Abstract
Background:
The high prevalence of airway hyperresponsiveness (AHR) among children with sickle cell anemia (SCA) remains unexplained.
Methods:
To determine the relationship between AHR, features of asthma, and clinical characteristics of SCA, we conducted a multicenter, prospective cohort study of children with SCA. Dose response slope (DRS) was calculated to describe methacholine responsiveness, because 30% of participants did not achieve a 20% decrease in FEV1 after inhalation of the highest methacholine concentration, 25 mg/mL. Multiple linear regression analysis was done to identify independent predictors of DRS.
Results:
Methacholine challenge was performed in 99 children with SCA aged 5.6 to 19.9 years (median, 12.8 years). Fifty-four (55%) children had a provocative concentration of methacholine producing a 20% decrease in FEV1 < 4 mg/mL. In a multivariate analysis, independent associations were found between increased methacholine responsiveness and age (P < .001), IgE (P = .009), and lactate dehydrogenase (LDH) levels (P = .005). There was no association between methacholine responsiveness and a parent report of a doctor diagnosis of asthma (P = .986). Other characteristics of asthma were not associated with methacholine responsiveness, including positive skin tests to aeroallergens, exhaled nitric oxide, peripheral blood eosinophil count, and pulmonary function measures indicating airflow obstruction.
Conclusions:
In children with SCA, AHR to methacholine is prevalent. Younger age, serum IgE concentration, and LDH level, a marker of hemolysis, are associated with AHR. With the exception of serum IgE, no signs or symptoms of an allergic diathesis are associated with AHR. Although the relationship between methacholine responsiveness and LDH suggests that factors related to SCA may contribute to AHR, these results will need to be validated in future studies.
Airway hyperresponsiveness (AHR) is believed to reflect inflammation in the airways that is relatively specific for asthma.1 The diagnostic evaluation for asthma includes measures of AHR, most commonly using methacholine provocation. In children with sickle cell disease (SCD), AHR is present in up to 78% of those tested,2,3 whereas the prevalence of asthma in children with SCD is 20% to 48%.4-6 Given that AHR is more prevalent than asthma in children with SCD, mechanisms other than asthma-related inflammation may be causing this high rate of AHR.
Among children with HbSS or sickle cell anemia (SCA), a doctor diagnosis of asthma has been associated with an increased rate of pain, acute chest syndrome, and death.4,5,7,8 Although these data imply that asthma has a significant disease-modifying effect in children with SCA, characteristics of SCA, such as episodes of acute chest syndrome,9 pulmonary function abnormalities,10-12 and chronic respiratory symptoms, add complexity to the diagnosis of asthma. Thus, it is not clear if descriptions of asthma in these children reflect the presence of two distinct comorbid conditions or an asthma-like phenotype secondary to SCA. Determining the cause of AHR in children with SCA may provide insight into the pathogenesis of asthma descriptions in this patient population. In this study, we propose to test whether among children with SCA hyperresponsiveness to methacholine is associated with an allergic diathesis, similar to children without SCA.13-15
Materials and Methods
Study Design
Children with SCA who were 4 to 20 years of age were identified at three clinical centers and enrolled into the Sleep and Asthma Cohort Study (SAC). Children were enrolled without regard to past morbidity or doctor diagnosis of asthma. Children receiving long-term transfusion or participation in a clinical trial evaluating hydroxyurea therapy were excluded. SAC is a National Heart, Lung, and Blood Institute-funded prospective, observational cohort study designed to evaluate the contribution of asthma and sleep abnormalities to SCA-related morbidity. Institutional approval was obtained from participating sites in St. Louis, Missouri; Cleveland, Ohio; and London, England. Informed written parental consent was obtained, and children were consented or assented according to institutional policies on enrollment.
As part of this study, participants completed a questionnaire on respiratory tract symptoms and underwent the following tests: spirometry before and after bronchodilator, methacholine airway challenge, exhaled nitric oxide (eNO), total serum IgE concentrations, allergy skin testing, and peripheral blood eosinophil count. Pulmonary function testing, methacholine airway challenge, eNO, and allergy skin testing were performed by SAC-certified technicians, and procedures were adopted with permission from methods used in the Childhood Asthma Research and Education Network16 (see e-Appendix 1 for details on testing procedures, including testing of methacholine concentrations at each of the three centers). Response to the questionnaires, bronchodilator responsiveness, skin testing, eNO, IgE concentrations, and eosinophil count were assessed at visit 1. Methacholine airway challenge was performed at visit 2, 6 months after visit 1. Methacholine airway challenge was performed when the child was well.
Special Considerations for Performing Methacholine Challenges in Children with SCA
The tests were not performed if (1) resting daytime oxygen saturation was < 85% on room air; (2) the child had a temperature ≥ 37.9°C; (3) there was a new finding on physical examination, such as chest crackles; or (4) the child reported pain requiring opioid therapy in the last 48 h or an increased dose of opioid therapy if the child was taking these medicines long-term. Tests were performed at the discretion of the supervising physician if a child reported symptoms of asthma (eg, wheezing, coughing, or chest symptoms) or if the baseline FEV1 was < 80% of FEV1 obtained from visit 1. Oxygen saturation was monitored continuously throughout the procedure. A nasal cannula was placed on participants and supplemental oxygen was given if oxygen saturation was < 92%.
Questionnaire
Participants and parents were administered questions pertaining to chest symptoms from the American Thoracic Society/Division of Lung Diseases (ATS/DLD) questionnaire.17 Questions about wheezing were read to parents as presented in the ATS/DLD questionnaire, without additional instruction about the definition of wheezing.
Asthma was defined as an answer of “yes” to any of the following three questions at the time of study consent: “Has a doctor ever said that the participant has asthma?” “Does the participant take any asthma medications?” “Does the participant still have asthma?”
Overreading of Spirometry, Methacholine Challenge Results, eNO, and Allergy Skin Testing
To ensure ATS criteria were met across the three participating sites for spirometry and methacholine challenge, test results were overread using the criteria detailed in e-Appendix 1. Similarly, allergy skin tests were remeasured by a single investigator to ensure that test results were valid. Any invalid tests were excluded from analyses.
Statistical Analysis
To describe the methacholine responsiveness of all study participants, including those with a negative provocative concentration of methacholine producing a 20% decrease in FEV1 (PC20) (ie, those who did not have a 20% decrease in FEV1 after inhalation of the highest methacholine concentration, 25 mg/mL), dose response slopes (DRSs) were calculated.18 The numerator of the slope was the percent decrease in FEV1 from the postsaline value to the value after the last concentration of methacholine used in the challenge. The denominator was the cumulative dose of methacholine calculated from all the doses used in the challenge. Each dose was calculated from the concentration (mg/mL) multiplied by time (2 min of tidal breathing) multiplied by the delivery rate (0.13 mL/min). For example, if a 20% decrease in FEV1 was obtained after a concentration of 0.78 mg/mL, the cumulative dose would be 0.381 mg and the DRS was 52.5 (20/0.381); however, if a 20% decrease in FEV1 had not been obtained at 25 mg/mL, then the cumulative dose would be 12.675 mg, and if at this stage the participant only had a 9% change in FEV1, the DRS would be 0.7. The slope for each study participant was then ranked (highest DRS corresponding to the lowest PC20 was given the highest rank), and the ranked values were used for all analyses.
Spearman rank correlation was used to determine the association between methacholine responsiveness (ranked DRS) and continuous variables. Student t test was used to examine the relationship between yes or no responses to the ATS/DLD questionnaire and DRS. To determine the independent association of clinical features in children with SCA and DRS, variables with an association in univariate analyses of at least P < .10 were entered into a multiple linear regression model where DRS was the dependent variable. Age, lactate dehydrogenase (LDH) level, hemoglobin level, IgE concentration, FEV1 % predicted, % change in FEV1 after administration of bronchodilator, FEV1/FVC % predicted, eNO, eosinophil count, number of positive allergy skin tests, and WBC count were tested in a univariate model with a Spearman rank correlation to determine the relationship with DRS. LDH values were ranked for all analyses. Data analysis was performed in SAS version 9.1 (SAS Institute; Cary, North Carolina).
Results
Demographics and Baseline Asthma Characteristics
Our cohort consisted of 99 children with SCA who underwent successful methacholine airway challenge (Table 1).19 The mean age of the cohort was 13 years and 52% were girls. Inhaled corticosteroids were used by 22% of children on entry into the study, intranasal steroids were used by 12%, and antihistamines were used by 4%. Hydroxyurea was used by 22% of the study cohort. Skin test reactivity to at least one aeroallergen was found in 46% of children, and of the aeroallergens tested, the most prevalent positive test was grass (24%). The geometric mean of IgE concentration was 47 U, and the geometric mean of eNO was 10 parts per billion. A positive bronchodilator response (increase in FEV1 ≥ 12%) was observed in 15% of participants.
Table 1.
—Relationship of Age, Laboratory Data, Allergy Skin Tests, eNO, Baseline Spirometry, and Bronchodilator Responsiveness to the DRS of the PC20 in Children With SCA (n = 99)
| Characteristic | Median of Outcome | Range | r | P Value |
| Age, y (n = 99) | 12.8 | 5.6, 19.9 | −0.512 | < .001 |
| LDH, IU/L (n = 90) | 392.5 | 14, 1221 | 0.260 | .013 |
| Hemoglobin, g/dL (n = 97) | 8.1 | 6.0, 12.0 | −0.137 | .180 |
| IgE, kU/I (n = 95) | 32.0 | 2, 1870 | 0.222 | .031 |
| FEV1, % predicteda (n = 99) | 88.1 | 55.7, 122.6 | −0.085 | .413 |
| Bronchodilator response, % change FEV1 (n = 98) | 5.8 | −4.0, 26.2 | 0.129 | .211 |
| FEV1/FVC, % predicteda (n = 99) | 96.0 | 73.6, 109.8 | −0.045 | .664 |
| eNO, ppb (n = 92) | 9.9 | 3.0, 68.0 | −0.101 | .338 |
| Eosinophil, % (n = 97) | 3.0 | 0, 14 | −0.042 | .683 |
| Number of positive skin tests (n = 96) | 0.0 | 0, 8 | −0.051 | .621 |
| WBC, k/mm3 (n = 98) | 11.3 | 1.9, 70.6 | 0.041 | .690 |
DRS = dose response slope; eNO = exhaled nitric oxide; LDH = lactate dehydrogenase; PC20 = provocative concentration of methacholine producing a 20% decrease in FEV1; ppb = parts per billion; SCA = sickle cell anemia.
Reference values for pulmonary function based on equations from Wang et al.19
Safety of Methacholine Airway Challenge
Ninety-eight methacholine airway challenges were performed without a serious adverse event. However, the 99th child tested had a pain episode that was temporally related to a methacholine challenge. He was not hypoxic during the procedure, but he reported chest tightness that resolved with albuterol. The following morning the chest tightness recurred and did not resolve with albuterol. Three days later he was admitted to the hospital for a pain episode.20 Following this serious adverse event, no further methacholine challenges were performed per recommendations of the data safety monitoring board.
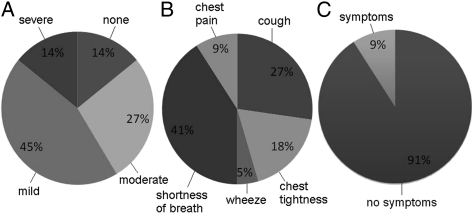
Children were asked to grade the severity of their symptoms (mild, moderate, or severe) at the point of the highest methacholine concentration administered. Respiratory symptoms were common in our cohort during methacholine airway challenge and most symptoms were mild (Fig 1). Few children reported severe respiratory symptoms during testing. Of those who did report severe symptoms, shortness of breath was the most common complaint. Symptoms persisted in only 9% of children after being treated with albuterol and were all rated as mild.
Figure 1.
A, Severity of symptoms (none, mild, moderate, severe) during methacholine airway challenge in children with sickle cell anemia. B, Breakdown of severe symptoms during methacholine airway challenge. C, Percentage of children with persistent symptoms and no symptoms after reversal with albuterol. Notably, all of the persistent symptoms were mild.
Results of Methacholine Airway Challenge
Methacholine responsiveness occurred at a PC20 ≤ 2 mg/mL in 42% of participants, at > 2 to 4 mg/mL in 12% of participants, at > 4 to 8 mg/mL in 6% of participants, at > 8 to 25 mg/mL in 9% of participants, and was not reached at 25 mg/mL in 30% of participants. The DRS (% decline in FEV1/cumulative dose in mg) for methacholine responsiveness ranged from 535 to 0.01. Responsiveness was associated with age at testing (with younger children more responsive, r = 0.512, P < .001), but was not affected by hydroxyurea therapy (P = .411), inhaled corticosteroids (P = .488), intranasal corticosteroids (P = .391), antihistamines (P = .584), or sex (P = .361).
Univariate Correlations of Methacholine Responsiveness and Clinical Characteristics
Among children with SCA, methacholine responsiveness was associated with few features of asthma (Table 1). Although methacholine responsiveness was correlated with a higher serum IgE concentration, there was no relationship between responsiveness and FEV1 % predicted, FEV1/FVC % predicted, bronchodilator reactivity, eNO, allergy skin tests, or eosinophil count. However, increased methacholine responsiveness was correlated to higher LDH (P = .013), a measure of hemolysis.
Relationship of Methacholine Responsiveness to Respiratory Symptoms and Doctor Diagnosis of Asthma
Symptoms in the ATS/DLD questionnaire were largely unrelated to methacholine responsiveness in children with SCA (Table 2). Methacholine responsiveness was related to cough in the absence of a cold (P = .002). However, wheezing in the absence of a cold and wheezing associated with exercise, symptoms generally believed to be related to presence of asthma in children,21 were not associated with methacholine responsiveness.
Table 2.
—Relationship of American Thoracic Society/Division of Lung Diseases Asthma Questions to the DRS of PC20 in Children With SCA
| Symptom | % With Event | P Value |
| Does the participant usually have a cough with colds? | 75 | .473 |
| Does he/she usually have a cough without having a cold? | 30 | .002 |
| Does the participant usually seem congested in the chest or bring up phlegm with colds? | 63 | .585 |
| Does the participant usually seem congested in the chest or bring up phlegm without having a cold? | 16 | .184 |
| Does the participant’s chest ever sound wheezy or whistling when he/she has a cold? | 51 | .903 |
| Does the participant’s chest ever sound wheezy or whistling occasionally even without having a cold? | 19 | .181 |
| Has the participant ever had an attack of wheezing that has caused him/her to be short of breath? | 26 | .903 |
| Has the participant ever had attacks of wheezing after playing hard or exercising? | 41 | .172 |
See Table 1 for expansion of abbreviations.
There was no relationship between a doctor diagnosis of asthma and methacholine responsiveness (P = .986). Sixty-seven percent of children with PC20 values ≤ 2 mg/mL did not have a diagnosis of asthma. Conversely, 45% of children with a PC20 > 25 mg/mL did have a doctor diagnosis of asthma.
LDH, Serum IgE, and Age Are Independent Predictors of Methacholine Responsiveness
When significant univariate predictors of methacholine responsiveness were entered into a multivariate model, increased LDH and serum IgE concentrations predicted methacholine responsiveness independently of age, which was also associated (Table 3). Notably, age was not associated with either hydroxyurea (P = .813) or inhaled corticosteroid use (P = .856).
Table 3.
—Multivariate Regression Model Describing the Relationship Between Participant Characteristics and PC20, Including Univariate Predictors of the PC20 With a P Value < .10
| Characteristic | β | 95% CI | Significance |
| Age, y | 4.590 | −5.797, −3.384 | < .001 |
| LDH, IU/L | −0.257 | 0.082, 0.432 | .005 |
| IgE, kU/I | −0.021 | 0.005, 0.038 | .009 |
See Table 1 for expansion of abbreviations.
Discussion
AHR is a cardinal feature of asthma in children without SCD.13 In a cohort of children with SCA who underwent methacholine airway challenge, a diagnosis of asthma and most objective signs and symptoms of asthma were not associated with AHR. Only younger age, higher serum IgE concentration, and higher LDH level were independently associated with AHR.
Few characteristics of asthma were associated with methacholine responsiveness in our cohort of children with SCA. Among children with well-characterized asthma without SCD, there are strong relationships between AHR and FEV1, FEV1/FVC, IgE, eosinophilia, eNO, and lower respiratory tract symptoms.13,22-24 Moreover, an elevated eosinophil count and serum IgE concentration are strongly associated with AHR regardless of an asthma diagnosis.22,25 In our study participants with SCA, elevated serum IgE concentration was the only feature of asthma that was an independent predictor of AHR. Because few allergic features or symptoms of asthma are related to AHR in our study, testing for AHR as part of an evaluation for respiratory symptoms is unlikely to discriminate between children with SCA with and without asthma.
LDH is a marker of hemolysis that is highly correlated with nitric oxide (NO) consumption in patients with SCD.26 In contrast to the lack of association between AHR and asthma characteristics, LDH was associated with methacholine responsiveness, providing preliminary evidence that hemolysis contributes to AHR in SCA. Although our data are consistent with the established role for dysregulation of NO homeostasis in the mechanism of AHR in asthma,27-30 further studies are necessary to examine the relationship between AHR, hemolysis, and low NO availability in patients with SCD.
Younger age was associated with AHR in the current study of children with SCA. Few investigators have examined the relationship between age and airway responsiveness in children without SCD. Le Souëf et al31 reported that responsiveness to methacholine was lower in both boys and girls in the upper quartile for height than in those in the lowest quartile independent of age from 9 to 15 years. Ownby et al32 studied children 6 to 8 years of age and found no significant association between methacholine responsiveness and either height or age. Analysis of the relationship between age and methacholine responsiveness among children 5 to 12 years of age with mild to moderate asthma enrolled in the Childhood Asthma Management Program Study found no relationship between age and responsiveness (unpublished data presented in e-Table 1). Taken together, these data show that the association between age and airway responsiveness present in our cohort of children with SCA has not been found in the general pediatric population.
Few children in our study had severe symptoms during a methacholine airway challenge. In a study of 1,041 children with asthma in the general population, 7% of children reported severe symptoms associated with a methacholine challenge.33 The most common severe symptom in this large cohort of children with asthma was chest tightness. Our study of children with SCA shows a similar distribution of symptom severity when compared with children with asthma but without SCA. Although the majority of children with SCA had either no symptoms or mild symptoms during a methacholine challenge, the child who had a pain episode after undergoing testing demonstrates that there may be risks associated with performing a methacholine airway challenge in children with SCA.20
There are limitations present in our study. Our cohort is a convenience sample of children with SCA. They were selected from children attending clinics at three centers and they do not represent all children with SCA. However, we believe that our broad entry criteria and multi-institution study design achieved a nonbiased, representative sample. Another limitation is that some participants were treated with medications, such as inhaled corticosteroids or hydroxyurea, which may have lessened their signs and symptoms of asthma. However, there was no association between use of inhaled corticosteroids or hydroxyurea and methacholine responsiveness.
In summary, AHR is prevalent in children with SCA, but related to few characteristics of asthma or other atopic disorders. Higher LDH, a marker of hemolysis, is associated with AHR, suggesting that low NO bioavailability may contribute to AHR in children with SCA. Based on these results, assessing airway responsiveness may have a limited role in the diagnostic evaluation for asthma in children with SCA. Future studies are needed to examine the role of hemolysis and NO bioavailability in children with SCA who develop symptoms consistent with asthma.
Supplementary Material
Acknowledgments
Author contributions: Dr Field: contributed to analyzing data and writing the article.
Dr Stocks: contributed to designing the study, collecting data, and writing the article.
Dr Kirkham: contributed to designing the study, collecting data, and editing the article.
Dr Rosen: contributed to collecting data and editing the article.
Dr Dietzen: contributed to analyzing data and editing the article.
Ms Semon: contributed to collecting data and editing the article.
Ms Kirkby: contributed to collecting data and editing the article.
Ms Bates: contributed to collecting data and editing the article.
Dr Seicean: contributed to collecting data and editing the article.
Dr DeBaun: contributed to designing the study and writing the article.
Dr Redline: contributed to designing the study and editing the article.
Dr Strunk: contributed to designing the study, analyzing data, and writing the article.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank the SAC investigative teams in St. Louis, Cleveland, and London; Allan Doctor, MD, for his critical reading of the manuscript; and Mark Van Natta, PhD, and the Childhood Asthma Management Program Research Group for sharing the data on relationship between age and methacholine responsiveness.
Additional information: The e-Appendix and e-Table can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/139/3/563/suppl/DC1.
Abbreviations
- AHR
airway hyperresponsiveness
- ATS/DLD
American Thoracic Society/Division of Lung Diseases
- DRS
dose response slope
- eNO
exhaled nitric oxide
- LDH
lactate dehydrogenase
- NO
nitric oxide
- PC20
provocative concentration of methacholine producing a 20% decrease in FEV1
- SAC
Sleep and Asthma Cohort Study
- SCA
sickle cell anemia
- SCD
sickle cell disease
Footnotes
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
Funding/Support: This study was supported by the National Heart, Lung, and Blood Institute [Grants R01 HL079937 to Drs Stocks, Kirkham, Rosen, DeBaun, Redline, and Strunk and K12 HL08710 to Dr Field].
References
- 1.Liem JJ, Kozyrskyj AL, Cockroft DW, Becker AB. Diagnosing asthma in children: what is the role for methacholine bronchoprovocation testing? Pediatr Pulmonol. 2008;43(5):481–489. doi: 10.1002/ppul.20801. [DOI] [PubMed] [Google Scholar]
- 2.Ozbek OY, Malbora B, Sen N, Yazici AC, Ozyurek E, Ozbek N. Airway hyperreactivity detected by methacholine challenge in children with sickle cell disease. Pediatr Pulmonol. 2007;42(12):1187–1192. doi: 10.1002/ppul.20716. [DOI] [PubMed] [Google Scholar]
- 3.Leong MA, Dampier C, Varlotta L, Allen JL. Airway hyperreactivity in children with sickle cell disease. J Pediatr. 1997;131(2):278–283. doi: 10.1016/s0022-3476(97)70166-5. [DOI] [PubMed] [Google Scholar]
- 4.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108(9):2923–2927. doi: 10.1182/blood-2006-01-011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd JH, Moinuddin A, Strunk RC, DeBaun MR. Asthma and acute chest in sickle-cell disease. Pediatr Pulmonol. 2004;38(3):229–232. doi: 10.1002/ppul.20066. [DOI] [PubMed] [Google Scholar]
- 6.Knight-Madden JM, Forrester TS, Lewis NA, Greenough A. Asthma in children with sickle cell disease and its association with acute chest syndrome. Thorax. 2005;60(3):206–210. doi: 10.1136/thx.2004.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glassberg J, Spivey JF, Strunk R, Boslaugh S, DeBaun MR. Painful episodes in children with sickle cell disease and asthma are temporally associated with respiratory symptoms. J Pediatr Hematol Oncol. 2006;28(8):481–485. doi: 10.1097/01.mph.0000212968.98501.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with increased mortality in individuals with sickle cell anemia. Haematologica. 2007;92(8):1115–1118. doi: 10.3324/haematol.11213. [DOI] [PubMed] [Google Scholar]
- 9.Castro O, Brambilla DJ, Thorington B, et al. The Cooperative Study of Sickle Cell Disease The acute chest syndrome in sickle cell disease: incidence and risk factors. Blood. 1994;84(2):643–649. [PubMed] [Google Scholar]
- 10.Koumbourlis AC, Zar HJ, Hurlet-Jensen A, Goldberg MR. Prevalence and reversibility of lower airway obstruction in children with sickle cell disease. J Pediatr. 2001;138(2):188–192. doi: 10.1067/mpd.2001.111824. [DOI] [PubMed] [Google Scholar]
- 11.Pianosi P, D’Souza SJ, Charge TD, Esseltine DE, Coates AL. Pulmonary function abnormalities in childhood sickle cell disease. J Pediatr. 1993;122(3):366–371. doi: 10.1016/s0022-3476(05)83418-3. [DOI] [PubMed] [Google Scholar]
- 12.Field JJ, DeBaun MR, Yan Y, Strunk RC. Growth of lung function in children with sickle cell anemia. Pediatr Pulmonol. 2008;43(11):1061–1066. doi: 10.1002/ppul.20883. [DOI] [PubMed] [Google Scholar]
- 13.Weiss ST, Van Natta ML, Zeiger RS. Relationship between increased airway responsiveness and asthma severity in the childhood asthma management program. Am J Respir Crit Care Med. 2000;162(1):50–56. doi: 10.1164/ajrccm.162.1.9811005. [DOI] [PubMed] [Google Scholar]
- 14.Sunyer J, Muñoz A. Concentrations of methacholine for bronchial responsiveness according to symptoms, smoking and immunoglobulin E in a population-based study in Spain. Spanish Group of the European Asthma Study. Am J Respir Crit Care Med. 1996;153(4 Pt 1):1273–1279. doi: 10.1164/ajrccm.153.4.8616553. [DOI] [PubMed] [Google Scholar]
- 15.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320(5):271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 16.Strunk RC, Szefler SJ, Phillips BR, et al. Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol. 2003;112(5):883–892. doi: 10.1016/j.jaci.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118(6 pt 2):1–120. [PubMed] [Google Scholar]
- 18.O’Connor G, Sparrow D, Taylor D, Segal M, Weiss S. Analysis of dose-response curves to methacholine. An approach suitable for population studies. Am Rev Respir Dis. 1987;136(6):1412–1417. doi: 10.1164/ajrccm/136.6.1412. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15(2):75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 20.Knight-Perry JE, Field JJ, Debaun MR, Stocks J, Kirkby J, Strunk RC. Hospital admission for acute painful episode following methacholine challenge in an adolescent with sickle cell disease. Pediatr Pulmonol. 2009;44(7):728–730. doi: 10.1002/ppul.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gergen PJ, Mullally DI, Evans R., III National survey of prevalence of asthma among children in the United States, 1976 to 1980. Pediatrics. 1988;81(1):1–7. [PubMed] [Google Scholar]
- 22.Jansen DF, Rijcken B, Schouten JP, et al. The relationship of skin test positivity, high serum total IgE levels, and peripheral blood eosinophilia to symptomatic and asymptomatic airway hyperresponsiveness. Am J Respir Crit Care Med. 1999;159(3):924–931. doi: 10.1164/ajrccm.159.3.9804024. [DOI] [PubMed] [Google Scholar]
- 23.Motomura C, Odajima H, Tezuka J, et al. Effect of age on relationship between exhaled nitric oxide and airway hyperresponsiveness in asthmatic children. Chest. 2009;136(2):519–525. doi: 10.1378/chest.08-2741. [DOI] [PubMed] [Google Scholar]
- 24.Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998;53(2):91–95. doi: 10.1136/thx.53.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325(15):1067–1071. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 26.Kato GJ, McGowan V, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107(6):2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vercelli D. Arginase: marker, effector, or candidate gene for asthma? J Clin Invest. 2003;111(12):1815–1817. doi: 10.1172/JCI18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmermann N, King NE, Laporte J, et al. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest. 2003;111(12):1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer A, Folkerts G, Geppetti P, Groneberg DA. Mediators of asthma: nitric oxide. Pulm Pharmacol Ther. 2002;15(2):73–81. doi: 10.1006/pupt.2001.0332. [DOI] [PubMed] [Google Scholar]
- 30.Maarsingh H, Zaagsma J, Meurs H. Arginine homeostasis in allergic asthma. Eur J Pharmacol. 2008;585(2-3):375–384. doi: 10.1016/j.ejphar.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 31.Le Souëf PN, Sears MR, Sherrill D. The effect of size and age of subject on airway responsiveness in children. Am J Respir Crit Care Med. 1995;152(2):576–579. doi: 10.1164/ajrccm.152.2.7633710. [DOI] [PubMed] [Google Scholar]
- 32.Ownby DR, Peterson EL, Johnson CC. Factors related to methacholine airway responsiveness in children. Am J Respir Crit Care Med. 2000;161(5):1578–1583. doi: 10.1164/ajrccm.161.5.9812156. [DOI] [PubMed] [Google Scholar]
- 33.Covar RA, Colvin R, Shapiro G, Strunk R. Safety of methacholine challenges in a multicenter pediatric asthma study. J Allergy Clin Immunol. 2006;117(3):709–711. doi: 10.1016/j.jaci.2006.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.