Abstract
Epidemiological studies have shown a strong link between air pollution and the increase of cardio-pulmonary mortality and morbidity. In particular, inhaled airborne particulate matter (PM) exposure is closely associated with the pathogenesis of air pollution-induced systemic diseases. In this study, we exposed C57BIV6 mice to environmentally relevant PM in fine and ultra fine ranges (diameter < 2.5 μm, PM2.5) using a “real-world” airborne PM exposure system. We investigated the pathophysiologic impact of PM2.5 exposure in the animal model and in cultured primary pulmonary macrophages. We demonstrated that PM2.5 exposure increased the production of reactive oxygen species (ROS) in blood vessels in vivo. Furthermore, in vitro PM2.5 exposure experiment suggested that PM2.5 could trigger oxidative stress response, reflected by an increased expression of the anti-oxidative stress enzymes superoxide dismutase-1 (SOD-1) and heme oxygenase-1(HO-1), in mouse primary macrophages. Together, the results obtained through our “real-world” PM exposure approach demonstrated the pathophysiologic effect of ambient PM2.5 exposure on triggering oxidative stress in the specialized organ and cell type of an animal model. Our results and approach will be informative for the research in air pollution-associated physiology and pathology.
Keywords: Air pollution, airborne particulate matter, oxidative stress, reactive oxygen species, animal model
Introduction
Air pollution is a sustained problem of public health for the general population in urban areas, especially for those who live in areas of intensive traffic or industrial activity. Accumulating evidence has suggested a significant association between exposure to airborne PM and the increase of morbidity and mortality associated with cardiovascular diseases [1-5]. Airborne PM2.5 is a complex mixture of chemical and/or biological elements, such as metals, salts, carbonaceous material, volatile organic compounds, polycyclic aromatic hydrocarbons and endotoxins, depending upon their natural and/or anthropogenic emission sources [6, 7]. Particularly, airborne PM in fine and ultrafine ranges (diameter < 2.5 mm, PM2.5) has been implicated to play a detrimental role in the pathogenesis of the disease. It has been demonstrated that PM2.5 has an incremental capacity to penetrate the most distal airway units and potentially into systemic circulation with diminishing sizes [8, 9]. Recent studies from different experimental systems have suggested that the impacts of airborne PM on signaling pathways related to redox homeostasis and inflammation [5, 10-13]. Our study here provides new evidence that “real-world” exposure of environmentally relevant PM25 can trigger oxidative stress in blood vessels in an animal model and in cultured primary pulmonary macrophages.
Our PM exposure system, the “Ohio's Air Pollution Exposure System for the Interrogation of Systemic Effects (OASIS-1)", is a mobile trailer versatile aerosol concentration enrichment system [14-16]. In this system, only PM2.5 particles, which include fine and ultrafine particles, are concentrated from the ambient air and exposed to the in-house-bred mice [15, 17, 18]. It has been demonstrated that the PM2.5 particle size and composition distribution did not change before (ambient) and after the VACES system [17, 18]. This system is one of several limited prototypic systems in the United States, which allows us to use animal models to recapitulate real world exposure to environmental relevant PM2.5, and to perform systemic analysis of PM2.5 -associated physiology and pathology.
Materials and methods
Animals
Male mice of C57BL/6 strain background at six-week-old were purchased from the Jackson Laboratories (Bar Harbor, ME), and were equilibrated for 2 weeks prior to the exposure experiment. The mice were housed in cages with regular chow. The Committees on Use and Care of Animals at the Ohio State University approved all experimental procedures.
Exposure to ambient PM2.5
Animals were exposed to concentrated ambient PM2.5 or filtered air (FA) for a total duration of 10 weeks in a mobile trailer “Ohio's Air Pollution Exposure System for the Interrogation of Systemic Effects (OASIS)-1” composed of the mid-western regional background in Columbus, OH, on the Ohio State University campus. The concentrated PM2.5 in the exposure chamber was generated using a versatile aerosol concentration enrichment system [14, 15]. The mice were exposed to PM2.5 at nominal 10 times of ambient concentrations for 6 hours per day, 5 days per week (Monday to Friday) for a total period of 10-weeks [4, 16]. The control mice were exposed to an identical protocol in filtered air chambers in which a high-efficiency particulateair (HEPA) filter positioned in the inlet valve position to remove all the PM2.5.
Primary macrophage isolation, culture, and in vitro exposure to PM2.5
C57BL/6 mice at 3-month of age were anesthetized with pentobarbital. A 5ml-syringe with a blunt needle containing 1.5 ml PBS (Ca/Mg free) was inserted into the lung through the trachea for flushing off the bronchoalveolar macro-phages. Isolated macrophages were cultured in FG12 media supplemented with L-glutamine and 10% fetal bovine serum. For in vitro PM2.5 exposure experiment, PM2.5 particles were collected at the time when the mice were exposed. The stock PM2.5 solution (5 mg/ml in PBS) was stored in -80 “C. The frozen PM2.5aliquots were thawed and briefly sonicated before adding into macrophage cell culture media for the in vitro experiment. Macrophages were treated with PM2.5 at the concentration of 50 yg/ml, and the same volume of PBS was added as the control.
Quantitative real-time reverse-transcription (RT)-PCR analysis
Total cellular RNA was prepared using TRIzol reagent as instructed by the manufacturer (Invitrogen Corp.). Total RNA was reverse-transcribed to cDNA using a random primer (Applied Biosystems). The quantitative real-time PCR analysis was performed with a Stratagene MX3000P Real-Time PCR System (Stratagene) following the standard procedure [19]. Realtime PCR primer sequences are: Heme oxygenase 1 (HO-1): 5'-CACGCATATACCCGCTACCT-3’ and 5'- CCAGAGTGTTCATTCGAGA-3'; super-oxide dismutase 1 (S0D-1): 5'- GCGGTGAAC CAGTTGTGTTGTC-3’ and 5'-CAGTCACATTGCCCA GGTCTCC-3'. Fold changes of mRNA levels were determined after normalization to internal control /3-act/n RNA levels.
Dihydroethidium (DHE) fluorescence staining of liver tissue
DHE (Sigma-Aldrich), an oxidative fluorescent dye, was used to detectsuperoxide (O2− in segments of frozen carotid artery as described previously [20]. Briefly, fresh segments of the common carotid artery were frozen in OCT compound, and transverse sections (10 μm) were generated with a cryostat and placed on glass slides. Sections were then incubated in chamber with 10 μmol/L DHE (Molecular Probes) for 30 minutes at room temperature. Images were obtained with a fluorescent microscope. The excitation wavelength was 488 nm, and emission fluorescence was detected with the use of a 585 nm filter.
Results and discussion
To investigate the pathophysiological impact of airborne PM2.5 exposure, in-house bred C57BL/6 mice were exposed to concentrated ambient PM2.5 for 10 weeks in the mobile trailer “0ASIS-1” exposure system located in Columbus (Ohio State University campus), Ohio, where most of the PM2.5 was attributed to long-range transport [4, 16]. The State of Ohio is one of many “perfect” states to study the effects of PM2.5 on human health, since “Ohio has a serious and widespread air pollution problem, and had failed to meet the National ozone and particulate matter annual standards” [21]. The ambient PM2.5 in Columbus is representative of regional background PM2.5 of the megalopolis that extends from Detroit to Cincinnati. Traffic-related PM2.5 is a complex mixture of particles and gases from gasoline and diesel engines, together with dust from wear of road surfaces, tires, and brakes [6, 7]. The composition analysis indicated that the distribution of concentrated PM2.5 collected from the exposure system can truly reflect that of non-concentrated PM2.5 present in the ambient air (data not shown) [16]. During the exposure period, the mean PM2.5 concentration inside the exposure chamber was 74.6 ug/m3. Because the mice were exposed 6 hours a day, 5 days a week, the equivalent PM2.5 concentration to which the mice were exposed in the chamber “normalized” over the 10-week period was 11.6 ug/m3. These calculations have taken into account non-exposed time and weekends. The equivalent PM2.5 exposure concentration in this study is well within the annual average PM2.5 National Ambient Air Quality Standard of 15.0 μg/m3 [21]. As controls, the mice were exposed to an identical protocol with the exception of HEPA filters installed in the PM chambers to remove all the PM2.5.
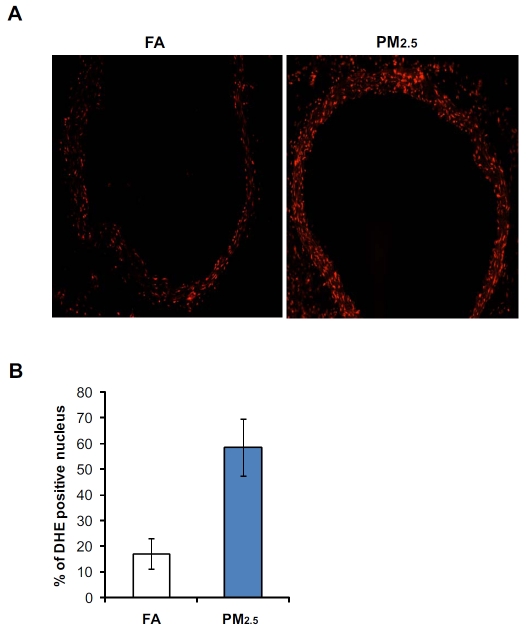
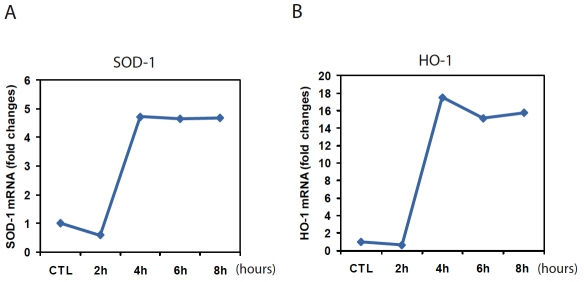
PM2.5 has been shown to stimulate generation of reactive oxygen species (ROS) in the cells due to its features of small diameters and high surface area [4, 5]. To test whether PM2.5 exposure can trigger ROS production in vivo, we examined the redox states in the blood vessels of mice exposed to PIVb.sor filtered air (FA). To evaluate the in situ levels of superoxide (O2−), we stained frozen mouse blood vessel tissue sections with DHE, an oxidative fluorescent dye. DHE staining showed that O2− production was significantly increased in the aorta endothilium of PM2.5-exposed mice compared to that in FA-exposed mice (Figure 1A-B). O2− activity in the aortic segments of mice exposed to PM2.5 was approximately 3.5-times higher than that of the FA-exposed controls (Figure IB). To further evaluate the effect of PM2.5 on triggering oxidative stress, we exposed mouse primary macro-phages to PM2.5 collected from the “OASIS-1” system at the time when the mice were exposed. Quantitative real-time RT-PCR analysis showed that expression of anti-oxidant enzymes, including SOD -1 and HO-1, was significantly increased in the primary macrophages after 4 hours of the PM2.5 treatment (Figure 2A-B). Taken together, the results obtained with our “real-world” PM exposure system indicated that PM2.5 is a trigger of oxidative stress in vivo and in vitro.
Figure 1.
Exposure to PM2.5 cause increased ROS in mouse blood vessel. (A-B) DHE staining of aortic tissue sections from the mice exposed to PM2.5 or FA for 10 weeks. Frozen aortic tissue sections were stained with DHE (10 μmol/L). The oxidative red fluorescence was analyzed by a fluorescent microscope. (C) DHE signals were quantified by counting the number of positive stained nuclei in 10 random fields. Microscopic interference contrast was used to exclude positive signals from non-cell origin. The percentages of DHE-positive nuclei (compared to total nuclei) were shown. Data are shown as mean ± SEM for 6 animals per group.
Figure 2.
Expression of the mRNAs encoding the anti-oxidative enzymes SOD-1 and HO-1 was increased in the primary macrophages after PM2.5 treatment. (A-B) Quantitative real-time RT-PCR analysis of the expression of mRNAs encoding SOD-1 and HO-1 in mouse primary macrophages in response to in vitro exposure to PM2.5. Primary macrophages were isolated from wild-type C57BL/6 mice. The primary macrophages were incubated with PM2.5 particles (50 μg/mL), which were collected by the “OASIS-1” system at the time when the mice were exposed, for 2, 4, 6 or 8 hours. The macrophages were treated with the vehicle buffer PBS as the control (CTL). Fold changes of mRNA levels were determined after normalization to internal control β-actin RNA levels.
It has been proposed that several proposed biological pathways are involved in PM-associated adverse cardiovascular outcomes [5, 12, 22, 23]: (1) systemic inflammatory response induced by PM exposure that impacts blood vessels; (2) extra-pulmonary translocation of fine and ultrafine particles that activate vasculature; (3) ROS generated by particles that result in cardiovascular dysfunction; (4) central nerve system manipulation of cardiovascular function following PM exposure. Our study indicated that exposure to PM2.5 indeed caused oxidative stress in blood vessels and in primary macrophages of the mouse model. Furthermore, our results also suggested that the “real-world” PM2.5 exposure with this mouse model is a useful approach for investigating the pathophysiologic impact of PM2.5 on the cardiovascular system. Future studies with this system should provide significant insights into the role and mechanism of PM 25 on air pollution-associated physiology and pathology.
Acknowledgments
This work was partly supported by new faculty research funding from the Wayne State University (to KZ) and K01ES016588 from NIH (to QS). The whole body exposure was performed in facilities at The Ohio State University that was supported by NIH grants R01ES013406 and R01ES015146 (to SR). KZ is a recipient of American Heart Association Scientist Development Award.
References
- 1.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 2.Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 3.Brook RD, Jerrett M, Brook JR, Bard RL, Finkelstein MM. The relationship between diabetes mellitus and traffic-related air pollution. J Occup Environ Med. 2008;50:32–38. doi: 10.1097/JOM.0b013e31815dba70. [DOI] [PubMed] [Google Scholar]
- 4.Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- 5.Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang JS, Zweier JL, Chen LC, Rajagopalan S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vase Biol. 2008;28:1760–1766. doi: 10.1161/ATVBAHA.108.166967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfaro-Moreno E, Martinez L, Garcia-Cuellar C, Bonner JC, Murray JC, Rosas I, Rosales SP, Osornio-Vargas AR. Biologic effects induced in vitro by PM10 from three different zones of Mexico City. Environ Health Perspect. 2002;110:715–720. doi: 10.1289/ehp.02110715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soukup JM, Becker S. Human alveolar macrophage responses to air pollution particulates are associated with insoluble components of coarse material, including particulate endotoxin. Toxicol Appl Pharmacol. 2001;171:20–26. doi: 10.1006/taap.2000.9096. [DOI] [PubMed] [Google Scholar]
- 8.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 9.Nemmar A, Hoylaerts MF, Hoet PH, Dinsdale D, Smith T, Xu H, Vermylen J, Nemery B. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am J Respir Crit Care Med. 2002;166:998–1004. doi: 10.1164/rccm.200110-026OC. [DOI] [PubMed] [Google Scholar]
- 10.Sun Q, Yue P, Kirk Rl, Wang A, Moatti D, Jin X, Lu B, Schecter AD, Lippmann M, Gordon T, Chen LC, Rajagopalan S. Ambient air particulate matter exposure and tissue factor expression in atherosclerosis. Inhal Toxicol. 2008;20:127–137. doi: 10.1080/08958370701821482. [DOI] [PubMed] [Google Scholar]
- 11.Yang W, Omaye ST. Air pollutants, oxidative stress and human health. Mutat Res. 2009;674:45–54. doi: 10.1016/j.mrgentox.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Mejiba SE, Zhai Z, Akram H, Pye QN, Hensley K, Kurien BT, Scofield RH, Ramirez DC. Inhalation of environmental stressors & chronic inflammation: Autoimmunity and neu-rodegeneration. Mutat Res. 2009;674:62–72. doi: 10.1016/j.mrgentox.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient Air Pollution Exaggerates Adipose Inflammation and Insulin Resistance in a Mouse Model of Diet-Induced Obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sioutas C, Koutrakis P, Burton RM. A technique to expose animals to concentrated fine ambient aerosols. Environ Health Perspect. 1995;103:172–177. doi: 10.1289/ehp.95103172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen LC, Nadziejko C. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. V. CAPs exacerbate aortic plaque development in hyperlipidemic mice. Inhal Toxicol. 2005;17:217–224. doi: 10.1080/08958370590912815. [DOI] [PubMed] [Google Scholar]
- 16.Ying Z, Yue P, Xu X, Zhong M, Sun Q, Mikolaj M, Wang A, Brook RD, Chen LC, Rajagopalan S. Air pollution and cardiac remodeling: a role for RhoA/Rho-kinase. Am J Physiol Heart Circ Physiol. 2009;296:H1540–1550. doi: 10.1152/ajpheart.01270.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maciejczyk P, Zhong M, Li Q, Xiong J, Nadziejko C, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. II. The design of a CAPs exposure system for biometric telemetry monitoring. Inhal Toxicol. 2005;17:189–197. doi: 10.1080/08958370590912743. [DOI] [PubMed] [Google Scholar]
- 18.Su Y, Sipin MF, Spencer MT, Qin X, Moffet RC, Shields LG, Prather KA, Venkatachari P, Jeong C, Kim E, Hopke PK, Gelein RM, Utell MJ, Oberdorster G, Berntsen J, Devlin RB, Chen LC. Real-Time Characterization of the Composition of Individual Particles Emitted From Ultrafine Particle Concentrators. Aerosol Science and Technology. 2006;40:19. [Google Scholar]
- 19.Zhang K, Kaufman RJ. Identification and characterization of endoplasmic reticulum stress -induced apoptosis in vivo. Methods Enzymol. 2008;442:395–419. doi: 10.1016/S0076-6879(08)01420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 21. American Lung Association: State of the Air 2009 ( http://www.lungusa2.org/sota/2OO9/S0TA-2009-Full-Print.pdf); 2008 Highlights of Recent Research on Particulate Air Pollution: Effects of Long-Term Exposure ( http://www.lungusa.org/site/cdvLUK900E/b.36864/k.9F49/State_of_the_Air.htm) [Google Scholar]
- 22.Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health ef fects. Lancet. 1995;345:176–178. doi: 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 23.Goto Y, Ishii H, Hogg JC, Shih CH, Yatera K, Vincent R, van Eeden SF. Particulate matter air pollution stimulates monocyte release from the bone marrow. Am J Respir Crit Care Med. 2004;170:891–897. doi: 10.1164/rccm.200402-235OC. [DOI] [PubMed] [Google Scholar]