Abstract
Immunological analyses of aged human brain tissues are widely used in characterizing the physiology or pathophysiology of brain aging or neurological diseases such as Alzheimer's disease. The primary antibodies used in immunological detection mainly originate from rabbit and mouse species. In the present study, we showed an unexpected cross-immunoreactivity between anti-rabbit immunoglobulin G and diffuse lipofuscin-associated protein(s) in aged human brain tissues. In immunoblotting analysis of aged human brain samples, anti-rabbit secondary antibody alone produces a sharp band of approximately 180 kDa, whereas anti-mouse antibody does not show this cross-reaction. Immunohistochemical localization of cross-immunoreactivity found that the cross-reactive protein(s) were mainly associated with diffuse and weak autofluorescence in the cytoplasm of neurons. This nonspecific cross-immunoreactivity produces sufficient intensity of non-specific immunostaining signals that are easily mis-recognized as specific immunoreactivity, and can generate misleading data. The above nonspecific cross-reactivity with anti-rabbit secondary antibody in aged human brain tissues could be significantly reduced or abolished by pretreating tissues with 1% sodium borohydride or/and adding 0.5% Tween-20 into the secondary antibody dilution buffer. When these modifications are included in the protocol, specific immunoreactivity (such as phospho-tau pT231) was unaffected, or slightly improved. Our study suggests that caution should be taken when performing immunological analyses on aged human brain samples with rabbit polyclonal antibody, and that modification of the experimental protocol is generally required to minimize the aforementioned nonspecificity.
Keywords: Immunoblotting, immunohistochemistry, aging, human brain tissues, nonspecificity
Introduction
Immunological analyses of postmortem brain tissues are widely employed in the areas of brain aging and neurological disease research. This type of study is especially important when a suitable animal model of a neurological disease is not available, or when the available animal models do not mimic the unique features of human pathology well. Particularly, such studies could be a primary way in characterizing the complicated and detailed features of certain age-dependent diseases, and provide guidance for further animal and cell biology studies.
Currently, the majority of primary antibodies used in immunological detections originate from rabbit and mouse. We regularly employed aged and diseased human brain samples in our Alzheimer's disease (AD) studies [1, 2] and unexpectedly detected an immunological cross-reaction between anti-rabbit secondary antibody and a lipofuscin-associated protein. The nonspecific cross-reactivity appeared with both immunoblotting and immunohistochemistry. We describe procedural modifications to alleviate such undesirable labeling when rabbit polyclonal antibodies are applied to aged human brain tissues.
Materials and methods
Human and mouse brain tissues
In this study, samples of the human hippocampus and parietal cortex from 17 cases (10 AD cases and 7 controls) were obtained from the University of Kentucky Alzheimer's Disease Research Center (ADRC). The ages of patients at death ranged from 66 – 95 years. The postmortem intervals ranged from 1.8 - 4.5 hours. All AD subjects met the clinical and neuropathological criteria for the diagnosis of AD, while control subjects were individuals without evidence of neurological disorders. Paraffin-embedded blocks were prepared by sequential dehydration in graded ethanol and vacuum-infiltrated in paraffin and serial sectioning to a thickness of 8 μm. Unfixed tissue samples were rapidly frozen in liquid nitrogen at the time of autopsy, stored at -80oC, and subsequently used for immunoblotting analysis.
Adult senescence-accelerated prone 8 (SAMP8) mice were purchased from Vital River Laboratory Animal Technology Co., Ltd (Beijing, China), and maintained until 8-12 months old. The brain tissues were processed and immunostained as described elsewhere [3]. All animal experiments were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
Immunoblotting analysis
Cellular proteins were extracted from tissue blocks of parietal cortex grey matter. Tissues were homogenized in the extraction buffer (10 mM Tris PH 7.4, 150 mM NaCl, 1 mM NaVO4, 0.3% Triton X-100, and protease inhibitor cocktail at1:1000, Sigma) with a Dounce tissue grinder and put on ice for 1 hour. Soluble extracts were separated by centrifugation at 10,600 g for 10 min. Protein samples (20μg) were mixed with the standard loading buffer, boiled for 5 min, resolved by 10% SDS-polyacrylamide gel, and transferred to nitrocellulose filters. The blots were blocked with 5% skim milk in TBST, and then incubated with HRP -conjugated goat anti-rabbit (1:5000), goat anti-mouse (1:3000), or rabbit anti-goat (1:6000) secondary antibody for 1 hour. Primary antibodies were not applied to immunoblots prior to incubation with the secondary antibody. Chemiluminescent detection was used for visualization of signals (ECL+ plus, Amersham). Goat anti-rabbit secondary antibodies from different vendors (Sigma and Bio-Rad) and different lots had been tested for cross-immunoreactivity. To test whether nonspecific cross-reaction can be blocked, additional immunoblots were subjected to enhanced blockings such as 5% skim milk and 5% goat serum in TBST buffer (containing 0.05% Tween-20) for 2 hours prior to incubation with goat anti-rabbit secondary antibody.
Autofluorescence and immunohistochemical Staining
Paraffin-embedded human brain sections were subjected to conventional deparaffinization. Autofluorescent signals were observed under the fluorescein isothiocyanate (FITC) and rhodamine filter sets using an Axioplan 2 imaging microscope (Carl Zeiss, Inc., Thornwood, NY). The optical filters used were as follows: rhodamine (ex 546 ±6 nm, em >570 nm) and FITC (ex 470 ±20 nm, em >515 nm). Images were captured using an Axiocam digital camera connected to a computer equipped with Axiovision 3.0 software [1].
After autofluorescence images from various brain regions were acquired, the sections were subjected to immunostaining. Briefly, deparaffinized brain sections were incubated at room temperature in 1% H2O2 for 10 min to reduce the background staining. Sections were placed in blocking buffer consisting of 0.1M phosphate buffer, 0.4% Triton X-100, and 5% goat or horse serum (matched with the source of secondary antibody) for 1 hour. To mimic actual immunostaining (with primary antibodies) procedure, antigen retrieval was applied in a subset of sections using Antigen Unmasking solution (Vector laboratories, Burlingame, CA) before the blocking step. To assess nonspecific cross-immunoreactivity, sections were incubated for 2 hours with biotinylated goat anti-rabbit or horse anti-mouse secondary antibodies (Vector Laboratories), with no prior incubation of primary antibodies. Sufficient washes with PBS were taken between each incubation step. The avidin -biotin immunoperoxidase method with 3,3'-diaminobenzidine tetrahydrochloride (DAB) as the chromogen was used to visualize immunoreactive cells (ABC kits; Vector Laboratories). The coverslipped sections were then photographed under bright field using a Zeiss Axioplan 2 imaging microscope. The same fields were identified and photographed with the help of biological cues on stained sections, in order to compare lipofuscin autofluorescence and DAB stained signals. After the images of immunostaining were captured, a subset of sections was counterstained with hematoxylin. To test whether such cross-immunoreactivity appeared in aged animal brain tissues, the brain sections of SAMP8 mice were examined with Alexa 488 conjugated goat anti-rabbit antibody (1:200, Molecular Probes).
A couple of protocol modifications had been tested to minimize the cross-immunoreactivity, such as pretreatment with 0.2% glycine, permanganate oxidation, 1% sodium borohydride, or including 0.5% Tween-20 in the secondary antibody solution. To examine the influence of the protocol modifications on specific immunoreactivity, additional sections were incubated overnight in polyclonal primary antibody against phospho-Thr231 tau (pT231, dilution 1:300, Biosource), then incubated in biotinylated goat anti-rabbit secondary antibody (Vector Laboratories) with or without selected pretreatments. The effects of various pretreatments on the quality of immunostains were compared with adjacent or nearby sections.
Results
Nonspecific cross-reaction with anti-rabbit antibody in the immunoblots of aged human brain samples
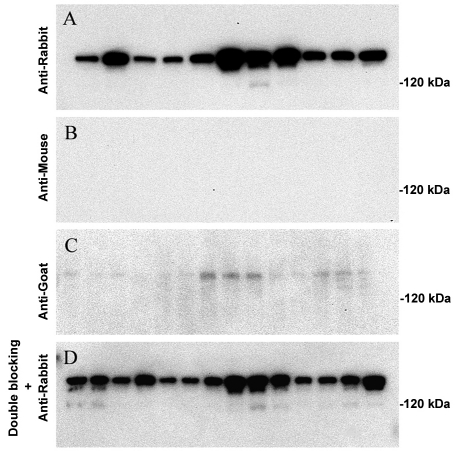
When we did immunoblotting analyses using postmortem tissues of aged human brain tissue, an unexpected band, around 180 kDa, was frequently seen on the blots, if primary antibodies were rabbit-originated polyclonal antibodies. This result directed us to examine and characterize this unexpected immunoreactivity carefully. When the primary antibodies were omitted, this unexpected band was still present, both in control and AD samples (Figure 1A). This nonspecific cross-reaction was observed with anti-rabbit secondary antibodies from different vendors (Sigma and Bio-Rad) or different lots of the same vendor (data not shown). The cross-immunoreactivity is not seen with anti-mouse secondary antibody (Figure 1B), while it is very weak with anti-goat secondary antibody (Figure 1C). Furthermore, enhanced blocking with 5% nonfat milk or 5% goat serum did not eliminate this unexpected band (Figure 1D).
Figure 1.
Immunoblotting analysis of proteins from control and AD patient brain tissues. A: Immunoblotting with anti-rabbit secondary antibody alone detected high cross-immunoreactivity in aged human cortices of both the control group (lane 1∼5) and the AD group (lane 6∼11). B: Immunoblotting with anti-mouse secondary antibody alone showed no cross-immunoreactivity in all cases. C: Immunoblotting with anti-goat secondary antibody alone detected weak cross-immunoreacticity in both the control group (lane 1∼7) and the AD group (lane 8∼14). D: Treatment with a blocking buffer containing both 5% milk and 5% goat serum does not prevent nonspecific immunoreactivity with anti-rabbit secondary antibody (same lane sequence as panel C).
Nonspecific immunoreactivity is associated with diffuse autofluorescence in immunohistochemistry
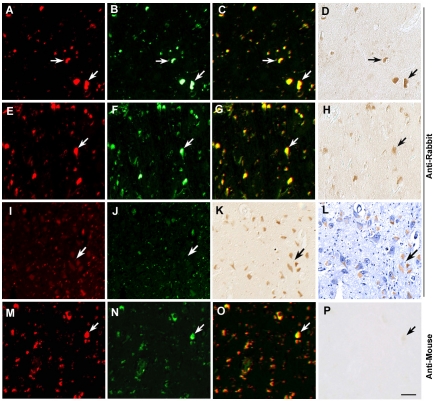
We examined the aforementioned cross immunoreactivity on brain sections where tissue structure and cell morphology were kept. In human brain tissues, a large amount of lipofuscin is generated and accumulated in neurons along with the increase of age [4]. Our preliminary experiments indicated that the cross -reaction signals seemed to be associated with lipofuscin granules. Since the autofluorescence of lipofuscin is visible under fluorescent microscopy with both rhodamine and FITC filter sets [5], we examined the possible co-localization of lipofuscin granules and cross-immunoreactivity of anti-rabbit IgG by sequential capturing of these two types of signals. Indeed, we found that immunostaining produced by anti-rabbit secondary antibody corresponded with diffuse autofluorescence. Bright autofluorescence was associated with moderate cross-immunoreactivity (Figure 2A-H), whereas weak but diffuse autofluorescence was associated with high cross- immunoreactivity (Figure 2 I-K). Counterstaining with hematoxylin showed that the nonspecific immunoreactivity was localized to the cytoplasm (Figure 2L). Thus, we infer that lipofuscin-associated protein (or protein complex) cross-reacts with anti-rabbit IgG, which may be responsible for the nonspecific band seen on immunoblots. In contrast to anti-rabbit secondary antibody, no remarkable cross-immunoreactivity was detected with anti-mouse secondary antibody (Figure 2, M-P).
Figure 2.
Autofluorescent characterization and immunohistochemical localization of the cross-immunoreactivity with anti-rabbit secondary antibody in aged human brain tissues. Bright autofluorescence (arrows) in hippocampal regions (A-C), and parietal cortex (E-G). Lipofuscin autofluorescence (arrows) is detected in both Cy3 and FITC filter channels (in a yellow color on the merged images), and produces cross-immunoreactivity with anti-rabbit antibody in the absence of any primary antibody (D and H). In the aged human cortices, high autofluorescence is associated with moderate cross-immunoreactivity (A-H), whereas diffuse and weak autofluorescence is associated with high cross-immunoreactivity (I-K). Counterstaining with hematoxylin (L) demonstrates that nonspecific immunoreactivity is localized to the cytoplasm. No cross-immunoreactivity is detected with anti-mouse antibody (M-P). Scale Bar 30 μm.
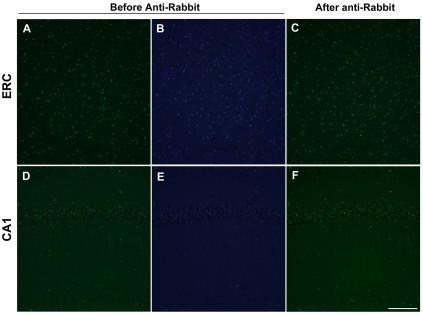
In the brains of 10-month old SAMP8 mice, the autofluorescence of lipofuscin exhibited as granular or speckled appearance (Figure 3, A and B, D and E). After incubating with anti-rabbit antibody conjugated with Alexa 488 for 2 hours, no remarkable cross-immunoreactivity was detected in the mouse cortex and hippocampus (Figure 3, C and F). Therefore the cross-immunoreactivity with anti-rabbit antibody is unique to aged human brain tissues.
Figure 3.
Examination of the cross-immunoreactivity of anti-rabbit antibody in aging-accelerated mouse brain. Autofluorescence of granule-like lipofuscin was detected in entorhinal cortex (ERC, A and B) and hippocampus (D and E) of 10-month old SAMP8 mice. After incubating with anti-rabbit antibody conjugated with Alexa 488, remarkable cross-immunoreactivity was not detected, as the intensity of green fluorescence did not change significantly. Scale Bar 100 μm.
Pretreatment with Tween-20 or sodium borohydride can reduce nonspecific immunoreactivity
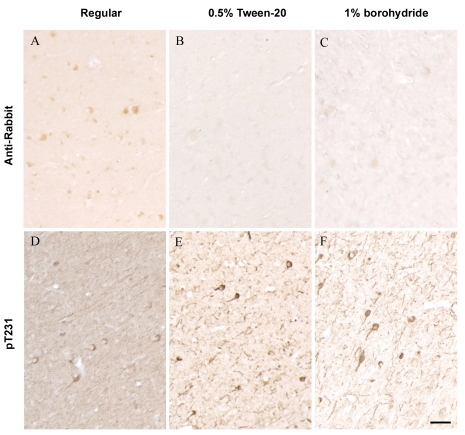
We tested a couple of agents in order to minimize or eliminate the above cross-immunoreactivity. Treatment of deparaffinized brain sections with sodium borohydride (1% in PBS buffer) reduced the overall nonspecific backgrounds. Particularly, adding 0.5% Tween-20 into the secondary antibody dilution buffer significantly reduced nonspecific staining with anti-rabbit secondary antibody. On the other hand, immunohistochemistry using the poly-clonal antibody of phosphorylated tau at Thr231 (pT231) showed that specific pT231 immunoreactivity is preserved, or even improved, when these modifications were integrated into the conventional immunostaining methods (Figure 4).
Figure 4.
Suppressing the nonspecific cross-immunoreactivity and improving specific immunostaining in aged human brain tissues. A and D: with regular immunostaining protocol, nonspecific staining with anti-rabbit secondary antibody (A) or phospho-tau at Thr231 (D, pT231) was detected. The nonspecific staining confused with specific pT231 immunostaining (D). With modified protocols either by using a secondary antibody buffer containing 0.5% Tween-20 (B and E) or tissue pretreatment with 1% sodium borohydride (C and F), little cross-immunoreactivity was detected with anti-rabbit secondary antibody alone (B, C), and phospho-tau immunostaining showed clear and specific pT231 immunoreactivity with less background (E, F). Scale Bar 30 μm.
Discussion
In this study, we identified a nonspecific immunoreactivity generated by using anti-rabbit secondary antibodies. We also suggest certain modifications of the immunohistochemical protocol, when immunological detection is done in aged human brain tissue samples to improve the specificity of data. For immunoblotting analysis, this high molecular weight band of cross-immunoreactivity may not generate severe problems, if the size of target protein differs largely from that nonspecific band. If the size of target proteins coincides with the nonspecific band, we would suggest selection of a monoclonal antibody, if it is available. As postmortem human brain tissues are widely used in neurological research, unawareness of this non-specificity could cause mis-interpretation of data in particular cases.
In immunostaining sections, this cross-immunoreactivity is largely associated with lipofuscin autofluorescence. We suspect that protein components of lipofuscin granules share epitopes of rabbit IgG that can be recognized by anti-rabbit antibody [6]. Lipofuscin is a lysosomal substance composed mainly of lipids and cross-linked protein residues. Although the exact mechanisms are unknown, lipofuscin may be formed due to iron-catalyzed oxidative processes and may accumulate over time due to incomplete degradation and/or removal from the cell by exocytosis [7, 8]. Post-mitotic cells such as neurons, cardiac myocytes, skeletal muscle fibers, and retinal pigment epithelial cells progressively accumulate a pigmented, autofluorescent material lipofuscin [7].
Interestingly, a moderate increase of lipofuscin was detected in the neurons free of NFTs, but not in tangle-bearing neurons, in AD brain [9]. Since we have not examined cross-reaction between the crossed protein(s) and human auto -antibody, the pathophysiological role of this cross-immunoreaction remains unknown.
In certain cases, this nonspecific cross-reactivity may confuse with specific positive stains and/or reduce signal-to-noise ratio. Therefore, modification of immunohistochemtry protocol would be needed to prevent the possible confusion due to this cross reactivity. Sodium borohydride (NaBH4) solution has been used to reduce background autofluorescence in paraformaldehyde-, formaldehyde- [2, 6], or glutaraldehyde-fixed brain tissue [7]. This reagent is known to neutralize Schiff's bases through reduction of amine-aldehyde compounds into non-fluorescent salts [10]. In this study, we found that sodium borohydride pretreatment could alleviate cross-immunoreactivity and overall background by anti-rabbit antibody in human brain sections. However, sodium borohydride pretreatment is not sufficient to abolish this nonspecificity completely.
Non-ionic detergent Tween-20 is most often used to block vacant non-specific binding sites on the surface of 96-well plates in enzyme-linked immunosorbent assay (ELISA) [11]. Tween-20 was also used to reduce background staining in human urothelium [12]. In this study, we found that adding 0.5% Tween-20 into the secondary antibody dilution buffer significantly reduced the aforementioned cross-immunoreactivity in aged human brain tissues. Importantly, this modification of protocol will not hinder the detection of a specific antigen. On the contrary, immunohistochemical detection of specific antigens such as phospho-T231 has actually been improved. The reasons for the improvement are unknown. Dilution of the antisera with a suitable detergent may prevent hydrophobic interactions of IgGs with some molecules [13]. On the other hand, certain antigen epitopes are embedded in membranes, and detergent treatment may enhance antigen exposure to antibodies. Therefore, pretreatment with Tween-20 may lead to a better presentation of antigen and/or better tissue permeability.
In summary, we suggest that caution should be taken when performing immunological analyses on aged human brain samples with rabbit polyconal antibodies. To minimize undesirable nonspecific cross-reactivity, we recommend the use of 1% sodium borohydride pretreatment and adding 0.5% Tween-20 in the diluting solution for the secondary antibodies in the immunohistochemical protocol.
Acknowledgments
We appreciate Dr. William Markesbery at The Sanders-Brown Center on Aging, the University of Kentucky for providing human brain samples in this study. This work was partially supported by grants from the National Natural Science Foundation of China (30470594, 30772282).
References
- 1.Sun A, Liu M, Nguyen XV, Bing G. P38 MAP kinase is activated at early stages in Alzheimer's disease brain. Exp Neurol. 2003;183:394–405. doi: 10.1016/s0014-4886(03)00180-8. [DOI] [PubMed] [Google Scholar]
- 2.Sun A, Nguyen XV, Bing G. A novel fluorescent method for direct visualization of neurofibrillary pathology in Alzheimer's disease. J Neurosci Methods. 2001;111:17–27. doi: 10.1016/s0165-0270(01)00434-4. [DOI] [PubMed] [Google Scholar]
- 3.Zhang QP, Zhang XG, Sun AY. Truncated tau at D421 is associated with neurodegeneration and tangle formation in the brain of Alzheimer transgenic models. Acta Neuropathol. 2009;117:687–697. doi: 10.1007/s00401-009-0491-6. [DOI] [PubMed] [Google Scholar]
- 4.Terman A, Brunk UT. Lipofuscin: mechanisms of formation and increase with age. APMIS. 1998;106:265–276. doi: 10.1111/j.1699-0463.1998.tb01346.x. [DOI] [PubMed] [Google Scholar]
- 5.Dowson JH. The evaluation of autofluorescence emission spectra derived from neuronal lipopigment. J Microsc. 1982;128:261–270. doi: 10.1111/j.1365-2818.1982.tb04628.x. [DOI] [PubMed] [Google Scholar]
- 6.Clancy B, Cauller LJ. Reduction of background autofluorescence in brain sections following immersion in sodium borohydride. J Neurosci Methods. 1998;83:97–102. doi: 10.1016/s0165-0270(98)00066-1. [DOI] [PubMed] [Google Scholar]
- 7.Baschong W, Suetterlin R, Laeng RH. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM) J Histochem Cytochem. 2001;49:1565–72. doi: 10.1177/002215540104901210. [DOI] [PubMed] [Google Scholar]
- 8.Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33:611–619. doi: 10.1016/s0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- 9.Stojanovic A, Roher AE, Ball MJ. Quantitative analysis of lipofuscin and neurofibrillary tangles in the hippocampal neurons of Alzheimer disease brains. Dementia. 1994;5:229–233. doi: 10.1159/000106728. [DOI] [PubMed] [Google Scholar]
- 10.Willingham MC. Alternative fixation processing method for preembedding ultrastructural immunocytochemistry of cytoplasmic antigens: the GBS (glutaraldehyde-borohydride-saponin) procedure. J Histochem Cytochem. 1983;31:791–889. doi: 10.1177/31.6.6404984. [DOI] [PubMed] [Google Scholar]
- 11.Steinitz M. Quantitation of the blocking effect of tween 20 and bovine serum albumin in ELISA microwells. Anal Biochem. 2000;282:232–238. doi: 10.1006/abio.2000.4602. [DOI] [PubMed] [Google Scholar]
- 12.Juhl BR, Norgaard T, Bjerrum OJ. The effect of Tween 20 on indirect immunoperoxidase staining of blood group antigen A in human urothelium. J Histochem Cytochem. 1984;32:935–941. doi: 10.1177/32.9.6205049. [DOI] [PubMed] [Google Scholar]
- 13.Pool CW, Buijs RM, Swaab DF, Boer GJ, van Leeuwen FW. On the way to specific immunocytochemical localization. In: Cuello AC, editor. Immunocytochemistry. John Wiley & Sons: Chichester, UK; 1983. pp. 1–46. [Google Scholar]