Abstract
Activation of NMDA receptors (NMDARs) can modulate excitatory synaptic transmission in the central nervous system by dynamically altering the number of synaptic AMPA receptors (AMPARs). The surface expression of NMDARs themselves is also subject to modulation in an activity-dependent manner. In addition to NMDAR-induced changes in AMPAR expression, AMPARs have also been found to regulate their own surface expression, independently of NMDARs. However, whether or not AMPARs and NMDARs might reciprocally regulate their surface expression has not previously been systematically explored. We utilized surface biotinylation assays and stimulation protocols intended to selectively stimulate various glutamate receptor subpopulations (e.g. AMPARs vs NMDARs; synaptic vs extrasynaptic). We reveal that activation of synaptic NMDARs increases the surface expression of both NMDAR and AMPAR subunits, while activation of extrasynaptic NMDAR produces the opposite effect. Surprisingly, we find that selective activation of AMPARs reduces the surface expression of not only AMPARs but also of NMDARs. These results suggest that both AMPARs and NMDARs at synaptic sites are subject to modulation by multiple signalling pathways in an activity-dependent way.
Keywords: AMPA receptor, NMDA receptor, trafficking, synaptic, extrasynaptic, surface expression
Introduction
The AMPA receptor (AMPAR), which underlies fast excitatory synaptic transmission, is a heterotetrameric channel composed of different combinations of GluA1-4 (formerly GluR1–4) subunits. At the majority of central synapses, AMPARs are rendered Ca2+ impermeable due to the inclusion of the GluA2 subunit within assembled heterotetramers. AMPARs display rapid lateral diffusion and high constitutive rates of trafficking between the cell surface and intracellular receptor pools. In addition, the number and subunit composition of surface AMPARs is dynamic and can be modulated in an activity-dependent manner [1-4]. Such activity-dependent alterations in the complement of surface expressed AMPARs underlie various forms of synaptic plasticity that are essential during neural development and learning and memory. In addition, changes in receptor surface expression may contribute to homeostatic mechanisms which maintain neuronal excitability within an effective dynamic range. Mechanistically, AMPAR surface expression is determined by the balance between clathrin-dependent internalization [5;6] and SNARE-dependent exocytosis [7].
The NMDA receptor (NMDAR) is a heteromeric ligand-gated ion channel composed of two GluN1 (formerly NR1) subunits and two GluN2 (GluN2A–D, formerly NR2A-D) subunits [8]. NMDARs display distinctive functional and biophysical properties. Indeed, channel opening requires not only the binding of both glutamate and glycine but also the relief of Mg2+ block by membrane depolarization. In addition, NMDAR channels have high permeability to Ca2+. This allows NMDARs to activate downstream signalling cascades responsible for the induction of synaptic plasticity (e.g. LTP and LTD). When compared to AMPARs, surface NMDARs are generally more stable at the cell surface due to a much lower rate of constitutive internalization. Nevertheless, activity can alter the surface expression of these receptors [9-18]. As with AMPARs, NMDARs are internalized in a clathrin-dependent manner [13] and likely trafficked to the cell surface through exocyst- and snare-dependent exocytosis [12;19].
In addition to their synaptic localization, both AMPARs and NMDARs are expressed in extrasynaptic compartments. These extrasynaptic receptors not only provide a pool of reserve receptors available to the synapse through lateral diffusion but may also mediate distinct functional consequences. This is especially true for NMDARs where synaptic receptors may play a neuroprotective role whereas extrasynaptic receptors may contribute to neurotoxicity. Given its important role as a gate for the induction of synaptic plasticity many of the studies examining changes in both AMPAR and NMDAR surface expression have focused on NMDAR-induced changes. Although it is known that AMPARs can initiate changes in AMPAR surface expression [5;20], whether they also regulate NMDARs has not yet been explored. With this in mind, in the present study we explored the specific contribution of glutamate receptor subpopulations to the regulation of surface expressed AMPARs and NMDARs.
Materials and methods
Cell cultures and surface biotinylation assay
Hippocampal and cortical cultures were prepared from 18 day embryonic mice as described [21]. Cultured neurons (15–17 div) were washed with bathing solution containing (in mM): NaCl 140, KCl 5, glucose 33; HEPES 10, CaCl2 1.3, MgCl2 1; glycine 0.001. The cells were adapted in bathing solution for 20 min at 36°C before treatment. The cells were then treated, washed and incubated at 36°C to allow receptor trafficking. Following this, surface proteins were biotinylated with Sulfo-NHS-LC-biotin (0.5 mg/ml; Pierce, Brockville, ON) in bath solution for 30 min at 4°C. Cells were washed, harvested and homogenized. Avidin beads (Sigma, Oakville, ON) were used to precipitate biotinylated proteins for Western blotting.
Gel electrophoresis and western blot analysis
Membrane homogenates were subjected to SDS-PAGE using 10% or 7.5% gels. Proteins were transferred to nitrocellulose membranes. The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween for 1 hr at room temperature or overnight at 4°C, incubated in primary antibodies (anti-GluA1 (0.5 μg/ml), anti-GluN1 (1:2000), anti-GluN2A (1:1000) and anti-GluN2B (1:1000), Chemicon, Mississauga, ON) for 1 hr, washed three times for 15 min each, incubated with HRP-conjugated secondary antibodies for 1 hr, washed three times, and bound antibodies were visualized by the enhanced chemiluminescence method. Densitometric analysis of Western blots was performed using the Kodak Image Station 2000R software. Data are presented as mean ± SE. Statistical significance was analyzed by Student's t test.
Results
Hypertonic sucrose stimulation increases the surface expression of GluA1
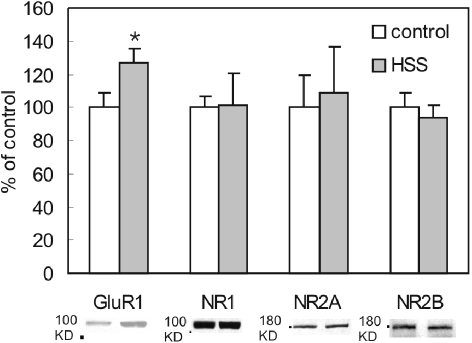
We began by examining the effects of synaptic glutamate receptor stimulation on surface expressed receptors. We have previously shown that stimulation of synaptic NMDARs with glycine (in the absence of Mg2+) could induce LTP and was associated with an increased insertion of AMPARs [7]. However, in the glycine model TTX was used to prevent action potential-dependent transmitter release. Thus, while the glycine culture model allows transient and selective stimulation of synaptic NMDARs, the stimulation is limited by the rate of spontaneous vesicular glutamate release and is restricted to NMDARs only. In order to elicit greater stimulation of both synaptic AMPARs and NMDARs we utilized brief applications of a hypertonic sucrose solution, which triggers synaptic vesicle exocytosis from the same pool from which action potentials provoke release [22]. As a result, hypertonic sucrose should allow robust and spatially restricted (i.e. synaptic) release of glutamate at nearly every cultured neuron synapses. Hypertonic sucrose was applied to the entire dish for 20 sec following which surface proteins were labelled with biotin for 30 min. Subsequently surface biotinylated proteins were isolated and immobilized on nitrocellulose membranes and an antibody against GluA1 was used to assess AMPAR labelling. Consistent with results previously reported using the glycine model, we observed an increase of 26.9 ± 8.9% in the surface expression of GluA1 (Figure 1). Surprisingly however, there was no detectable change in surface expression of GluN1 (1.6 ± 19.0%), GluN2A (8.3 ± 28.4%) or GluN2B subunits (-6.1 ± 7.9 %).
Figure 1.
Surface expression of GluA1 subunits was modified by hypertonic sucrose treatment. Surface proteins were biotinylated, isolated and immobilized on nitrocellulose membranes for quantitative Western blotting. Treatment with hypertonic sucrose induced a potentiation of surface GluA1 subunits (n = 4). Surface expression of GluN1 (P = 0.955, n = 4), GluN2A (P = 0.836, n = 4) or GluN2B (P = 0.807, n = 4) subunits was unchanged by HSS treatment.
AMPAR activation produces loss of surface expression of NMDA and AMPA receptors by a Ca2+-independent mechanism
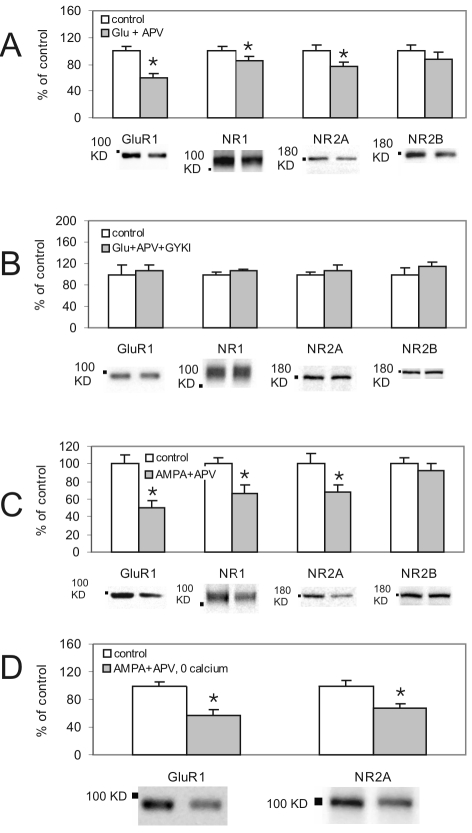
The lack of effect of hypertonic sucrose on NMDAR surface expression indicated that NMDARs are either immobile or that opposing mechanisms were recruited; the net effect of which was to leave the total surface expression unchanged. For example, the activation of non-NMDA glutamate receptors might alter the expression of NMDARs in a way which opposes the effect produced by the predominant activation of synaptic NMDARs. To investigate the role of non-NMDA receptors in glutamate receptor trafficking, we initially used brief applications (20 sec) of glutamate (100 μM) in the presence of Mg2+ (1 mM) and APV (100 μM) to block NMDARs. Interestingly, non-NMDA receptor stimulation caused a reduction in surface GluA1 (-41.0 ± 7.7%), GluN1 (-15.0 ± 5.8%) and GluN2A (-23.2 ± 6.5%) signals (Figure 2A). No change in GluN2B was observed (-13.4 ± 10.7%).
Figure 2.
AMPA receptors were involved in the regulation of both AMPAR and NMDAR surface expression. A: Cells were treated for 20 sec with glutamate (100 μM) in the presence of Mg2+ (1 mM) and APV (100 μM) to block NMDAR activation. There was a significant reduction in surface GluA1 (P = 0.025, n = 6) GluN1 (P = 0.0.032, n = 6) and GluN2A (P = 0.035, n = 6). GluN2B signals were not significantly reduced (P = 0.368, n = 6) B: When both NMDARs and AMPARs were blocked with APV (100 μM) and GYKI53655 (50 μM), respectively, glutamate (100 μM) failed to cause significant change in the surface signal for GluA1 (P = 0.771, n = 6), GluN1 (P = 0.211, n = 6), GluN2A (P = 0.590, n = 6) and GluN2B (P = 0.339, n = 6). C: Brief (20 sec) application of AMPA in the presence of APV (100 μM), Mg2+ (1 mM) and TTX (0.5 μM) caused a decrease in surface GluA1 signal (P = 0.011, n = 6). Interestingly, the surface expression of GluN1 and GluN2A subunits was also reduced (P = 0.013, 0.05, respectively, n = 6). There was no change in surface GluN2B signal (P = 0.511, n = 6). D: The effect of AMPA on AMPAR and NMDAR trafficking was independent of extracellular calcium, since AMPAR activation in Ca2+-free solution still caused reduction in surface GluA1 (P = 0.028, n = 6) and GluN2A (P = 0.012, n = 6) subunits.
Among non-NMDA receptors the activation of AMPARs as well as metabotropic glutamate receptors have previously been implicated in regulating the surface expression of glutamate receptors [14;20]. We initially tested the specific involvement of AMPARs by treating neurons with glutamate (100 μM) for 20 sec in the presence of APV (100 μM) and the highly selective AMPAR antagonist, GYKI53655 (50 μM). Under these conditions there was no change in the signal for surface GluA1 (6.0 ± 11.1%), GluN1 (7.5 ± 3.1%), GluN2A (7.2 ± 9.6%) and GluN2B (15.4 ± 6.7%, Figure 2B). These results suggest that selective AMPAR activation mediates reduced surface expression of GluA1, GluN1 and GluN2A.
If AMPARs mediate a downregulation of AMPA and NMDA receptor surface expression, then the selective activation of these receptors should produce a similar effect. Therefore we examined the effect of briefly (20 sec) applying AMPA (50 μM). These experiments were performed in the presence of APV (100 μM) and Mg2+ (1 mM) to prevent secondary activation of NMDA receptors by glutamate released as a consequence of the AMPA-induced depolarization. TTX (0.5 μM) was also included to minimize neuronal activity. Cells in the control group were mock-treated with solution containing APV, Mg2+ and TTX. Selective AMPA receptor stimulation produced a similar reduction of GluA1 (-50.1 ± 9.1%), GluN1 (-34.1 ± 10.6%) and GluN2A (- 32.3 ± 8.6%) without changing GluN2B (-7.8 ± 8.9%) surface expression (Figure 2C).
Ehlers [20] previously reported that reduced surface expression of AMPARs following AMPAR activation occurred independently of Ca2+. We therefore examined the Ca2+-dependence of the AMPAR-mediated downregulation of surface expressed AMPARs and NMDARs. To test this, we treated cells with AMPA (50 μM) in calcium-free (0 added Ca2+ plus 10 mM EGTA) solution containing APV (100 μM), Mg2+ (1 mM) and TTX (0.5 μM). We still saw reductions in surface GluA1 (-43.7 ± 9.7%) and GluN2A (-32.9 ± 5.8%) subunits (Figure 2D) demonstrating that AMPAR stimulation causes internalization of AMPARs and NMDARs through a Ca2+- independent mechanism.
Activation of synaptic NMDA receptors produces reliable enhancement of surface NMDA and AMPA receptors
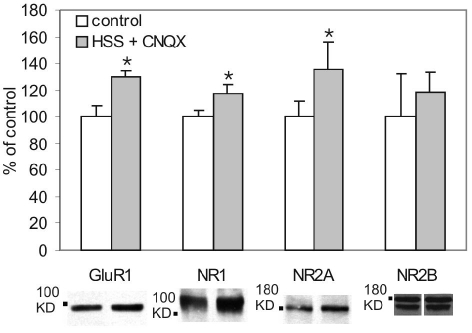
As shown previously (Figure 1), whereas GluA1 surface expression was increased by hypertonic sucrose, the surface expression of NMDAR subunits was unchanged. Having shown that AMPA receptors can reduce the surface expression of both NMDA and AMPA receptors we examined whether the inability to observe a change in NMDARs might have been due to coincident activation, by hypertonic sucrose, of AMPARs which might oppose a potential upregulation mediated by NMDARs. We therefore examined whether the activation of synaptic NMDARs by hypertonic sucrose, applied in the presence of an AMPAR antagonist, can enhance the surface expression of both AMPARs and NMDARs. We challenged the cells with Mg2+-free hypertonic sucrose in the presence of the AMPAR antagonist CNQX (100 μM). Under these conditions, the sucrose-induced release of glutamate produced an increase in GluA1 (30.4 ± 4.0%), GluN1 (17.4 ± 7.2%) and GluN2A (35.5 ± 21.1) signals (Figure 3) while the GluN2B (18.6 ± 15.3%) surface signal was not affected.
Figure 3.
Activation of synaptic NMDARs increased surface expression of AMPARs and NMDARs. The cells were challenged with HSS in the presence of CNQX (100 μM) to eliminate the opposing effect produced by AMPAR activation. Sucrose-induced release of glutamate produced an increase in GluA1 (P = 0.027, n = 5), GluN1 (P = 0.042, n = 5) and GluN2A (P = 0.028, n = 13) surface signals, while GluN2B surface signal was not affected (P = 0.669, n = 4).
Selective activation of extrasynaptic receptors produces broad reductions of glutamate receptor subunits
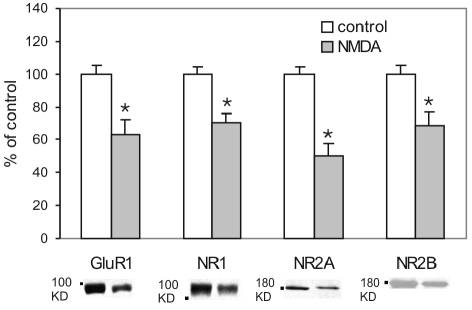
Neither synaptic NMDA nor AMPA receptor stimulation could influence the surface expression of GluN2B subunits. In mature neurons, GluN2B subunits are proposed to predominate at extrasynaptic sites [23-25]. Changes in their surface expression may therefore require the specific activation of extrasynaptic NMDARs (e.g. autoregulation). We have previously demonstrated that extrasynaptic NMDAR stimulation produces an LTD of mEPSCs [7] but we did not examine the consequence of such stimulation on the surface expression of glutamate receptors. We therefore used the following strategy to block synaptic NMDA receptors and allow the selective activation of extrasynaptic NMDA receptors. A high potassium (50 mM) solution was first applied in order to release nerve terminal glutamate and preferentially activate synaptic NMDARs. This was done in a very high concentration of MK801 (100 μM) so that synaptic NMDARs were preferentially opened and then very rapidly blocked by this open channel blocker. EGTA (10 mM) was also applied in order to prevent influx of Ca2+ and therefore the induction of potentiation through activated synaptic NMDA receptors. The cells were then washed extensively to remove unbound MK801. Synaptically activated receptors will remain blocked because of the extremely slow rate of dissociation of MK801. Subsequently, the cells were exposed to NMDA (50 μM) for 20 sec in order to stimulate the extrasynaptic NMDAR population and permit preferential Ca2+ entry via this subpopulation of receptors. As anticipated there was a consistent decrease in the surface GluA1 (-36.6 ± 8.8%) expression (Figure 4). Importantly, the expression of GluN1 (-29.6 ± 5.4%), GluN2A (-49.7 ± 7.2%) and GluN2B (- 31.1 ± 8.0%) were significantly reduced as compared to control cells (also subject to preferential block of synaptic NMDA receptors).
Figure 4.
Activation of extrasynaptic NMDA receptors reduced surface AMPA and NMDA receptors. Ca2+-free high potassium (50 mM) solution was used to depolarize the cells to release glutamate from nerve terminals in the presence of MK801 (100 μM) and EGTA (10 mM). Under these conditions, synaptic NMDARs will preferentially be activated and then rapidly blocked by the open channel blocker, MK801. The cells were then treated with NMDA (50 μM) for 20 sec which will preferentially activate the extrasynaptic NMDAR population. There was a significant decrease in GluA1 (P = 0.014, n = 5), GluN1 (P = 0.005, n = 5), GluN2A (P = 0.001, n = 5) and GluN2B (P = 0.021, n = 5) surface expression.
Discussion
Most protocols currently used for studying receptor trafficking in neurons involve the bath application of agonists or antagonists which indiscriminately activate or block entire populations of a specific receptor subtype. Since the traditionally held view is that synaptic plasticity exists along a continuum whereby weak NMDAR activation causes LTD and strong NMDAR activation causes LTP, it seemed somewhat paradoxical that bath applications of NMDA, expected to cause strong NMDAR activation, has only been shown to induce LTD. We have reported that rather than being strictly dependent on the strength of NMDAR stimulation, and consequently on the amount of Ca2+ entry, the functional outcome of NMDAR stimulation depends on the localization of the activated NMDARs [7]. Thus, glycine stimulation of synaptic NMDARs, activated by spontaneously released glutamate, induces LTP of mEPSCs that is associated with rapid insertion of AMPARs at the surface of dendritic membranes. Whereas, stimulation of extrasynaptic NMDARs by bath applied NMDA provokes LTD of mEPSCs. Our current data confirms that robust activation of synaptic NMDARs is associated with an increase in the surface expression of GluA1. Using a protocol which allows robust and co-incident activation of both AMPARs and NMDARs we now extend these finding in a number of ways which may have important implications for synaptic plasticity mediated through changes in surface expression of glutamate receptors. We demonstrate that the selective activation of AMPARs decreases surface expressed GluA1, GluN1 and GluN2A while in contrast the selective activation of synaptic NMDARs increases the surface expression of not only GluA1 but also GluN1 and GluN2A. Finally, we demonstrate that stimulation of extrasynaptic NMDARs, which are thought to be predominantly composed of GluN2B subunits, reduces the surface expression of not only GluN2B but also GluA1, GluN1 and GluN2A.
Transient activation of AMPA receptors reduce the surface expression of both AMPA and NMDA receptors
The changes which we observed could be induced following brief (20 sec) stimulation of AMPARs. While it was impractical to examine shorter time points, our results suggest that mechanisms contributing to the feedback regulation of AMPARs by AMPAR activation may operate on a time scale which allows them to contribute to the changes in synaptic efficacy underlying LTD and LTP. Additionally, while NMDARs were previously believed to regulate AMPARs as well as themselves, they were not believed to be influenced by activity-dependent changes induced downstream of AMPARs. The finding that selective AMPAR stimulation reduces the surface expression of not only GluA1 but also GluN1 and GluN2A, as illustrated in Figure 5C, suggests a previously unrecognized reciprocal regulation between these two receptors subtypes. Similar to the findings of Ehlers (2000) we found that the effects of AMPAR stimulation upon AMPA and NMDA receptor surface expression occurred independently of extracellular Ca2+. The present study does not allow us to distinguish whether such changes occurred as a result of increased internalization or reduced insertion of receptors.
Figure 5.
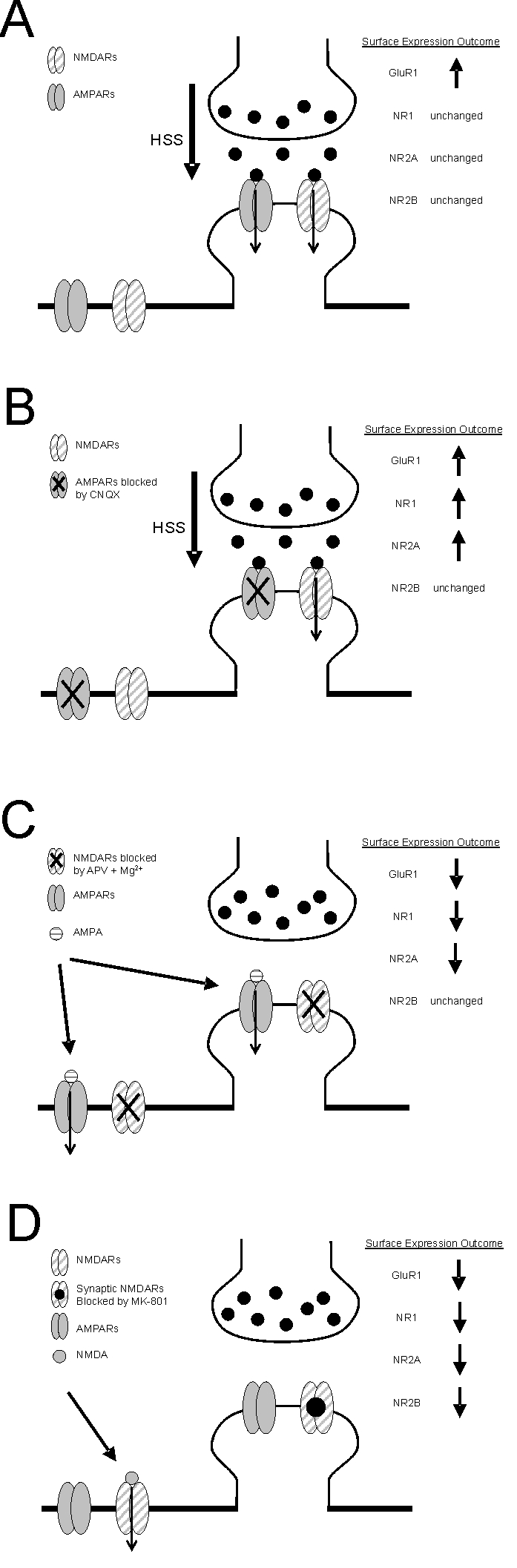
Summary diagram illustrating the changes in glutamate receptor surface expression following the selective activation of various subtypes and populations of glutamate receptors. The activation of selected populations of postsynaptic glutamate receptors was achieved as follows: A) predominantly synaptic AMPARs and NMDARs were co-incidently activated by hypertonic sucrose solution (HSS), B) predominantly synaptic NMDARs were activated by HSS in the presence of CNQX, C) the entire complement of surface AMPARs were activated by bath applied AMPA in the presence of APV and Mg2+ and D) extrasynaptic NMDARs were activated by applied NMDA after having first blocked synaptic NMDARs with MK-801. Downward arrows superimposed on glutamate receptor subunits illustrate receptor populations activated by each protocol. In all panels, the outcome of the various treatments on the surface expression of glutamate receptors is summarized as either increased (upward arrow), decreased (downward arrow) or unchanged.
Co-incident activation of synaptic AMPA and NMDA receptor mediate opposing effects on synaptic plasticity
Interestingly, under conditions that allowed the activation of both AMPA and NMDA receptors we observed that while GluA1 surface expression was increased by hypertonic sucrose treatment, NMDAR subunits were not altered (illustrated in Figure 5A). When hypertonic sucrose was applied in the presence of an AMPAR antagonist, the resulting selective activation of synaptic NMDARs resulted in an increased surface expression of not only GluA1 but also GluN1 and GluN2A (illustrated in Figure 5B). While hypertonic sucrose stimulates synaptic vesicle exocytosis and thus should predominantly activate synaptic receptor we cannot completely exclude the possibility that hypertonic sucrose activated perisynaptic as well as some extrasynaptic NMDA receptors. However, we reason that synaptic NMDARs were predominantly stimulated since our previous study as well as the present results demonstrate that strong stimulation of extrasynaptic NMDARs receptors mediates LTD and a reduced surface expression of glutamate receptors. Consequently, and quite surprisingly, our results suggest that robust simultaneous activation of synaptic AMPA and NMDA receptors recruit opposing AMPAR-mediated downregulating and NMDAR-mediated upregulating mechanisms.
Since hypertonic sucrose alone produced an increase in GluA1, the potentiating actions of synaptic NMDAR activation appear to dominate over the depressant actions of AMPAR under conditions where coincident stimulation of both receptor types occurs. This may involve a signalling crosstalk whereby strong synaptic NMDARs negatively interferes with the AMPAR mediated regulation of GluA1 subunits. In contrast, NMDAR subunits were unchanged under these conditions presumably due to the offsetting effects of coincident NMDAR and AMPAR activation.
Relative strength of AMPA and NMDA receptor activation determines direction of activity-dependent plasticity
Experimental manipulations allowed us to demonstrate that selective activation allows synaptic AMPA and NMDA receptors to induce differential changes in glutamate receptor surface expression. It is obviously unlikely that such selective activation would occur in a physiological, or for that matter, pathophysiological setting. Thus, our results suggest that it is the relative strength with which each receptor (i.e. synaptic AMPA vs NMDA receptors) is activated that determines the direction of an activity-induced change in synaptic plasticity rather than strictly depending on the level of activation of NMDARs. Although speculative, such a model might allow significant new insight into mechanisms contributing to the induction of LTD and LTP of AMPAR-mediated responses. For example, due to the voltage-dependent Mg2+ block of NMDARs low-frequency stimulation, such as those typically utilized for inducing LTD, should favour AMPAR activation. Thus, AMPAR-mediated downregulation of surface expressed AMPARs might contribute to LTD. Interestingly, there is evidence to suggest that antagonism of AMPARs can prevent the establishment of LTD in hippocampal as well as in cerebellar slices [26;27]. Combined with the wealth of evidence for NMDAR-dependence of LTD, these results may suggest that coincident activation, but which favours AMPAR activation, is required for the establishment of LTD. In contrast, during high frequency tetanic stimulation commonly used to induce LTP, rapid relief of Mg2+ block coupled with more prominent desensitization of AMPARs might shift the balance in favour of synaptic NMDAR-mediated enhancement of AMPAR surface expression.
Implications of extrasynaptic NMDAR-mediated plasticity
Yet another level of complexity is suggested by the results obtained with stimulation of extrasynaptic NMDARs (illustrated in Figure 5D). While we did not explore the subunit composition directly using pharmacological means (e.g. ifenprodil) considerable evidence has accumulated to suggest that in mature cultured neurons (> 14 DIV) extrasynaptic receptors are predominantly composed of GluN2B subunits [23-25]. It is interesting to note that only when we selectively stimulated extrasynaptic receptors did we observe any change in GluN2B surface expression. While definitive proof is needed, these results could suggest that under these conditions GluN2B containing receptors were strongly stimulated resulting in a feedback control of their own expression. As mentioned previously since treatment of neurons with hypertonic sucrose alone caused LTP and increased surface expression of GluA1 without a change in NMDAR subunits, spillover of synaptically released glutamate is unlikely to cause sufficient activation of extrasynaptic GluN2B containing receptors. Thus, these receptor populations are more likely to be activated during pathophysiological insults (e.g. seizure activity and excitotoxicity). As a result, the broad down-regulation of glutamate receptor subunits and resulting LTD of synaptic transmission might serve as a homeostatic mechanism attempting to restore the balance between excitation and inhibition.
Physiological implications of correlated surface expression of AMPA and NMDA receptors
It is well known that blockade of NMDAR activation causes redistribution of surface NMDARs [9;28]. However, in these experiments global treatment with NMDAR antagonists likely involves blockade of both extrasynaptic and synaptic NMDARs, which may produce opposing effects and complicate interpretation of the physiological role of receptor trafficking. The finding that AMPA and NMDA receptor-mediated plastic changes are more likely to offset one another with respect to NMDARs may help partially explain the long held belief that NMDARs are less mobile than AMPARs and suggests that the conditions of glutamate receptor activation be carefully considered when interpreting biochemical and electrophysiological analysis of NMDAR plasticity.
Acknowledgments
We thank E. Czerwinska, W. Czerwinski and L. Brandes for technical assistance and also thank Drs. M. W. Salter and W. Ju for reading the manuscript and providing suggestions. We are grateful to Dr. Y.T. Wang for his technical advice with surface biotinylation assays. This work was supported by a grant to JFM from the Canadian Institutes of Health Research (CIHR) and by a Canadian Stroke Network/Heart and Stroke Foundation of Canada/CIHR fellowship to MFJ.
References
- 1.Wang YT. Probing the role of AMPAR endocytosis and long-term depression in behavioural sensitization: relevance to treatment of brain disorders, including drug addiction. Br J Pharmacol. 2008;153(Suppl 1):S389–S395. doi: 10.1038/sj.bjp.0707616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 3.Groc L, Choquet D. AMPA and NMDA glutamate receptor trafficking: multiple roads for reaching and leaving the synapse. Cell Tissue Res. 2006;326:423–438. doi: 10.1007/s00441-006-0254-9. [DOI] [PubMed] [Google Scholar]
- 4.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 5.Carroll RC, Beattie EC, Xia H, scher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci USA. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 7.Lu WY, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of Synaptic NMDA Receptors Induces Membrane Insertion of New AMPA Receptors and LTP in Cultured Hippocampal Neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 8.Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- 9.Crump FT, Dillman KS, Craig AM. cAMP-Dependent Protein Kinase Mediates Activity-Regulated Synaptic Targeting of NMDA Receptors. J Neurosci. 2001;21:5079–5088. doi: 10.1523/JNEUROSCI.21-14-05079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat Neurosci. 2001;4:587–596. doi: 10.1038/88404. [DOI] [PubMed] [Google Scholar]
- 12.Skeberdis VA, Lan JJ, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci USA. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roche KW, Standley S, McCallum J, Dune LC, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- 14.Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 15.Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- 16.Prybylowski K, Fu Z, Losi G, Hawkins LM, Luo J, Chang K, Wenthold RJ, Vicini S. Relationship between availability of NMDA receptor subunits and their expression at the synapse. J Neurosci. 2002;22:8902–8910. doi: 10.1523/JNEUROSCI.22-20-08902.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- 18.Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Nature. 2003;422:302–307. doi: 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- 19.Sans N, Prybylowski K, Petralia RS, Chang K, Wang YX, Racca C, Vicini S, Wenthold RJ. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat Cell Biol. 2003;5:520–530. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- 20.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 21.Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- 22.Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 23.Li B, Chen N, Luo T, Otsu Y, Murphy TH, Raymond LA. Differential regulation of synaptic and extra-synaptic NMDA receptors. Nat Neurosci. 2002;5:833–834. doi: 10.1038/nn912. [DOI] [PubMed] [Google Scholar]
- 24.Li JH, Wang YH, Wolfe BB, Krueger KE, Corsi L, Stocca G, Vicini S. Developmental changes in localization of NMDA receptor subunits in primary cultures of cortical neurons. Eur J Neurosci. 1998;10:1704–1715. doi: 10.1046/j.1460-9568.1998.00169.x. [DOI] [PubMed] [Google Scholar]
- 25.Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemp N, Bashir ZI. Induction of LTD in the adult hippocampus by the synaptic activation of AMPA/kainate and metabotropic glutamate receptors. Neuropharmacology. 1999;38:495–504. doi: 10.1016/s0028-3908(98)00222-6. [DOI] [PubMed] [Google Scholar]
- 27.Hemart N, Daniel H, Jaillard D, Crepel F. Receptors and second messengers involved in long-term depression in rat cerebellar slices in vitro: a reappraisal. Eur J Neurosci. 1995;7:45–53. doi: 10.1111/j.1460-9568.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 28.Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]