excitatory Na+ current [3]. This channel, ASIC1a, was just the first member of a small family of H+ -gated channels. Thanks to recent work in many laboratories, we now have a clearer picture of the physiological function of these H+-gated channels and there are clear indications that ASICs contribute to the outcome of several pathophysiological states.
ASIC1a had been cloned by its homology to the amiloride-sensitive epithelial Na+ channel (ENaC), with which it shares approximately 25% amino acid sequence identity [3]. Other homo-logues of ASICs include peptide-gated channels from polyps [4] and mollusks [5] and mech-anosensitive channels (the degenerins or “DEG” channels) from C. elegans [6]. Thus, ASICs define a branch of the DEG/ENaC family of ion channels. Since the most ancient DEG/ENaC channels that have been characterized so far are peptide-gated channels from the cnidarian Hydra, the HyNaCs [4], and since HyNaCs are close relatives of ASICs within the DEG/ENaC gene family [4], it is conceivable that H+-gated ASICs evolved from peptide-gated channels.
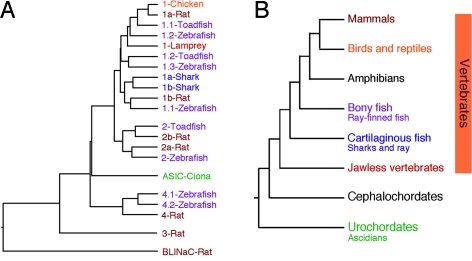
So far, ASICs have been identified exclusively in chordates (Figure 1). A single ASIC gene is already found in the genome of Ciona intestinalis [7], an urochordate that belongs to the earliest branch in the chordate phylum. Moreover, ASICs have been cloned from a jawless vertebrate (lamprey) [8], from cartilaginous fish (shark) [8], from bony fish (toadfish and zebrafish) [9, 10], from chicken [8] and different mammals (mouse, rat, human) (Figure 1).
Figure 1.
A) Phylogenetic tree illustrating the relationship of selected ASICs. Note that a clear ASIC1 ortholog is present in all vertebrate lineages, whereas ASIC3 has so far only been identified in mammalian species. The urochordate Ciona contains a single asic gene [7], whereas in mammals the number of asic genes has increased to four. Amino acid sequences of selected ASICs were aligned and the tree for the cladogram established by neighbor joining using ClustalX (DNAstar software); sequences at the N- and C-termini had been deleted. The related brain-liver-intestine Na+ channel (BLINaC) form rat [156] was included for comparison. B) Phylogenetic tree of the chordate phylum. Main chordate clades are shown. The color corresponds to the color of ASICs from organisms of these clades in (A). So far, no ASICs have been reported from cephalochordates and amphibians; their existence in these clades is, however, likely.
ASICs are defined by their sequence homology; functionally, some ASICs, like ASIC2b and ASIC4, are not sensitive to acid [11-13] and the function of those subunits is not always known. ASIC2b contributes to proton-sensing by forming heteromeric channels with other ASICs [11]; the function of ASIC4, however, is unknown and current evidence suggests it is not involved in H+-sensing [12]. On the other hand, some DEG/ ENaC members, which do not belong to the ASIC branch, are sensitive to H+. For example, acid-sensitive channel, degenerin-like (ACD-1) from C. elegans, which is most closely related to degenerins, is inhibited by intra- and extracellular acidification [14]. Another protein from C. elegans with sequence homology to DEG/ENaC channels has been dubbed ASIC-1 [15]. Since this channel does not belong to the ASIC branch of the DEG/ENaC family and functional expression, let alone H+-sensitivity, has not been described, this name is misleading and the protein should be renamed.
In the present review we will focus on ASIC1. ASIC1 is the most abundant ASIC subunit in the mammalian central nervous system (CNS). In addition, in the CNS it is the ASIC with the highest H+-affinity; ASIC3 is equally sensitive to H+ but restricted to the peripheral nervous system (PNS) [16]. However, one recent study has reported expression of ASIC3 in rat hypothalamus [17]. Moreover, homomeric ASIC1a is the only ASIC that is slightly permeable to Ca2+ [3, 18, 19]. The importance of ASIC1a in the CNS is underlined by the fact that it is apparently involved in a number of neurodegenerative diseases (see below). Finally, chicken ASIC1 is the only ASIC that has been crystallized [20] and the gating mechanism of ASIC1 has been elucidated in some detail. The ASIC1 gene gives rise to two variants - ASIC1a and ASIC1b [19, 21]. The splice variant ASIC1b is restricted to the PNS [21], like ASIC3, and its physiological functions are largely unknown. Moreover, although an ASIC1b gene is present in the human genome, clear evidence for an ASIC1b cDNA in humans is lacking. Therefore, we will discuss ASIC1b only where it sheds further light on the properties of ASIC1a.
We will start with a short description of the biophysical properties of ASIC1a. We will then discuss current models of the gating mechanism of ASIC1a, review its pharmacology and will then briefly introduce what we know about the physiological functions of ASIC1a and its implications in the pathogenesis of neurodegenera-tive diseases. Most of the discussion will be on homomeric ASIC1a; it is known, however, that heteromers containing ASIC1a also mediate legated currents in many neurons. In the CNS, homomeric ASIC1a and heteromeric ASIC1a/2a are the major ASICs [22-24].
Biophysical properties of ASIC1a
In most studies, ASIC1a starts to open at pH 6.9 [3, 25]; current amplitude then increases with increasing H+ concentrations down to pH 6.0, where ASIC current amplitude starts to saturate. The H+-concentration response curve can be fit by a Hill function and yields half-maximal activation at pH 6.5 with a Hill-coefficient of approximately 3 [3, 25]. Very recently, it has been shown that in neurons acutely dissociated from the amygdala, ASICs containing the ASIC1a sub-unit are even more sensitive to H+: channels opened at pH 7.2 [26]. Whether this extreme sensitivity to tiny variations in the H+ concentrations is due to formation of heteromeric channels, or, more likely, to some modulation by accessory proteins or other unknown factors, awaits further studies.
ASIC1a desensitizes completely with a time constant of 1 - 2 sec [3, 19]. Complete desensitization means that ASIC1a cannot encode sustained acidification. It recovers from desensiti-zation within a few seconds [25]. Desensitization of ASIC1a starts at pH values slightly below pH 7.4 and during prolonged acidification to pH values below 7.1 all ASIC channels are in the desensitized state [25]. Thus, ASIC1a can enter the desensitized state without apparent opening. This steady-state desensitization (SSD) can also be fit by a Hill function: for rat ASIC1a it is half-maximal at pH 7.25 and has a Hill coefficient of approximately 10 [25]; for mouse and human ASIC1a, it is half-maximal at pH 7.15 and 6.90, respectively [27]. Thus, SSD seems to be a process completely separate from activation, with a higher H+ affinity.
This conclusion is not necessarily true, however. At low H+ concentrations (for rat, between pH 7.4 and 6.9) channels may open with a low probability. The unconcerted opening (and subsequent desensitization) of individual channels would not lead to visible currents in whole cell measurements and pass unnoticed. In agreement with such a scenario, SSD takes much longer than open state desensitization (70 sec, at pH 7.05, rather than 1-2 sec) [25]. In addition, in most mutagenesis studies where mutations changed the apparent H+ affinity of ASIC1a, mutations also affected H+ affinity of SSD [25], arguing that both processes are indeed intimately linked. Moreover, for the rapidly desensitizing ATP receptor P2X1, it has been shown that desensitization masks a higher ATP affinity of P2X1 activation [28, 29], suggesting that the “real” H+ affinity of ASIC1a is that of SSD. The E79A substitution in ASIC3 is an example of a mutation that shifts the SSD curve to 10-fold lower H+ concentrations without shifting the activation curve [30], suggesting that this mutation uncouples SSD from activation gating. However, since the same mutation also enhanced desensitization 4-fold [30], it is not excluded that the faster desensitization may mask a higher H+ affinity of ASIC3 activation. In summary, whether ASIC1a channels can reach the desensitized state directly from the closed state and if so, to which extent this closed state desensitization contributes to SSD at slight acidification are open questions. Irrespective of this uncertainty, all observations suggest that ASIC1a is an exquisitely sensitive H+ receptor; if H+ affinity were only slightly higher, the channel would be chronically desensitized at rest.
Recently, it has been shown that the endogenous opioid peptides dynorphin A and big dynor-phin shift the SSD curve of ASIC1a to lower H+ concentrations limiting the impact of SSD [31]; probably dynorphin directly binds to the extracellular loop of ASIC1a. This result suggests that ASIC1 should be considered as a new nonopioid target for dynorphin action in the CNS.
For ASIC3 it has been shown that the curves of H+ activation and SSD overlap and that in the window of overlap ASIC3 carries sustained currents [32]. This property endows ASIC3 with the capacity to encode also the sustained acidification in the window of overlap between pH 7.3 and 6.7. ASIC1a, however, carries no such window currents [32] and can, therefore, encode only the acidic transients as they may occur duringsynaptic transmission.
Once gated open, the ASIC1a pore has a slight selectivity for Na+ over K+ (PNa/PK = 10) [3, 19], so that at a membrane potential of -70 mV, which is much closer to the K+ than the Na+ equilibrium potential, ASIC1a mainly carries an excitatory inward current of Na+. In addition, homomeric ASIC1a is the only ASIC that has a slight permeability to Ca2+ (PNa/Pca = 15) [18, 19, 33]. The fractional Ca2+ current is, however, very small [34]. Therefore, the Ca2+ influx through ASIC1a per se will significantly raise the intracellular Ca2+ concentrations only in a micro-domain close to the channel, if at all. Ca2+ is a permeant blocker of ASIC1a, permeating through the channel but also blocking it [3, 35].
Finally, ASIC1a is not only gated by H+ but also permeable to H+[3, 36]. Not unexpected, relative H+ permeability is higher than Na+ permeability (PNa/PH < 0.3) [36] but due to the very small H+ concentrations (1 μM at pH 6.0) relative to the Na+ concentration (∼100 mM), the fractional H+ current will again be tiny. However, it could lead to slight acidification in a microdo-main surrounding the intracellular mouth of the channel.
Tachyphylaxis is another peculiar feature of homomeric ASIC1a that is specific to this subtype [36]. Tachyphylaxis means that repeated activation of the channel leads to ever-smaller current amplitudes even though the time interval between individual activation is sufficient for recovery from normal desensitization. Thus, tachyphylaxis uncovers a second long-lived desensitized state, from which the channel may not recover [36]. Tachyphylaxis is linked to H+ influx though ASIC1a and its Ca2+ permeability [36]. Since tachyphylaxis is especially pronounced at pH < 6 [36], it is unclear to which extent it limits ASIC1a activity in vivo.
The structure of an ASIC: pre-crystal era
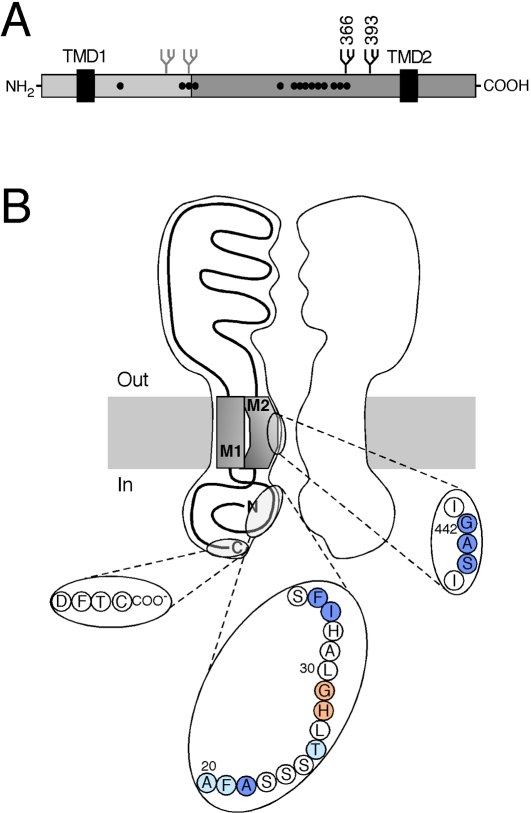
ASICs consist of 500 to 560 amino acids. The primary structure of each ASIC subunit is characterized by two hydrophobic regions of approximately 20 amino acids, long enough to span the lipid bilayer, rather short termini (35 - 90 amino acids) and a large domain between the two hydrophobic domains (approximately 370 amino acids) (Figure 2A). The transmembrane topology of the related ENaC had been established in three independent studies [37-39] and revealed that the two termini are located in the cytoplasm, whereas the domain between the two hydrophobic domains, each of which spans the membrane once, forms a large extracellular domain (ECD). It was confirmed that ASICs have the same transmembrane topology [40] (Figure 2B). The first exon, which is different between ASIC1a and ASIC1b, thus comprises the intracellular N-terminus, the first transmembrane domain (TMD) and approximately the first one-third of the ECD (Figure 2A).
Figure 2.
A) Linear model of ASIC1a. The first one-third of the protein, which is different between ASIC1a and lb, is indicated in light grey. The two J-COOH hydrophobic domains are shown as black boxes. The position of the two glycosylation sites of ASIC1a are indicated by a branched symbol and the two additional glycosylation sites, which are specific for ASIC1b, are shown in grey. The 14 cysteines that are conserved in ASICs are shown as black dots; 10 of them cluster in one region. B) Model of the transmembrane topology of ASIC1a from the pre-crystal era. TMD2 faces the ion pore, the three residues (G-A-S) that probably form the ion-selective part of the molecular sieve [53, 55, 59] are magnified in the inset Residues within the intracellular N-terminus that have been implicated in contributing to the internal pore of ASIC1a [65] or ASIC2 [66] are marked in the inset in dark and light blue, respectively. Amino acids in this region also determine Ca2+-permeability of ASIC1a [18]. The amino acid sequence from rat ASIC1a is shown. The two invariably conserved amino acids (H-G) in the cytoplasmic N-terminus that are involved in gating of ENaC [67, 68] are marked in orange. The four amino acids (D-F-T-C) at the cytoplasmic C-terminus that bind to the PDZ domain of PICK1 [69, 70] are also magnified in an inset.
A large ECD is not specific to ASICs, but shared by all DEG/ENaC channels, although its size can vary. Homology between different ASICs is largest in the second two-thirds of the ECD, whereas some parts of the first one-third are characterized by considerable sequence divergence. All ASICs have 14 conserved cysteines in their ECD (Figure 2A), which likely stabilize the structure of the ECD by forming disulphide-bonds [41]. As almost every membrane protein, ASICs carry at least one N-glycan in the ECD. ASIC1a has two glycans in the distal part of the ECD, whereas ASIC1b carries four glycans: two in the distal part, which is identical to ASIC1a, and two additional glycans in the proximal part [42] (Figure 2A), which is different between ASIC1a and ASIC1b. It has been proposed that the two proximal glycans help in shaping the structure of the proximal ECD of ASIC1b [42], which may therefore be substantially different between ASIC1a and ASIC1b.
The number of subunits in a functional channel complex has been experimentally addressed in several studies, most of the studies focusing on ENaC and one study on FaNaC [43]. These studies came to conflicting results, most of them proposing tetramers [43-48] and some nonatriers [49, 50]; one recent study found identical numbers of the three different ENaC subunits (α, β, γ), making a trimeric structure a minimal subunit composition [51].
Several studies, most on the ENaC [52-64] and some on ASICs [35, 65], suggested that the second TMD, especially the first, outer half contributes to the outer entrance and the selectivity filter of the ion pore (Figure 2B). ENaC, which is characterized by a high Na+ selectivity of its pore (PNa/PK > 100), selects ions by molecular sieving [54], largely excluding cations bigger than Na+. The narrowest part of the pore, representing the ion-selective part of this molecular sieve, is formed by three amino acids, which are in the middle of the second TMD [54, 56, 60]. In all ASICs, these three amino acids are (from N-to C-terminus or outside to inside) Gly-Ala-Ser (Gly442–Ser444 in rat ASIC1a; Figure 2B), amino acids with small side chains. For ENaC, it was proposed that the side chains of these three amino acids do not point toward the lumen of the pore but rather towards subunit-subunit interfaces [61]. Thus, the backbone of the pep-tide would face the lumen of the selectivity filter and help to coordinate permeating ions; a similar situation is found in the P-loop of K-channels. The inner entrance to the ASIC pore is much less characterized [66].
Surprisingly, the second TMD is identical between ASIC1a and ASIC1b, yet ASIC1a is permeable to Ca2+ whereas ASIC1b is not [19]. The difference in Ca2+-selectivity has been attributed to the different intracellular N-terminus [19], suggesting that this part of the protein helps to shape the structure of the ion pore, perhaps by long-range allosteric effects. Other studies also demonstrate a role for the amino terminus in ion selectivity [67] and participation in the internal pore [66] of ASICs (Figure 2B).
Two amino acids (His-Gly) at the intracellular N-terminus (Figure 2B) are invariably conserved in all DEG/ENaC channels. Also their distance to the beginning of the first TMD (approximately 15 amino acids) is within one or two amino acids invariable, suggesting that these two amino acids have some crucial function for all DEG/ ENaC channels. In ENaC, these two amino acids plus a few surrounding amino acids, which are also similar across the whole gene family, are crucial for the gating of this channel [68, 69], defining a “gating domain". It is likely that they have a similar function in ASICs.
The function of the cytoplasmic C-terminus for ASICs is less well established. Actually the C-terminus can be quite short (>40 amino acids) in some ASICs. Although the ASIC C-terminus is not indispensable for channel activity (see below), it may be quite important for the physiological regulation of ASIC function. For example, ASIC1a interacts with its extreme C-terminus (Figure 2B) with the PDZ domain of the protein interacting with C kinase (PICK1) [70, 71]. No interactions have been directly demonstrated for ASIC1b, but since the C-terminus of ASIC1b is identical to that of ASIC1a, in principle at least, this subtype can also interact with PICK1. In addition to protein-protein interactions mediated by the C-terminus of ASIC1a, in dorsal root ganglion neurons ASIC1a physically interacts with its N-terminus with annexin light chain p11, increasing surface-expression of ASIC1a [72].
In summary, in the pre-crystal era we had a picture of ASICs being made of probably four sub-units, each having an overall simple topology with two transmembrane domains, short cytoplasmic termini and a large ECD. Although it was clear that the ECD has a defined and probably intricate structure, what this structure would exactly look like was completely elusive.
The structure of an ASIC: post-crystal era
A deletion mutant of chicken ASIC1 has been crystallized and the crystal structure has been resolved at a resolution of 1.9 A [20]. Since rat and human ASIC1a are 90 % identical to chicken ASIC1, it is very likely that many structural details are conserved in mammalian ASIC1. Therefore and to have a common reference sequence, in this review all amino acids are numbered based on the sequence of rat ASIC1a. Initially, the chicken ASIC1 structure was obtained from a deletion mutant where most of the N- and C-termini had been deleted [20]. Subsequently, a functional channel, in which only the C-terminus was deleted but not the N-terminus with its essential “gating domain", had been crystallized at a lower resolution (3 A) [73]. The main difference between the first crystal of a non-functional channel and the second crystal of a functional channel was the symmetric order of the two transmembrane domains in the functional channel [73]. Thus, the high-resolution structure revealed the precise structure of the ECD, whereas the low-resolution structure added the structure of the transmembrane domains and the ion pore. Both proteins were crystallized at low pH (pH 5-6 and 6.5, respectively), where chicken ASIC1 is desensitized. Therefore, most likely both structures represent the desensitized conformation of the channel.
It came as a big surprise that chicken ASIC crystallized in units of two trimers [20]. Since crystals of the functional chicken ASIC also contained trimers as the basic unit [73], it is highly likely that functional ASICs, and probably all functional DEG/ENaC channels, are made of three subunits. In the meanwhile, a trimeric structure has been confirmed for ASIC1a by atomic force microscopy imaging [74].
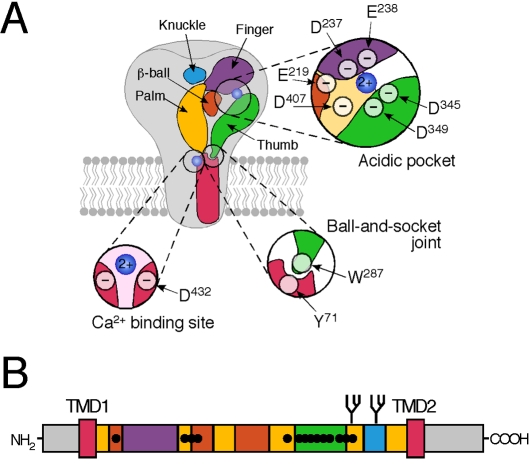
The ECD of each subunit resembles a clenched hand, which is linked to the TMDs via an apparently flexible wrist [20]. The hand domain can be divided in five subdomains: palm, finger, thumb, and knuckle domains and a β-ball domain (Figure 3A). The contacts between the subunits are in particular between different palm domains and the palm domain of one sub-unit and the thumb domain of another. Prominent subunit interactions are 1) hydrogen bonds between Asp78 (all numbers referring to rat ASIC1a) of one subunit and His73 and Gin420 of an adjacent subunit, 2) interactions between the loops of β-strands in the palm domain and the C terminus of helix α5 of the thumb domain of an adjacent subunit, and 3) interactions between helix α6 of the knuckle that is enveloped by a concave surface on the neighboring finger.
Figure 3.
A) Model of the transmembrane topology of ASIC1a from the post-crystal era (based on reference [19]). Three features of the structure and their crucial amino acids are magnified in the insets: the acidic pocket, the ball-and-socket joint and the Ca2+ binding site in the ion pore. Binding of a Ca2+ ion in the acidic pocket is hypothetical. Note that all amino acids are numbered based on the sequence of rat ASIC1a. B) Linear model of ASIC1a highlighting the relation of the primary sequence to the five subdomains of the ECD. It becomes apparent that the α-helices of the finger, thumb and knuckle subdomains are contained within contiguous stretches of the poiypeptide chain, whereas the palm and (β-ball domains are formed by (β-sheets that are scattered over the poiypeptide chain. Those (β-sheets connect the finger, thumb and knuckle subdomains. Note also that most of the cysteines (black dots) cluster within the thumb subdomain (green), giving it a rigid structure. The structure of the cytopiasmic termini is unresolved.
Most of the disulphide bonds localize to the thumb (Figure 3B), giving it a rigid structure. Since the thumb domain has contact with the wrist junction via a conserved Trp (Trp287) residue (Figure 3A), its rigid structure may serve to faithfully transduce conformational changes to the transmembrane ion pore [20].
A peculiar feature of the structure was the clustering of several acidic amino acids within a small space, the so-called acidic pocket. This pocket is far (45 A) from the TMDs and is formed by intra-subunit contacts between the thumb, the β-ball and the finger domains, together with residues from the palm domain of an adjacent subunit [20] (Figure 3A). The acidic pocket contains three pairs of acidic residues (glutamate or aspartate), which form carboxyl-carboxylate interactions. The distance of the carboxyl groups is so small (2.8 - 3.0 A; [20]) that at least one of the carboxyl groups of each pair must be protonated. This means that the pKa value of one residue of each pair is several units larger than that of isolated Asp or Glu residues.
TMD1 and TMD2 of the three subunits form ex-helices [73]. Whereas in the crystal of the nonfunctional protein TMDs were asymmetric, in the crystal of the functional channel they are symmetric, providing the structure of the ion pore in the desensitized conformation. The TMD2 helices are closest to the three-fold axis, are tilted by ∼50° with respect to the membrane and cross each other in the middle of the membrane bilayer [73]. Thus, in line with previous functional studies [52-65], TMD2 helices probably line the ion pore. The TMD1 helices make most contacts with the lipid bilayer but also with TMD2 helices of the same subunit and the TMD1 and TMD2 helices of adjacent subunits.
In the desensitized conformation, two cation-binding sites have been revealed by soaking the crystal in solutions containing Cs+. In both sites, Cs+ is coordinated by six ligands arranged in a trigonal antiprism geometry. At site 1, Cs+ is coordinated by three side chain carboxyl groups of Asp432. At site 2, ligands consist of either main-chain carbonyl oxygen atoms from Gly431 or side chain oxygen atoms from Asp432. Moreover, it was suggested that main-chain carbonyl oxygen atoms, derived mainly from glycine residues, also coordinate permeant ions in the open channel. Thus, G431 and G435 could build a third ion-binding site in the open conformation of the channel [73]. Other candidates for gly-cines involved in ion coordination are G438 and G442 [73]. G442 is the first of the three residues proposed by earlier studies to form the selectivity filter [54, 56, 60] (Figure 2B).
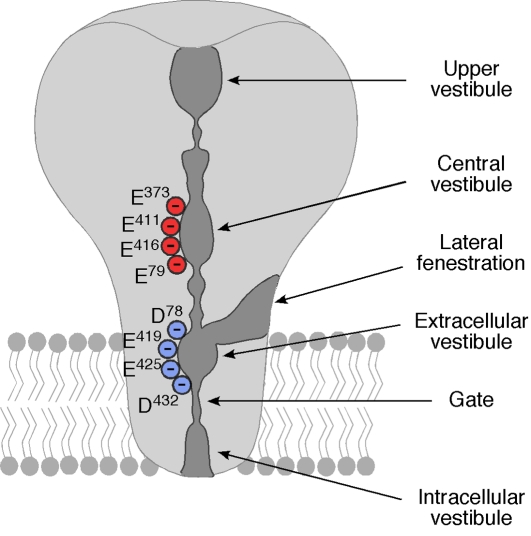
In the desensitized conformation, three vestibules can be discerned in the ECD, along the central axis of the channel [73] (Figure 4). The upper vestibule is in direct contact with the extracellular solution, whereas the central and the extracellular vestibule, which is situated at the height of the outer surface of the membrane, are separated from each other and the upper vestibule by constrictions. If these constrictions would be wider in the open channel, they would form a central tunnel, constituting a pathway for ions to the pore. Cations would be concentrated within the vestibules along the central tunnel by several acidic residues, clustered in the central and extracellular vestibules (Figure 4). In addition to this putative central pathway for ions, in the desensitized conformation, there is direct access to the extracellular vestibule through three lateral fenestrations at the wrist of the ECD.
Figure 4.
Model of ASIC1a showing the three vestibules in the ECD and the lateral access to the extracellular vestibule via a lateral fenestration (based on reference [72]). Acidic amino acids that line the central and extracellular vestibules are indicated; they are numbered based on the sequence of rat ASIC1a. The negative charge of their side chains could attract cations to the vestibules.
Even with the lateral access pathway to the extracellular vestibule, ions cannot permeate through the pore because, in the desensitized conformation, TMD2 helices cross each other in the middle of the membrane bilayer. Thus, the helices apparently change their conformation during desensitization, constricting the ion pore. This constriction therefore constitutes the desensitization gate - a physical block of the ion pore (Figure 4). Close packing of the three TMD2 helices at the constriction is made possible by Gly435.
The speed of the desensitization is determined by three amino acids in the proximal ectodo-main (S83-T85) [10]. Moreover, a residue in the immediate vicinity (E79) is modified by cysteine-modifying reagents in the closed but not the desensitized conformation [30], suggesting that this residue undergoes a conformation change during desensitization gating of the channel. Together these results suggest that the loop encompassing residues E79-T85, which localizes between the palm and the β-ball domains, undergoes a conformation change during desensitization gating that determines the speed of desensitization.
In summary, the crystal structure of chicken ASIC1 provides an invaluable picture of the intricate three-dimensional structure of the ECD and the ion pore. It suggests models for ion permeation and selectivity in these channels and, as we will see, it also suggests models for H+-gating of ASICs. One has to keep in mind, however, that the chicken ASIC1 structure is a snapshot of the desensitized state of the channel. A clear picture of the conformational changes accompanying ASIC gating needs additional crystal structures of, ideally, the closed and the open states. In the absence of these structures, the first crystal structure can nevertheless guide future structure-function studies and greatly refine models of ASIC gating.
How to gate an ASIC by H+
In principle, titration of a single amino acid of an ASIC, for example in the ECD, could induce a conformational change, opening the ion pore. Current models of ASIC gating, however, invoke a more complicated process to explain ASIC gating.
An initial observation providing a clue to ASIC gating came from the observation that extracellular Ca2+ ions modulate the apparent H+ affinity of ASIC1: high [Ca2+]e decreases apparent H+ affinity (higher H+ concentrations are needed to open the pore) and low [Ca2+]e increases apparent H+ affinity (lower H+ concentrations are needed to open the pore) [25]. To explain these findings, it was proposed that Ca2+ stabilizes the closed state of the channel and that H+ displaces Ca2+ from its binding site, effectively competing with Ca2+for this binding site [25]. This model was then extended for ASIC3 by proposing that a Ca2+ ion in fact blocks the open ASIC pore. According to this model, H+ would gate an ASIC open by unblocking the ion pore with no accompanying conformational change [75].
Substitution of D432 and E425 at the N-terminal end of TMD2 by neutral amino acids disrupted pore block of ASIC1a by Ca2+ [35]. The crystal structure of cASIC1 confirmed that D432 and E425 are accessible from the aqueous phase within the extracellular vestibule; moreover, D432 forms a putative binding site for cations at the bottom of the extracellular vestibule [73] (Figure 3A), suggesting that substitution of D432 and E425 disrupted the Ca2+ binding site in the outer pore of ASIC1a. In addition, voltage clamp fluorometric measurements showed that E425 undergoes a significant reorganization following extracellular acidification [76], in agreement with the idea that such a movement is associated with relief of Ca2+ blockade. This Ca2+ binding site mutant, therefore, allowed testing of the pore block model. According to this model, disruption of the Ca2+ binding site should have three effects: first and most importantly, the ion pore should be constitutively open, second, the channel should be no longer gated by H+ and third, Ca2+ should no longer modulate the apparent affinity for H+. All three predicted effects were not observed: mutant channels were not constitutively open, they were still gated by H+ and their apparent H+ affinity was still modulated by Ca2+ [35]. These results effectively rule out the possibility that pore block by Ca2+ can fully explain gating of ASIC1. Examination of single channel recordings of ASIC1 from toad-fish also argued against the Ca2+ pore block model and favored an allosteric model to explain ASIC gating [77]. Moreover, modulation by a spider toxin (psalmotoxin 1, see below) of chimeras between ASIC1a and lb suggested the existence of partiaIly-liganded open states of ASIC1 [78], a finding, which is in agreement with a Monod-Wyman-Changeux model for coop-erativity in allosteric proteins.
Channels with the Ca2+ binding site mutations (D432 and E425) had, however, reduced affinity for H+, a smaller Hill co-efficient and modulation by Ca2+ was less pronounced [35], suggesting that pore block by Ca2+ contributes to ASIC gating. This interpretation suggests the existence of two Ca2+ binding sites involved in ASIC gating: one at the outer entrance to the ion pore, crucial determinants are D432 and E425, and another one at a different location within the ECD. The acidic pocket is suspicious of such a second putative Ca2+ binding site within the ECD [20]. Pairs of acidic amino acids within the acidic pocket are D237-D349, E238-D345, and E219-D407 (Figure 3A); E79-E416 defines a fourth pair outside the acidic pocket [20].
Since the crystal probably represents the structure of the desensitized conformation, it has been hypothesized that, in the closed state, the acidic pocket held a Ca2+ ion that was displaced upon acidification when one of the acidic residues of each pair became protonated [20]. This is an attractive and plausible hypothesis that is supported by the experimental finding that E79, which is in an acidic pair, is inaccessible for cysteine-modifying reagents in the desensitized but not the closed state [30]. Moreover, molecular simulations of cation binding to the structure of chicken ASIC1 revealed that Ca2+ binds to the acidic pocket, but only when all three pairs of acidic residues are charged, and that H+ binding inhibits Ca2+ binding at this site [79]. Evidence for cation binding was especially strong for the two pairs D237-Q349 and E238-D345[79]. Individual mutations of amino acids of the acidic pocket have rather modest effects on ASIC gating [20, 80], however, and even combined substitution of all four amino acids from the two critical pairs (D237_D349 E23s_D345) did not disrupt proton gating of an ASIC [81]. These results suggest that there are further determinants of ASIC gating. Indeed molecular simulations suggested the surface residue pairs D298-D331 and E338-E342 as additional potential candidates for H+ sensing sites of ASIC1a [79]; these sites are not conserved in other H+-sensitive ASICs (e.g. ASIC3), however, suggesting that if they have a role in ASIC gating it must be a rather modulatory role.
In addition to titration of acidic residues within Ca2+-binding sites, H+ may also titrate amino acids that contribute to open gating of an ASIC by an allosteric mechanism. Indeed some years ago, a histidine that is highly conserved in ASICs has already been shown to be important for H+ gating of ASIC2a [82]. ASIC1a has two adjacent histidines at the corresponding position (H72/ H73) and in a large screen for amino acids involved in gating of ASIC1a, these same two histidines were found to be indispensable for ASIC1a gating [80], confirming the importance of these His residues. In this screen, D78 and E79 were also found to be important for ASIC1a gating, but only when combined with the Ca2+ binding site mutations in the pore (D432 and E425) [80]. Interestingly, in the desensitized conformation, D78 forms a hydrogen bond with H73 [20] and E79 forms a carboxyl-carboxylate pair with E416, supporting an important function of these two residues. Molecular simulations suggested that the E79-E416 pair, in contrast to the pairs from the acidic pocket (D237-D349, E238-D345, and E219-D407), preferentially binds Na+ over Ca2+ ions [79], suggesting a role in ion permeation for E79. Although their exact role is still unknown, collectively current evidence suggests a crucial role for H72/H73, D78, and E79 in ASIC gating. Indeed the presence of these amino acids has been successfully used to predict H+-sensitivity of ASIC1bfrom shark [83].
Several studies used chimeric approaches to identify the regions that are responsible for differential H+ sensitivity of different ASICs. Such regions do not necessarily contain crucial H+ binding sites, however. In one such approach with chimeras between rat ASIC1a and lb, two amino acids (K105N106) that localize to the finger domain were identified where substitutions convert the relatively high apparent H+ affinity of ASIC1a into the lower affinity of ASIC1b [25]. Molecular simulation also suggested that the finger domain moves during gating [79]. In another study with chimeras between rat ASIC1a and 2a, two larger regions (from amino acid 87 – 197 and from 323 – 431) were identified that specify the different H+ sensitivities of these two ASICs [84]. ASIC2a has a rather low H+ sensitivity, however, explaining the larger regions necessary to swap sensitivities.
Based on current evidence it has been proposed [80] that upon acidification, H73 (and perhaps H72) gets protonated, which induces a conformational change, facilitating the displacement of Ca2+ ions from its binding sites in the pore and the acidic pocket. Concomitantly, charged residues of the Ca2+ binding sites get protonated and form the carboxyl-carboxylate pairs. The conformational change is probably accompanied by a movement of the palm domain relative to the thumb domain. Movement of the thumb domain is then likely transferred to the TMDs to open the ion pore. Voltage clamp fluorometry indeed suggests that the pre-TMD2 region changes its conformation upon acidification [76]. The crucial role of the thumb domain in transferring the conformational changes of the ECD to the TMDs is suggested by the close contact of Tyr71 and Trp287 (at the base of the thumb in immediate contact to the wrist junction) in the cASIC crystal [20]. It has been proposed that these two residues form a ball-and-socket joint to transduce conformational changes of the ECD to the TMDs [20] (Figure 3A). Moreover, the importance of the interaction between Y71 and W287 for ASIC gating has recently been experimentally confirmed [81]. In addition, amino acid changes in the region around W287 determine the differential H+ sensitivity of SSD of mouse and human ASIC1a [27].
Desensitization would then be accompanied by a movement of the loop encompassing residues E79-T85, which is probably accompanied by rotation of the adjacent residues D78 towards H73 and E79 towards E416, and finally by constriction of the ion pore in the middle of TMD2. This is a tentative and hypothetical model. What is quite clear is that ASIC gating critically involves several residues scattered over the ECD, with residues at the wrist junction having a central role.
Pharmacology of ASIC1
The pharmacology of ASIC inhibitors and their therapeutic potentials have been reviewed previously [85-87]. Here we summarize recent findings from the view of structure-function relationships.
Chemically, there are three classes of ASIC inhibitors: metal ions, polypeptide toxins, and small-molecule inhibitors (table 1). Besides Ca2+ and Mg2*, a number of divalent and trivalent metal ions inhibit ASICs. PcTx1 is a cysteine-rich high-affinity polypeptide toxin purified from a tarantula venom. Amiloride, A-317567, nafamostat and diarylamidines are examples for synthetic, small molecule ASIC inhibitors.
Table 1.
Inhibitory constants (IC50) of different inhibitors at homomeric ASIC1a and ASIC currents in cultured neurons. ND, not determined
| Inhibitor | IC50 at ASIC1a [μM] | IC50 at ASIC in neurons [μM] | Reference |
|---|---|---|---|
| Zn2+ | 0.007 | ∼0.015 | [87] |
| Cu2+ | ND | ∼50 | [90] |
| Pb2+ | ∼5 | ∼10 | [91] |
| Cd2+ | no inhibition | ND | [92] |
| Ni2+ | ∼600 | ND | [92] |
| PcTx1 | ∼0.001 (at pH 7.4) | ND | [95] |
| amiloride | 10 | 10-50 | [3] |
| nafamostat | ∼15 | ND | [121] |
| ibuprofen | ∼350 | ND | [122] |
| flurbiprofen | ∼350 | ND | [122] |
| A-317567 | ND | 2-30 | [123] |
| DAPI | ND | 3 | [124] |
| pentamidine | ND | 40 | [124] |
| hydroxystilbamidine | ND | 1.5 | [124] |
| diminazene | 2-3 | 0.3 | [124] |
Metal ions
Currently zinc, copper, lead, nickel, and cadmium have been documented to affect ASIC1a. Zinc (Zn2+), an endogenous trace element, inhibits homomeric ASIC1a and heteromeric ASIC1a/2a with nanomolar affinity, without affecting currents mediated by homomeric ASIC1b, ASIC2a, or ASIC3 [88]. In agreement with high-affinity Zn2+-inhibition of the major ASICs in the CNS (ASIC1a and ASIC1a/2a), che-lation of trace amounts of Zn2+ by TPEN, a Zn2+/ Fe2+ chelator with low affinity for Ca2+, potentiates approximately two-fold ASIC currents in cultured mouse cortical neurons [88]. Likewise, sulfhydryl-containing reducing agents like dithio-threitol potentiate ASIC currents in hippocampal neurons, mainly by chelating metal ions that tonically inhibit ASIC1a and ASIC1a/2a [89]. Homomeric ASIC1a is more strongly inhibited by Zn2+ than heteromeric ASIC1a/2a [88]. At much higher (micromolar) concentrations, Zn2+ has an opposite effect and potentiates currents through homomeric ASIC2a and heteromeric ASIC1a/2a (EC50: ∼100 μM), but not through homomeric ASIC1a [82]. In addition, such high concentrations of Zn2+ slow the decay time constant of ASIC currents in hippocampal neurons [90]. High-affinity Zn2+-block depends on K133 in the ECD of ASIC1a [88] whereas Zn2+-potentiation depends on H162 and H339 in the ECDofASIC2a[82].
Micromolar concentrations of copper (Cu2+) inhibit ASIC currents in cultured hypothalamic neurons (IC50: ∼50 μM) [91] and the neurotoxic metal ion lead (Pb2+) strongly and reversibly blocks ASIC1a-containing homo- and hetero-meric channels (IC50: ∼5 μM) [92]. Nickel (Ni2+) and cadmium (Cd2+) affect some ASIC currents at high concentrations (1 mM) [93]. At this concentration, Ni2+ inhibits homomeric ASIC1a and heteromeric ASIC1a/2a [93]. In contrast, Cd2+, up to 1 mM, does not affect homomeric ASIC1a currents [93]. Since Cd2+ blocks voltage-gated calcium channels [94, 95], it can be used as a pharmacological tool to distinguish between ASIC1a and voltage-gated calcium channels as a source of acid-evoked calcium influx in neurons.
Polypeptide toxins
Psalmotoxin 1 (PcTx1) is a polypeptide toxin isolated from the venom of the South American tarantula Psalmopoeus Cambridge] and specifically inhibits homomeric ASIC1a with an IC50 of ∼ 1 nM. PcTx1 does not inhibit other ASIC subtypes or voltage-gated ion channels [96] and has already proven to be a useful pharmacological tool for probing the functions of ASIC1a in vivo [97-100]. PcTx1 consists of 40 amino acids, containing 6 cysteines that form three disul-fide bridges. The solution structure of PcTx1 has been determined [101] and, interestingly, PcTx1 is structurally similar to a class of gating modifier toxins of voltage-gated ion channels, such as Hanatoxin 1 [101]. Like Hanatoxin 1, PcTx1 has a canonical inhibitor cysteine knot (ICK) scaffold, which possesses an embedded ring formed by two disulfide bonds and their connecting backbone segments which is threaded by a third disulfide bond [101, 102]. Spider toxins most often modify the voltage dependence of voltage-gated ion channels [103-106] or slow their inactivation kinetics [107, 108]. PcTx1 inhibits ASIC1a in a similar manner: it increases the apparent ligand affinity of ASIC1a [109]. Since ASIC1a is activated by H+ at concentrations that are only slightly higher than the resting H+ concentrations, this increase in H+ affinity is sufficient to transfer ASIC1a channels into the desensitized state. Since ASIC1 has been implicated in the neurodegeneration accompanying ischemic stroke and since it contributes to ax-onal degeneration in autoimmune inflammation, this finding implies a new approach to intervene with ASIC1a activity: to pharmacologically facilitate its steady-state desensitization by slightly increasing its H+ affinity. Such an approach is hampered, however, by the comparatively low H+ affinity of SSD of human ASIC1a [27].
PcTx1 does not inhibit ASIC1b but rather increases the proton affinity of ASIC1b and shifts its pH-activation curve to higher pH values. PcTx1 directly opens ASIC1b under slightly acidic conditions. In addition, PcTx1 significantly slows the desensitization process of ASIC1b, but not of ASIC1a. These data strongly support a model of state dependent binding, i.e. PcTx1 has different affinities for the closed, open, and desensitized states of the channels. For ASIC1a, it stabilizes the desensitized state promoting SSD, whereas for ASIC1b, it stabilizes the open state promoting channel opening [78].
First hints on the PcTx1 binding site came from mutagenesis studies, in which chimeras between ASIC1a and ASIC2a and/or lb were assessed for binding of radiolabelled PcTx1 [110] or for PcTx1-inhibition of proton-gated currents [78, 110]. One of these studies identified amino acids 167-185 in the ECD of ASIC1a that determines a high PcTx1 affinity of the desensitized state of ASIC1a [78]. Another study identified the same region plus a second region (amino acids 271-369) in the ECD [110]. The involvement of both domains in PcTx1 binding was recently confirmed by computational docking simulations [111, 112], which revealed that PcTx1 binds to a pocket in the ECD of ASIC1a which is formed by four structural motifs: the two stretches identified with the chimeric channels (amino acids 167-185 and the distal part of stretch 271-369), an additional region comprising amino acids 113-129, and E219 [111, 112]. Moreover, molecular docking revealed that PcTx1 binds at the interface of two sub-units and that the domains constituting the high -affinity binding site are located on two adjacent subunits [111, 112]. The crucial role of the distal part of the stretch from amino acids 271-369 was recently confirmed by the finding that the F350L substitution (F352 in human ASIC1a) completely eliminated the modulation of ASIC1a by PcTx1 [84]. Since PcTx1 competes with dynorphin for binding to ASIC1a [31], the binding site for dynorphin may overlap with the PcTx1 binding site.
Recently, it was demonstrated that PcTx1 strongly activates chicken ASIC1a even at neutral pH [34]. Therefore, PcTx1 functions as an allosteric agonist for cASIC1, providing a novel tool for elucidating the gating mechanism of cASIC1. This finding also provides an explanation why a tarantula venom contains a toxin that targets ASIC1. From an evolutionary point of view, PcTx1 may have been generated by the tarantula as an activator rather than as an inhibitor of ASICs so as to ward off its potential predators (such as birds) because activating ASICs would cause pain or inflammation. Similar examples from nature are vanillotoxins that are contained within the venom of the same tarantula species (Psalmopoeus Cambridgei). Vanillotoxins share high homology and a conserved cysteine bridge pattern with PcTx1 and directly open TRPV1 channels [113] to cause pain as a defending strategy. The result that PcTx1 directly gates chicken or rat ASIC1, thus, supports the idea that ASICs are involved in pain perception. Therefore, blocking ASIC1 may have beneficial effects in relieving pain. Indeed it has been shown that PcTx1 has potent analgesic properties against thermal, mechanical, chemical, inflammatory and neuropathic pain in rodents [100].
Small-molecule inhibitors
The diuretic amiloride is an open channel blocker plugging the ion pore [35] and is viewed as a prototype blocker of ASIC channels. Amiloride blocks ASIC1a currents with an IC50 of 10 μM [16, 114]. It is not very selective and inhibits, among others, ENaC, T-type calcium channels, and the sodium-proton exchanger [86]. In peripheral sensory neurons, amiloride has been used to suppress acid-induced pain [115, 116], whereas in CNS neurons, it reduces acid-mediated ischemic neuronal injury and axonal degeneration [97, 98]. Both effects are potentially mediated by the inhibition of ASICs.
Nafamostat, a synthetic, competitive serine protease inhibitor that is clinically used for the treatment of disseminated intravascular coagulation and acute pancreatitis [117-121], has recently been identified as an inhibitor of ASICs [122]. In contrast to amiloride, nafamostat inhibits ENaC (IC50: ∼1 mM) less potently than ASICs (IC50: ∼2 – 70 μM, depending on the subtype). Non-steroidal anti-inflammatory drugs, such as diclofenac and ibuprofen, directly inhibit ASICs in nociceptors but with relatively low potency [123]. Ibuprofen and its analogue flur-biprofen inhibit ASIC1a with an IC50 of 350 μM, whereas diclofenac inhibits ASIC3 (IC50: 100 MM) but not ASIC1a [123]. A-317567, a novel non-diuretic blocker, inhibits three distinct types of native ASIC currents of acutely dissociated adult rat dorsal root ganglion (DRG) neurons [124].
Through a small scale screen, anti-protozoa I diarylamidines, including diminazene, hydroxys-tilbamidine (HSB), and pentamidine were recently identified as potent inhibitors of ASICs in primary cultured hippocampal neurons as well as of ASICs over-expressed in CHO cells or in Xenopus oocytes [125]. Diarylamidines have been widely used for the treatment of protozoa I diseases such as trypanosomiasis and leishma-niasis since the 1930s [126-128]. Diarylamidines such as diminazene are lead compounds that point to a new route to identify new potent and selective inhibitors of ASICs. Diminazene is the most potent small molecule inhibitor of ASICs known so far (IC50: 0.3 μM, measured in cultured hippocampal neurons). It has distinct potency on different ASIC subtypes. It blocks homomeric ASIC currents expressed in CHO cells with a rank order of potency 1b > 3 > 2a > 1a. Intriguingly, anti-parasitic diarylamidines comprise more than 30 members and the family number is rapidly increasing due to medical needs in the treatment of pneumocystosis, African trypanosomiasis and leishmaniasis. Thus, it is feasible to select these compounds from the chemical family of diarylamidines to test their inhibitory effects on ASICs and to possibly identify new selective and potent ASIC blockers with therapeutic potential.
Comparison of nafamostat with diarylamidines reveals that both are dications which are governed by amidine/guandine groups at their termini, with variable bulk (like benzene rings) on the outer edge of the molecule linked by molecular bridges of varying length and composition. Their difference lies in that nafamostat has a guanidine group at one terminus and an amidine group at the other, while diarylamidines have amidine groups at both ends. It is not surprising that dicationic diarylamidines or nafamostat block ASIC potently as the ectodomain of ASICs has a large number of negatively charged amino acids [80].
A “patchdock” computation technique was used to dock nafamostat, diminazene, HSB and pen-tamidine, respectively, to the ASIC1 crystal structure and to analyze possible binding domains of these compounds [125]. This analysis suggests that these dicationic compounds likely bind to a groove formed by part of the β-ball and palm domains of ASICs [125]. Specifically, residues in a stretch from F174 to L207 shape this groove [125]. Thus, this domain partly overlaps with the PxTc1 binding site. So far, there is no selective small-molecule inhibitor for ASICs, mainly because the potency of the known inhibitors is relatively low. Further structure-function studies and larger screens based on the cASIC1 crystal structure and the common template of diarylamidines may lead to the identification of more potent and subtype-specific blockers of ASICs.
Regarding the pharmacological classification of known ASIC inhibitors, they fall into three classes: gating modifiers (PcTx1), non-competitive antagonists (probably diminazene and nafamostat) and un-competitive antagonists (pore blocker amiloride). Ca2+ and Mg2+ might be competitive antagonists.
Physiological functions and pathophysiology of ASIC1
ASICs are predominantly, though not exclusively, expressed throughout the PNS and CNS. In the CNS, ASIC1a is abundantly present in the hippocampus, cortex, striatum, olfactory bulb and elsewhere, with a particularly high expression in the amygdala [129]. Low pH-induced ASIC currents are detected in virtually every cultured neuron from spinal dorsal horn [130], cortex [97], and hippocampus [22, 23]. In contrast, ASIC currents are not detectable in glial cells [131, 132] and we were unable to detect ASIC currents by puff-application of pH 5.8 to micro-glia in acute cortical slices (X.C., unpublished). However, the presence of ASIC-like currents has been documented in cells of the oligodendro-cyte lineage [133, 134] and in glioblastoma cells [132].
What is the function of ASICs in central neurons? Synaptic vesicles are acidic [135] and it is conceivable that the release of the content of the synaptic vesicles sufficiently acidifies the synaptic cleft [136] to activate ASICs. In agreement with such a scenario, in transfected cultured hippocampal neurons, ASIC1a has a synaptic pattern of distribution and co-localizes with the postsynaptic marker PSD95 but not with the axonal marker dephospho tau-1 [137]. ASIC1a transfected into hippocampal slices also has somatodendritic localization and is present in most spines [138]. Moreover, ASIC2, which can tether to PSD95 (in contrast to ASIC1a), facilitates ASIC1a localization at dendritic spines by direct association with ASIC1a [139]. In addition, there is evidence that ASIC1a modulates the density of dendritic spines [138]. The presumably postsynaptic location of ASIC1a together with its Na+-selectivity, thus, suggests that ASIC1a contributes to the excitatory postsynaptic current in many neurons.
In agreement with this hypothesis, knock out of the asic1 gene in mice reduces excitatory postsynaptic potentials during high-frequency electric stimulation and impairs long-term potentiation (LTP) at Schaffer collateral-CAl synapses in the hippocampus [137]. In contrast, baseline synaptic transmission is normal in those mice [137]. It is plausible that ASIC1a facilitates neu-ronal depolarization especially during high frequency stimulation and thus may promote the opening of NMDA receptors, activation of which is a central feature of CA1 LTP. However, the exact role of ASIC1a in LTP is not fully understood. Behaviorally, ASIC1a null mice display defective spatial learning and defective cerebellum-dependent eyeblink conditioning [137].
Consistent with the robust expression of ASIC1a in lateral and basolateral nuclei of the amygdala and in the hippocampus [129, 137, 140], mice lacking ASIC1 also exhibit deficits in multiple fear-related behaviors including unconditioned fear, and the acquisition and retention of conditioned fear [129, 140]. Conversely, over-expressing ASIC1a throughout the brain in a transgenic mouse enhances context fear conditioning [141]. Restoring ASIC1a function specifically in the amygdala of ASIC1 knockout mice with a viral vector rescues fear memory but not unconditioned fear responses [142]. Finally, ASIC1a was recently shown to be a chemosen-sor in the amygdala detecting carbon dioxide and acidosis that elicits fear behavior [26]. In line with an important role in the fear circuit, genetically disrupting or pharmacologically inhibiting ASIC1 has antidepressant-like effects [143], establishing ASIC1a as a new possible target to combat depression.
Somewhat contradictory to its excitatory role, some studies point to an inhibitory role of ASIC1a. One recent study suggested that ASIC1a could exert some protective effects in the brain under epileptic conditions [99]. This study combined genetic, pharmacological, and electrophysiological approaches with animal behavioral analysis to examine how ASIC1a works under epileptic conditions. It was found that ASIC1a facilitates termination of seizures rather than causing neuronal over-excitation [99]. ASIC1a reduces seizure severity, shortens seizure duration, and promotes seizure termination [99]. Transgenic over-expression of ASIC1a had beneficial effects without affecting seizure threshold, while ASIC1a ablation had opposite effects and increased seizure severity and reduced postictal depression of epilepsy and thus increased seizure-caused mortality [99]. Thus, there is solid evidence that ASIC1a mediates the antiepileptic effects of extracellular acidosis during epilepsy. But how can an excitatory channel dampen neuronal over-excitation? To explain this paradox, a mechanistic model was proposed that activation of ASIC1a in inhibitory interneurons of the hippocampus, which have larger ASIC currents than excitatory pyramidal neurons [99], could increase the inhibitory tone and thereby attenuate epileptic activities [99]. Thus, according to this model, ASIC1a is an excitatory ionotropic receptor, but its predominant expression in inhibitory neurons would increase the inhibitory tone in a network during ASIC1a activation.
A similar model may explain increased (rather than reduced) pain sensitivity in mice with a loss of ASIC function. A dominant-negative ASIC3 subunit assembles with other ASIC sub-units and transgenic expression of this subunit leads to a complete loss of ASIC-like currents to pH 5.0 stimulation in DRG neurons [144]. Unexpectedly, in these transgenic mice sensitivity to mechanic and inflammatory pain is increased rather than reduced [144]. Similarly, ASIC3 null mice show enhanced behavioral responses to high-intensity nociceptive stimuli [145]. This increased pain sensitivity may again be explained by the precise location of ASICs within the pain pathway.
But there are also observations suggesting that ASICs might down-regulate excitability of individual neurons. For example, presynaptic release probability is enhanced in hippocampal neurons from ASIC1 null mice [146]. Moreover, in the retina ASIC2a negatively modulates rod photo-transduction and protects the retina from light-induced degeneration [147]. ASIC1a, however, is a positive modulator of cone phototransduction and adaptation [148]. Although the exact role of different ASICs in signal transduction in the retina is unknown, these studies support the possibility that ASICs, under certain circumstances, might function as negative regulators instead of depolarizing elements in the course of synaptic transmission.
In addition to its roles in fear conditioning and seizure termination, ASIC1a apparently gets activated during the course of a number of neu-ropathological syndromes such as brain ischemia, autoimmune inflammation, or inflammation-associated pain. A prolonged acidosis is common to all these syndromes; how completely desensitizing ASIC1a gets activated during prolonged acidosis is unknown. Since extracellular acidosis increases neuronal cell calcium by activating ASIC1a [33, 138], ASIC1a activation might enhance brain injury, for example during the ischemia associated with stroke. Indeed ASIC1a was found to have a significant detrimental effect on neuronal survival under ischemic conditions [97]. In the focal ischemia model, intracerebroventricular injection of ASIC1a blockers (amiloride, PcTx1) or knockout of the ASIC1a gene protects the brain from ischemic injury [97]. Furthermore, post-ischemic administration of PcTx1 was found to be neuro-protective against brain ischemic injury in the transient middle cerebral artery occlusion model [149]. In addition and as mentioned before, there is evidence that ASIC1a functionally couples to NMDA receptors. Consequently, activation of ASIC1a may exacerbate neuronal death during ischemia also by coupling to NMDA receptors [150]. These findings identify ASIC1a as an emerging non-classical excitotoxic therapeutic target for combating stroke [151].
In a mouse model for experimental autoimmune encephalomyelitis (EAE), ASIC1a mRNA is up-regulated and genetic disruption of ASIC1 or pharmacological inhibition by amiloride ameliorates disease severity and axonal loss in EAE mice [98]. Axonal degeneration may be initiated by activation of ASIC1a localized to dendrites or cell bodies or alternatively, ASIC1a may be abnormally distributed to demyelinated axons in inflamed EAE lesions [98].
In the PNS, ASIC1a is expressed together with other ASICs (ASIC1b, 2a, 2b, and 3) in sensory neurons of the dorsal root ganglion (DRG) and trigeminal ganglion (TG) [123, 152, 153]. Moreover, homomeric ASIC1a and heteromeric ASIC1a/2a are the predominant ASICs in neurons of the spinal dorsal horn [130, 154]. During inflammation, ASIC1a expression increases in the DRGs [123, 155] and the spinal horn [114, 154]. Increased ASIC1a expression most likely contributes to enhanced pain during inflammation [100, 156]. In agreement with this hypothesis, spinal infusion of PcTx1 or ASIC1a antisense oligonucleotides attenuated thermal and mechanical hypersensitivity [154], suggesting that ASIC1a contributes to central sensitization.
In summary, in the last decade we learned a lot about the roles of ASIC1a in the central nervous system and in neuropathological conditions. Many aspects of its action remain mysterious, however, suggesting that the study of ASIC1a will lead to further exciting discoveries in the future.
Acknowledgments
We thank M. Schnizler for critical reading of part of this review and all present and former members of the Gründer lab for many helpful discussions. X.C. thanks Dr. John MacDonald for his continuous support. Work in the Gründer lab is supported by the DFG (grant GR1771/3).
References
- 1.Krishtal OA, Pidoplichko VI. Receptor for protons in the membrane of sensory neurons. Brain Res. 1981;214:150–154. doi: 10.1016/0006-8993(81)90446-7. [DOI] [PubMed] [Google Scholar]
- 2.Krishtal OA, Pidoplichko VI. A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience. 1981;6:2599–2601. doi: 10.1016/0306-4522(81)90105-6. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 4.Golubovic A, Kuhn A, Williamson M, Kalbacher H, Holstein TW, Grimmelikhuijzen CJ, Gründer S. A peptide-gated ion channel from the freshwater polyp Hydra. J Biol Chem. 2007;282:35098–35103. doi: 10.1074/jbc.M706849200. [DOI] [PubMed] [Google Scholar]
- 5.Lingueglia E, Champigny G, Lazdunski M, Barbry P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature. 1995;378:730–733. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- 6.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 7.Coric T, Passamaneck YJ, Zhang P, Di Gregorio A, Canessa CM. Simple chordates exhibit a proton-independent function of acid-sensing ion channels. Faseb J. 2008;22:1914–1923. doi: 10.1096/fj.07-100313. [DOI] [PubMed] [Google Scholar]
- 8.Coric T, Zheng D, Gerstein M, Canessa CM. Proton sensitivity of ASIC1 appeared with the rise of fishes by changes of residues in the region that follows TM1 in the ectodomain of the channel. J Physiol. 2005;568:725–735. doi: 10.1113/jphysiol.2005.087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paukert M, Sidi S, Russell C, Siba M, Wilson SW, Nicolson T, Gründer S. A family of acid-sensing ion channels from the zebrafish: widespread expression in the central nervous system suggests a conserved role in neuronal communication. J Biol Chem. 2004;279:18783–18791. doi: 10.1074/jbc.M401477200. [DOI] [PubMed] [Google Scholar]
- 10.Coric T, Zhang P, Todorovic N, Canessa CM. The extracellular domain determines the kinetics of desensitization in acid-sensitive ion channel 1. J Biol Chem. 2003;278:45240–45247. doi: 10.1074/jbc.M304441200. [DOI] [PubMed] [Google Scholar]
- 11.Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- 12.Gründer S, Geissler HS, Bassler EL, Rup-persberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport. 2000;11:1607–1611. doi: 10.1097/00001756-200006050-00003. [DOI] [PubMed] [Google Scholar]
- 13.Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport. 2000;11:2217–2222. doi: 10.1097/00001756-200007140-00031. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Apicella A, Jr, Lee SK, Ezcurra M, Slone RD, Goldmit M, Schafer WR, Shaham S, Driscoll M, Bianchi L. A glial DEG/ENaC channel functions with neuronal channel DEG-1 to mediate specific sensory functions in C. elegans Embo J. 2008;27:2388–2399. doi: 10.1038/emboj.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voglis G, Tavernarakis N. A synaptic DEG/ENaC ion channel mediates learning in C. elegans by facilitating dopamine signalling. Embo J. 2008;27:3288–3299. doi: 10.1038/emboj.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- 17.Meng QY, Wang W, Chen XN, Xu TL, Zhou JN. Distribution of acid-sensing ion channel 3 in the rat hypothalamus. Neuroscience. 2009;159:1126–1134. doi: 10.1016/j.neuroscience.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 18.Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Gründer S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem. 2001;276:33782–33787. doi: 10.1074/jbc.M104030200. [DOI] [PubMed] [Google Scholar]
- 20.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 21.Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci U S A. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocam-pal neurons. J Physiol. 2002;539:485–494. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- 24.Vukicevic M, Kellenberger S. Modulatory effects of acid-sensing ion channels on action potential generation in hippocampal neurons. Am J Physiol Physiol Cell. 2004;287:C682–690. doi: 10.1152/ajpcell.00127.2004. [DOI] [PubMed] [Google Scholar]
- 25.Babini E, Paukert M, Geisler HS, Gründer S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1) J Biol Chem. 2002;277:41597–41603. doi: 10.1074/jbc.M205877200. [DOI] [PubMed] [Google Scholar]
- 26.Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherwood TW, Askwith CC. Endogenous arginine-phenylalanine-amide-related peptides alter steady-state desensitization of ASIC1a. J Biol Chem. 2008;283:1818–1830. doi: 10.1074/jbc.M705118200. [DOI] [PubMed] [Google Scholar]
- 28.Rettinger J, Schmalzing G. Activation and desensitization of the recombinant P2X1 receptor at nanomolar ATP concentrations. J Gen Physiol. 2003;121:451–461. doi: 10.1085/jgp.200208730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rettinger J, Schmalzing G. Desensitization masks nanomolar potency of ATP for the P2X1 receptor. J Biol Chem. 2004;279:6426–6433. doi: 10.1074/jbc.M306987200. [DOI] [PubMed] [Google Scholar]
- 30.Cushman KA, Marsh-Haffner J, Adelman JP, McCleskey EW. A conformation change in the extracellular domain that accompanies desensitization of acid-sensing ion channel (ASIC) 3. J Gen Physiol. 2007;129:345–350. doi: 10.1085/jgp.200709757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J Neurosci. 2009;29:14371–14380. doi: 10.1523/JNEUROSCI.2186-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99:501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- 33.Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci USA. 2004;101:6752–6757. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samways DS, Harkins AB, Egan TM. Native and recombinant ASIC1a receptors conduct negligible Ca2+ entry. Cell Calcium. 2009;45:319–325. doi: 10.1016/j.ceca.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paukert M, Babini E, Pusch M, Gründer S. Identification of the Ca2+ blocking site of acid-sensing ion channel (ASIC) 1: implications for channel gating. J Gen Physiol. 2004;124:383–394. doi: 10.1085/jgp.200308973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Gründer S. Permeating protons contribute to tachyphylaxis of the acid-sensing ion channel (ASIC) 1a. J Physiol. 2007;579:657–670. doi: 10.1113/jphysiol.2006.120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renard S, Lingueglia E, Voilley N, Lazdunski M, Barbry P. Biochemical analysis of the membrane topology of the amiloride-sensitive Na+ channel. J Biol Chem. 1994;269:12981–12986. [PubMed] [Google Scholar]
- 38.Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol. 1994;267:C1682–1690. doi: 10.1152/ajpcell.1994.267.6.C1682. [DOI] [PubMed] [Google Scholar]
- 39.Snyder PM, McDonald FJ, Stokes JB, Welsh MJ. Membrane topology of the amiloride-sensitive epithelial sodium channel. J Biol Chem. 1994;269:24379–24383. [PubMed] [Google Scholar]
- 40.Saugstad JA, Roberts JA, Dong J, Zeitouni S, Evans RJ. Analysis of the membrane topology of the acid-sensing ion channel 2a. J Biol Chem. 2004;279:55514–55519. doi: 10.1074/jbc.M411849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Firsov D, Robert-Nicoud M, Gründer S, Schild L, Rossier BC. Mutational analysis of cysteine -rich domains of the epithelium sodium channel (ENaC). Identification of cysteines essential for channel expression at the cell surface. J Biol Chem. 1999;274:2743–2749. doi: 10.1074/jbc.274.5.2743. [DOI] [PubMed] [Google Scholar]
- 42.Kadurin I, Golubovic A, Leisle L, Schindelin H, Gründer S. Differential effects of N-glycans on surface expression suggest structural differences between the acid-sensing ion channel (ASIC) 1a and ASIC1b. Biochem J. 2008;412:469–475. doi: 10.1042/BJ20071614. [DOI] [PubMed] [Google Scholar]
- 43.Coscoy S, Lingueglia E, Lazdunski M, Barbry P. The Phe-Met-Arg-Phe-amide-activated sodium channel is a tetramer. J Biol Chem. 1998;273:8317–8322. doi: 10.1074/jbc.273.14.8317. [DOI] [PubMed] [Google Scholar]
- 44.Firsov D, Gautschi I, Merillat AM, Rossier BC, Schild L. The heterotetrameric architecture of the epithelial sodium channel (ENaC) EMBO J. 1998;17:344–352. doi: 10.1093/emboj/17.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosari F, Sheng S, Li J, Mak DO, Foskett JK, Kleyman TR. Subunit stoichiometry of the epithelial sodium channel. J Biol Chem. 1998;273:13469–13474. doi: 10.1074/jbc.273.22.13469. [DOI] [PubMed] [Google Scholar]
- 46.Berdiev BK, Karlson KH, Jovov B, Ripoll PJ, Morris R, Loffing-Cueni D, Halpin P, Stanton BA, Kleyman TR, Ismailov II. Subunit stoichiometry of a core conduction element in a cloned epithelial amiloride-sensitive Na+ channel. Biophys J. 1998;75:2292–2301. doi: 10.1016/S0006-3495(98)77673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dijkink L, Hartog A, van Os CH, Bindels RJ. The epithelial sodium channel (ENaC) is in-tracellularly located as a tetramer. Pflugers Arch. 2002;444:549–555. doi: 10.1007/s00424-002-0855-4. [DOI] [PubMed] [Google Scholar]
- 48.Anantharam A, Palmer LG. Determination of epithelial Na+ channel subunit stoichiometry from single-channel conductances. J Gen Physiol. 2007;130:55–70. doi: 10.1085/jgp.200609716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder PM, Cheng C, Prince LS, Rogers JC, Welsh MJ. Electrophysiological and biochemical evidence that DEG/ENaC cation channels are composed of nine subunits. J Biol Chem. 1998;273:681–684. doi: 10.1074/jbc.273.2.681. [DOI] [PubMed] [Google Scholar]
- 50.Staruschenko A, Medina JL, Patel P, Shapiro MS, Booth RE, Stockand JD. Fluorescence resonance energy transfer analysis of subunit stoichiometry of the epithelial Na+ channel. J Biol Chem. 2004;279:27729–27734. doi: 10.1074/jbc.M404169200. [DOI] [PubMed] [Google Scholar]
- 51.Staruschenko A, Adams E, Booth RE, Stockand JD. Epithelial Na+ channel subunit stoichiometry. Biophys J. 2005;88:3966–3975. doi: 10.1529/biophysj.104.056804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waldmann R, Champigny G, Lazdunski M. Functional degenerin-containing chimeras identify residues essential for amiloride-sensitive Na+ channel function. J Biol Chem. 1995;270:11735–11737. doi: 10.1074/jbc.270.20.11735. [DOI] [PubMed] [Google Scholar]
- 53.Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the alpha, beta, and gamma subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kellenberger S, Gautschi I, Schild L. A single point mutation in the pore region of the epithelial Na+ channel changes ion selectivity by modifying molecular sieving. Proc Natl Acad Sci USA. 1999;96:4170–4175. doi: 10.1073/pnas.96.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kellenberger S, Hoffmann-Pochon N, Gautschi I, Schneeberger E, Schild L. On the molecular basis of ion permeation in the epithelial Na+ channel. J Gen Physiol. 1999;114:13–30. doi: 10.1085/jgp.114.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snyder PM, Olson DR, Bucher DB. A pore segment in DEG/ENaC Na(+) channels. J Biol Chem. 1999;274:28484–28490. doi: 10.1074/jbc.274.40.28484. [DOI] [PubMed] [Google Scholar]
- 57.Langloh AL, Berdiev B, Ji HL, Keyser K, Stanton BA, Benos DJ. Charged residues in the M2 region of alpha-hENaC play a role in channel conductance. Am J Physiol Cell Physiol. 2000;278:C277–291. doi: 10.1152/ajpcell.2000.278.2.C277. [DOI] [PubMed] [Google Scholar]
- 58.Sheng S, Li J, McNulty KA, Avery D, Kleyman TR. Characterization of the selectivity filter of the epithelial sodium channel. J Biol Chem. 2000;275:8572–8581. doi: 10.1074/jbc.275.12.8572. [DOI] [PubMed] [Google Scholar]
- 59.Sheng S, Li J, McNulty KA, Kieber-Emmons T, Kleyman TR. Epithelial Sodium Channel Pore Region. STRUCTURE AND ROLE IN GATING. J Biol Chem. 2001;276:1326–1334. doi: 10.1074/jbc.M008117200. [DOI] [PubMed] [Google Scholar]
- 60.Sheng S, McNulty KA, Harvey JM, Kleyman TR. Second transmembrane domains of ENaC subunits contribute to ion permeation and selectivity. J Biol Chem. 2001;276:44091–44098. doi: 10.1074/jbc.M108522200. [DOI] [PubMed] [Google Scholar]
- 61.Kellenberger S, Auberson M, Gautschi I, Schneeberger E, Schild L. Permeability properties of ENaC selectivity filter mutants. J Gen Physiol. 2001;118:679–692. doi: 10.1085/jgp.118.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J, Sheng S, Perry CJ, Kleyman TR. Asymmetric organization of the pore region of the epithelial sodium channel. J Biol Chem. 2003;278:13867–13874. doi: 10.1074/jbc.M300149200. [DOI] [PubMed] [Google Scholar]
- 63.Kellenberger S, Gautschi I, Schild L. Mutations in the epithelial Na+ channel ENaC outer pore disrupt amiloride block by increasing its dissociation rate. Mol Pharmacol. 2003;64:848–856. doi: 10.1124/mol.64.4.848. [DOI] [PubMed] [Google Scholar]
- 64.Takeda AN, Gautschi I, van Bemmelen MX, Schild L. Cadmium trapping in an epithelial sodium channel pore mutant. J Biol Chem. 2007;282:31928–31936. doi: 10.1074/jbc.M700733200. [DOI] [PubMed] [Google Scholar]
- 65.Adams CM, Price MP, Snyder PM, Welsh MJ. Tetraethylammonium block of the BNC1 channel. Biophys J. 1999;76:1377–1383. doi: 10.1016/S0006-3495(99)77299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfister Y, Gautschi I, Takeda AN, van Bemmelen M, Kellenberger S, Schild L. A gating mutation in the internal pore of ASIC1a. J Biol Chem. 2006;281:11787–11791. doi: 10.1074/jbc.M513692200. [DOI] [PubMed] [Google Scholar]
- 67.Coscoy S, de Weille JR, Lingueglia E, Lazdunski M. The pre-transmembrane 1 domain of acid-sensing ion channels participates in the ion pore. J Biol Chem. 1999;274:10129–10132. doi: 10.1074/jbc.274.15.10129. [DOI] [PubMed] [Google Scholar]
- 68.Gründer S, Firsov D, Chang SS, Jaeger NF, Gautschi I, Schild L, Lifton RP, Rossier BC. A mutation causing pseudohypoaldosteronism type 1 identifies a conserved glycine that is involved in the gating of the epithelial sodium channel. EMBO J. 1997;16:899–907. doi: 10.1093/emboj/16.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gründer S, Jaeger NF, Gautschi I, Schild L, Rossier BC. Identification of a highly conserved sequence at the N-terminus of the epithelial Na+ channel alpha subunit involved in gating. Pflugers Arch. 1999;438:709–715. doi: 10.1007/s004249900119. [DOI] [PubMed] [Google Scholar]
- 70.Duggan A, Garcfa-Afioveros J, Corey DP. The PDZ domain protein PICK1 and the sodium channel BNaCl interact and localize at mech-anosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J Biol Chem. 2002;277:5203–5208. doi: 10.1074/jbc.M104748200. [DOI] [PubMed] [Google Scholar]
- 71.Hruska-Hageman AM, Wemmie JA, Price MP, Welsh MJ. Interaction of the synaptic protein PICK1 (protein interacting with C kinase 1) with the non-voltage gated sodium channels BNC1 (brain Na+ channel 1) and ASIC (acid-sensing ion channel) Biochem J. 2002;361:443–450. doi: 10.1042/0264-6021:3610443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donier E, Rugiero F, Okuse K, Wood JN. Annexin II light chain p11 promotes functional expression of acid-sensing ion channel ASIC1a. J Biol Chem. 2005;280:38666–38672. doi: 10.1074/jbc.M505981200. [DOI] [PubMed] [Google Scholar]
- 73.Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature. 2009;460:599–604. doi: 10.1038/nature08218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carnally SM, Dev HS, Stewart AP, Barrera NP, Van Bemmelen MX, Schild L, Henderson RM, Edwardson JM. Direct visualization of the trimeric structure of the ASIC1a channel, using AFM imaging. Biochem Biophys Res Commun. 2008;372:752–755. doi: 10.1016/j.bbrc.2008.05.100. [DOI] [PubMed] [Google Scholar]
- 75.Immke DC, McCleskey EW. Protons open Acid-sensing ion channels by catalyzing relief of ca(2+) blockade. Neuron. 2003;37:75–84. doi: 10.1016/s0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- 76.Passero CJ, Okumura S, Carattino MD. Conformational changes associated with protondependent gating of ASIC1a. J Biol Chem. 2009;284:36473–36481. doi: 10.1074/jbc.M109.055418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang P, Sigworth FJ, Canessa CM. Gating of acid-sensitive ion channel-1: release of Ca2+ block vs. allosteric mechanism. J Gen Physiol. 2006;127:109–117. doi: 10.1085/jgp.200509396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen X, Kalbacher H, Gründer S. Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent. J Gen Physiol. 2006;127:267–276. doi: 10.1085/jgp.200509409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shaikh SA, Tajkhorshid E. Potential cation and H+ binding sites in acid sensing ion channel-1. Biophys J. 2008;95:5153–5164. doi: 10.1529/biophysj.108.141606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paukert M, Chen X, Polleichtner G, Schindelin H, Gründer S. Candidate amino acids involved in H+ gating of acid-sensing ion channel 1a. J Biol Chem. 2008;283:572–581. doi: 10.1074/jbc.M706811200. [DOI] [PubMed] [Google Scholar]
- 81.Li T, Yang Y, Canessa CM. Interaction of the aromatics Tyr-72/Trp-288 in the interface of the extracellular and transmembrane domains is essential for proton gating of acid-sensing ion channels. J Biol Chem. 2009;284:4689–4694. doi: 10.1074/jbc.M805302200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baron A, Schaefer L, Lingueglia E, Champigny G, Lazdunski M. Zn2+ and H+ are coactivators of acid-sensing ion channels. J Biol Chem. 2001;276:35361–35367. doi: 10.1074/jbc.M105208200. [DOI] [PubMed] [Google Scholar]
- 83.Springauf A, Gründer S. An Acid-Sensing Ion Channel from Shark (Squalus Acanthias) Mediates Transient and Sustained Responses to Protons. J Physiol. 2010;588:809–820. doi: 10.1113/jphysiol.2009.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sherwood T, Franke R, Conneely S, Joyner J, Arumugan P, Askwith C. Identification of protein domains that control proton and calcium sensitivity of ASIC1a. J Biol Chem. 2009;284:27899–27907. doi: 10.1074/jbc.M109.029009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diochot S, Salinas M, Baron A, Escoubas P, Lazdunski M. Peptides inhibitors of acidsensing ion channels. Toxicon. 2007;49:271–284. doi: 10.1016/j.toxicon.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 86.Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol. 2008;8:25–32. doi: 10.1016/j.coph.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sluka KA, Winter OC, Wemmie JA. Acidsensing ion channels: A new target for pain and CNS diseases. Curr Opin Drug Discov Devel. 2009;12:693–704. [PMC free article] [PubMed] [Google Scholar]
- 88.Chu XP, Wemmie JA, Wang WZ, Zhu XM, Saugstad JA, Price MP, Simon RP, Xiong ZG. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci. 2004;24:8678–8689. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cho JH, Askwith CC. Potentiation of acidsensing ion channels by sulfhydryl compounds. Am J Physiol Cell Physiol. 2007;292:C2161–2174. doi: 10.1152/ajpcell.00598.2006. [DOI] [PubMed] [Google Scholar]
- 90.Gao J, Wu LJ, Xu L, Xu TL. Properties of the proton-evoked currents and their modulation by Ca2+ and Zn2+ in the acutely dissociated hippocampus CA1 neurons. Brain Res. 2004;1017:197–207. doi: 10.1016/j.brainres.2004.05.046. [DOI] [PubMed] [Google Scholar]
- 91.Wang W, Yu Y, Xu TL. Modulation of acidsensing ion channels by Cu(2+) in cultured hypothalamic neurons of the rat. Neuroscience. 2007;145:631–641. doi: 10.1016/j.neuroscience.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 92.Wang W, Duan B, Xu H, Xu L, Xu TL. Calcium -permeable acid-sensing ion channel is a molecular target of the neurotoxic metal ion lead. J Biol Chem. 2006;281:2497–2505. doi: 10.1074/jbc.M507123200. [DOI] [PubMed] [Google Scholar]
- 93.Staruschenko A, Dorofeeva NA, Bolshakov KV, Stockand JD. Subunit-dependent cadmium and nickel inhibition of acid-sensing ion channels. Dev Neurobiol. 2007;67:97–107. doi: 10.1002/dneu.20338. [DOI] [PubMed] [Google Scholar]
- 94.Swandulla D, Armstrong CM. Calcium channel block by cadmium in chicken sensory neurons. Proc Natl Acad Sci U S A. 1989;86:1736–740. doi: 10.1073/pnas.86.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thevenod F, Jones SW. Cadmium block of calcium current in frog sympathetic neurons. Biophys J. 1992;63:162–168. doi: 10.1016/S0006-3495(92)81575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem. 2000;275:25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- 97.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acidsensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 98.Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 99.Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA, 3rd, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11:816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mazzuca M, Heurteaux C, Alloui A, Diochot S, Baron A, Voilley N, Blondeau N, Escoubas P, Gelot A, Cupo A, Zimmer A, Zimmer AM, Eschalier A, Lazdunski M. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat Neurosci. 2007;10:943–945. doi: 10.1038/nn1940. [DOI] [PubMed] [Google Scholar]
- 101.Escoubas P, Bernard C, Lambeau G, Lazdunski M, Darbon H. Recombinant production and solution structure of PcTx1, the specific peptide inhibitor of ASIC1a proton-gated cation channels. Protein Sci. 2003;12:1332–1343. doi: 10.1110/ps.0307003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Escoubas P, Rash L. Tarantulas: eight-legged pharmacists and combinatorial chemists. Toxicon. 2004;43:555–574. doi: 10.1016/j.toxicon.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 103.Swartz KJ, MacKinnon R. An inhibitor of the Kv2.1 potassium channel isolated from the venom of a Chilean tarantula. Neuron. 1995;15:941–949. doi: 10.1016/0896-6273(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 104.Swartz KJ, MacKinnon R. Hanatoxin modifies the gating of a voltage-dependent K+ channel through multiple binding sites. Neuron. 1997;18:665–673. doi: 10.1016/s0896-6273(00)80306-2. [DOI] [PubMed] [Google Scholar]
- 105.McDonough SI, Lampe RA, Keith RA, Bean BP. Voltage-dependent inhibition of N- and P-type calcium channels by the peptide toxin omega-grammotoxin-SIA. Mol Pharmacol. 1997;52:1095–1104. doi: 10.1124/mol.52.6.1095. [DOI] [PubMed] [Google Scholar]
- 106.McDonough SI, Mintz IM, Bean BP. Alteration of P-type calcium channel gating by the spider toxin omega-Aga-IVA. Biophys J. 1997;72:2117–2128. doi: 10.1016/S0006-3495(97)78854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nicholson GM, Little MJ, Tyler M, Narahashi T. Selective alteration of sodium channel gating by Australian funnel-web spider toxins. Toxicon. 1996;34:1443–1453. doi: 10.1016/s0041-0101(96)00089-x. [DOI] [PubMed] [Google Scholar]
- 108.Szeto TH, Birinyi-Strachan LC, Smith R, Connor M, Christie MJ, King GF, Nicholson GM. Isolation and pharmacological characterisation of delta-atracotoxin-Hv1b, a vertebrate-selective sodium channel toxin. FEBS Lett. 2000;470:293–299. doi: 10.1016/s0014-5793(00)01339-9. [DOI] [PubMed] [Google Scholar]
- 109.Chen X, Kalbacher H, Gründer S. The tarantula toxin psaimotoxin 1 inhibits acid-sensing ion channel (ASIC) la by increasing its apparent H+ affinity. J Gen Physiol. 2005;126:71–79. doi: 10.1085/jgp.200509303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salinas M, Rash LD, Baron A, Lambeau G, Escoubas P, Lazdunski M. The receptor site of the spider toxin PcTx1 on the proton-gated cation channel ASIC1a. J Physiol. 2006;570:339–354. doi: 10.1113/jphysiol.2005.095810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pietra F. Docking and MD simulations of the interaction of the tarantula peptide psaimotoxin -1 with ASIC1a channels using a homology model. J Chem Inf Model. 2009;49:972–977. doi: 10.1021/ci800463h. [DOI] [PubMed] [Google Scholar]
- 112.Qadri YJ, Berdiev BK, Song Y, Lippton HL, Fuller CM, Benos DJ. Psalmotoxin-1 docking to human acid-sensing ion channel-1. J Biol Chem. 2009;284:17625–17633. doi: 10.1074/jbc.M109.003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, Basbaum AI, King D, Julius D. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444:208–212. doi: 10.1038/nature05285. [DOI] [PubMed] [Google Scholar]
- 114.Wu U, Duan B, Mei YD, Gao J, Chen JG, Zhuo M, Xu L, Wu M, Xu TL. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem. 2004;279:43716–43724. doi: 10.1074/jbc.M403557200. [DOI] [PubMed] [Google Scholar]
- 115.Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110:1185–1190. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB. Acid-induced pain and its modulation in humans. J Neurosci. 2004;24:10974–10979. doi: 10.1523/JNEUROSCI.2619-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fujii S, Hitomi Y. New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin. Biochim Biophys Acta. 1981;661:342–345. doi: 10.1016/0005-2744(81)90023-1. [DOI] [PubMed] [Google Scholar]
- 118.Hitomi Y, Ikari N, Fujii S. Inhibitory effect of a new synthetic protease inhibitor (FUT-175) on the coagulation system. Haemostasis. 1985;15:164–168. doi: 10.1159/000215139. [DOI] [PubMed] [Google Scholar]
- 119.Ikari N, Sakai Y, Hitomi Y, Fujii S. New synthetic inhibitor to the alternative complement pathway. Immunology. 1983;49:685–691. [PMC free article] [PubMed] [Google Scholar]
- 120.Kenji Okajima MU, Kazunori Murakamit. Nafamostat Mesilate. Cardiovascular Drug Reviews. 1995;13:51–65. [Google Scholar]
- 121.Mori S, Itoh Y, Shinohata R, Sendo T, Oishi R, Nishibori M. Nafamostat mesilate is an extremely potent inhibitor of human tryptase. J Pharmacol Sci. 2003;92:420–423. doi: 10.1254/jphs.92.420. [DOI] [PubMed] [Google Scholar]
- 122.Ugawa S, Ishida Y, Ueda T, Inoue K, Nagao M, Shimada S. Nafamostat mesilate reversibly blocks acid-sensing ion channel currents. Biochem Biophys Res Commun. 2007;363:203–208. doi: 10.1016/j.bbrc.2007.08.133. [DOI] [PubMed] [Google Scholar]
- 123.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain. 2005;117:88–96. doi: 10.1016/j.pain.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 125.Chen X, Qiu L, Li M, Durrnagel S, Orser BA, Xiong ZG, Macdonald JF. Diarylamidines: High Potency Inhibitors Of Acid-Sensing Ion Channels. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosenthal P. Mcgraw-Hill; 2007. Antiprotozoal Drugs. [Google Scholar]
- 127.Baraldi PG, Bovero A, Fruttarolo F, Preti D, Tabrizi MA, Pavani MG, Romagnoli R. DNA minor groove binders as potential antitumor and antimicrobial agents. Med Res Rev. 2004;24:475–528. doi: 10.1002/med.20000. [DOI] [PubMed] [Google Scholar]
- 128.Mishra J, Saxena A, Singh S. Chemotherapy of leishmaniasis: past, present and future. Curr Med Chem. 2007;14:1153–1169. doi: 10.2174/092986707780362862. [DOI] [PubMed] [Google Scholar]
- 129.Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baron A, Voilley N, Lazdunski M, Lingueglia E. Acid sensing ion channels in dorsal spinal cord neurons. J Neurosci. 2008;28:1498–1508. doi: 10.1523/JNEUROSCI.4975-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 132.Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ. Acid-sensing ion channels in malignant gliomas. J Biol Chem. 2003;278:15023–15034. doi: 10.1074/jbc.M300991200. [DOI] [PubMed] [Google Scholar]
- 133.Sontheimer H, Perouansky M, Hoppe D, Lux HD, Grantyn R, Kettenmann H. Glial cells of the oligodendrocyte lineage express proton-activated Na+ channels. J Neurosci Res. 1989;24:496–500. doi: 10.1002/jnr.490240406. [DOI] [PubMed] [Google Scholar]
- 134.Feldman DH, Horiuchi M, Keachie K, McCauley E, Bannerman P, Itoh A, Itoh T, Pleasure D. Characterization of acid-sensing ion channel expression in oligodendrocyte-lineage cells. Glia. 2008;56:1238–1249. doi: 10.1002/glia.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 136.Krishtal OA, Osipchuk YV, Shelest TN, Smirnoff SV. Rapid extracellular pH transients related to synaptic transmission in rat hippocampal slices. Brain Res. 1987;436:352–356. doi: 10.1016/0006-8993(87)91678-7. [DOI] [PubMed] [Google Scholar]
- 137.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 138.Zha XM, Wemmie JA, Green SH, Welsh MJ. Acid-sensing ion channel la is a postsynaptic proton receptor that affects the density of dendritic spines. Proc Natl Acad Sci U S A. 2006;103:16556–16561. doi: 10.1073/pnas.0608018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neurosci. 2009;29:8438–8446. doi: 10.1523/JNEUROSCI.1284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry. 2007;62:1140–1148. doi: 10.1016/j.biopsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 141.Wemmie JA, Coryell MW, Askwith CC, Lamani E, Leonard AS, Sigmund CD, Welsh MJ. Over-expression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc Natl Acad Sci U S A. 2004;101:3621–3626. doi: 10.1073/pnas.0308753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Coryell MW, Wunsch AM, Haenfler JM, Allen JE, McBride JL, Davidson BL, Wemmie JA. Restoring Acid-sensing ion channel-1a in the amygdala of knock-out mice rescues fear memory but not unconditioned fear responses. J Neurosci. 2008;28:13738–13741. doi: 10.1523/JNEUROSCI.3907-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, Light AR, Langbehn DR, Wemmie JA. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci. 2009;29:5381–5388. doi: 10.1523/JNEUROSCI.0360-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mogil JS, Breese NM, Witty MF, Ritchie J, Rainville ML, Ase A, Abbadi N, Stucky CL, Seguela P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci. 2005;25:9893–9901. doi: 10.1523/JNEUROSCI.2019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci U S A. 2002;99:8992–8997. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cho JH, Askwith CC. Presynaptic release probability is increased in hippocampal neurons from ASIC1 knockout mice. J Neurophysiol. 2008;99:426–441. doi: 10.1152/jn.00940.2007. [DOI] [PubMed] [Google Scholar]
- 147.Ettaiche M, Guy N, Hofman P, Lazdunski M, Waldmann R. Acid-sensing ion channel 2 is important for retinal function and protects against light-induced retinal degeneration. J Neurosci. 2004;24:1005–1012. doi: 10.1523/JNEUROSCI.4698-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ettaiche M, Deval E, Cougnon M, Lazdunski M, Voilley N. Silencing acid-sensing ion channel la alters cone-mediated retinal function. J Neurosci. 2006;26:5800–5809. doi: 10.1523/JNEUROSCI.0344-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–158. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- 150.Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48:635–646. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 151.Xiong ZG, Chu XP, Simon RP. Acid sensing ion channels-novel therapeutic targets for ischemic brain injury. Front Biosci. 2007;12:1376–1386. doi: 10.2741/2154. [DOI] [PubMed] [Google Scholar]
- 152.Olson TH, Riedl MS, Vulchanova L, Ortiz-Gonzalez XR, Elde R. An acid sensing ion channel (ASIC) localizes to small primary afferent neurons in rats. Neuroreport. 1998;9:1109–1113. doi: 10.1097/00001756-199804200-00028. [DOI] [PubMed] [Google Scholar]
- 153.Xie J, Price MP, Wemmie JA, Askwith CC, Welsh MJ. ASIC3 and ASIC1 Mediate FMRFamide-Related Peptide Enhancement of H+-Gated Currents in Cultured Dorsal Root Ganglion Neurons. J Neurophysiol. 2003;89:2459–2465. doi: 10.1152/jn.00707.2002. [DOI] [PubMed] [Google Scholar]
- 154.Duan B, Wu U, Yu YQ, Ding Y, Jing L, Xu L, Chen J, Xu TL. Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J Neurosci. 2007;27:11139–11148. doi: 10.1523/JNEUROSCI.3364-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mamet J, Baron A, Lazdunski M, Voilley N. ProInflammatory Mediators, Stimulators of Sensory Neuron Excitability via the Expression of Acid-Sensing Ion Channels. J Neurosci. 2002;22:10662–10670. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu TL, Duan B. Calcium-permeable acidsensingion channel in nociceptive plasticity: a new target for pain control. Prog Neurobiol. 2009;87:171–180. doi: 10.1016/j.pneurobio.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 157.Sakai H, Lingueglia E, Champigny G, Mattei MG, Lazdunski M. Cloning and functional expression of a novel degenerin-like Na+ channel gene in mammals. J Physiol (Lond) 1999;519(Pt 2):323–333. doi: 10.1111/j.1469-7793.1999.0323m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]