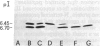Abstract
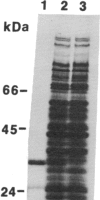
Crude extracts of a multiply peptidase-deficient strain of Salmonella typhimurium contain an aminopeptidase that specifically removes N-terminal methionine from peptides. This activity shows pronounced specificity for the peptide's second amino acid. Methionine is removed from peptides with alanine, threonine, or glycine in this position but not when the second amino acid is leucine or methionine. The activity is stimulated by Co2+ and is inhibited by EDTA. Mutations that lead to overproduction (up to 30-fold) of the activity have been obtained by selecting for growth on Met-Gly-Gly as a methionine source. These mutations map at approximately 3 map units, phage P22 cotransducible with leu. The overproducer mutations are dominant to wild type, and duplication of the wild-type allele of the locus leads to a gene dosage effect on peptidase levels. This suggests that the locus of the overproducer mutations may be the structural gene for the peptidase. NaDodSO4/PAGE shows an increased level of a single protein (34 kDa) in the overproducer mutant. This protein is highly enriched in a purified preparation of the peptidase. The specificity of this enzyme suggests that it is involved in the cleavage of methionine from newly synthesized peptide chains. This activity can specifically remove methionine from the N terminus of a completed protein. Treatment of purified, unprocessed (N-terminal methionine) interleukin 1 beta with the purified peptidase results in removal of N-terminal methionine with no additional alterations. N-terminal processing of at least this protein can occur after translation is complete. We propose to call this enzyme peptidase M (methionine-specific aminopeptidase).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M. On the release of the formyl group from nascent protein. J Mol Biol. 1968 May 14;33(3):571–589. doi: 10.1016/0022-2836(68)90307-0. [DOI] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Gene duplication in bacteria: alteration of gene dosage by sister-chromosome exchanges. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1083–1087. doi: 10.1101/sqb.1979.043.01.120. [DOI] [PubMed] [Google Scholar]
- Ben-Bassat A., Bauer K., Chang S. Y., Myambo K., Boosman A., Chang S. Processing of the initiation methionine from proteins: properties of the Escherichia coli methionine aminopeptidase and its gene structure. J Bacteriol. 1987 Feb;169(2):751–757. doi: 10.1128/jb.169.2.751-757.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. L. Purification and properties of dipeptidase M from Escherichia coli B. J Biol Chem. 1973 Jan 25;248(2):409–416. [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Davis M. A., Roberts D. E., Takeshita K., Kleckner N. Genetic organization of transposon Tn10. Cell. 1981 Jan;23(1):201–213. doi: 10.1016/0092-8674(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller C. G., Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):355–363. doi: 10.1128/jb.120.1.355-363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G. Peptidases and proteases of Escherichia coli and Salmonella typhimurium. Annu Rev Microbiol. 1975;29:485–504. doi: 10.1146/annurev.mi.29.100175.002413. [DOI] [PubMed] [Google Scholar]
- Miller C. G., Schwartz G. Peptidase-deficient mutants of Escherichia coli. J Bacteriol. 1978 Aug;135(2):603–611. doi: 10.1128/jb.135.2.603-611.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarimo S. S., Pine M. J. Taxonomic comparison of the amino termini of microbial cell proteins. J Bacteriol. 1969 May;98(2):368–374. doi: 10.1128/jb.98.2.368-374.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Carter T. H., Miller C. G. Overproduction of Salmonella typhimurium peptidase T. J Bacteriol. 1983 Nov;156(2):743–751. doi: 10.1128/jb.156.2.743-751.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Lenk J. B., Gamble B. L., Miller C. G. Oxygen regulation in Salmonella typhimurium. J Bacteriol. 1985 Feb;161(2):673–680. doi: 10.1128/jb.161.2.673-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Miller C. G. Isolation and characterization Salmonella typhimurium mutants lacking a tripeptidase (peptidase T). J Bacteriol. 1983 May;154(2):763–771. doi: 10.1128/jb.154.2.763-771.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. S., Matheson A. T. Purification and properties of a ribosomal peptidase from Escherichia coli B. Can J Biochem. 1965 Oct;43(10):1643–1652. doi: 10.1139/o65-181. [DOI] [PubMed] [Google Scholar]
- Tsunasawa S., Stewart J. W., Sherman F. Amino-terminal processing of mutant forms of yeast iso-1-cytochrome c. The specificities of methionine aminopeptidase and acetyltransferase. J Biol Chem. 1985 May 10;260(9):5382–5391. [PubMed] [Google Scholar]
- Vogt V. M. Purification and properties of an aminopeptidase from Escherichia coli. J Biol Chem. 1970 Sep 25;245(18):4760–4769. [PubMed] [Google Scholar]
- WALLER J. P. THE NH2-TERMINAL RESIDUES OF THE PROTEINS FROM CELL-FREE EXTRACTS OF E. COLI. J Mol Biol. 1963 Nov;7:483–496. doi: 10.1016/s0022-2836(63)80096-0. [DOI] [PubMed] [Google Scholar]
- Wingfield P. T., Graber P., Rose K., Simona M. G., Hughes G. J. Chromatofocusing of N-terminally processed forms of proteins. Isolation and characterization of two forms of interleukin-1 beta and of bovine growth hormone. J Chromatogr. 1987 Jan 30;387:291–300. doi: 10.1016/s0021-9673(01)94532-7. [DOI] [PubMed] [Google Scholar]
- Wingfield P., Payton M., Tavernier J., Barnes M., Shaw A., Rose K., Simona M. G., Demczuk S., Williamson K., Dayer J. M. Purification and characterization of human interleukin-1 beta expressed in recombinant Escherichia coli. Eur J Biochem. 1986 Nov 3;160(3):491–497. doi: 10.1111/j.1432-1033.1986.tb10066.x. [DOI] [PubMed] [Google Scholar]