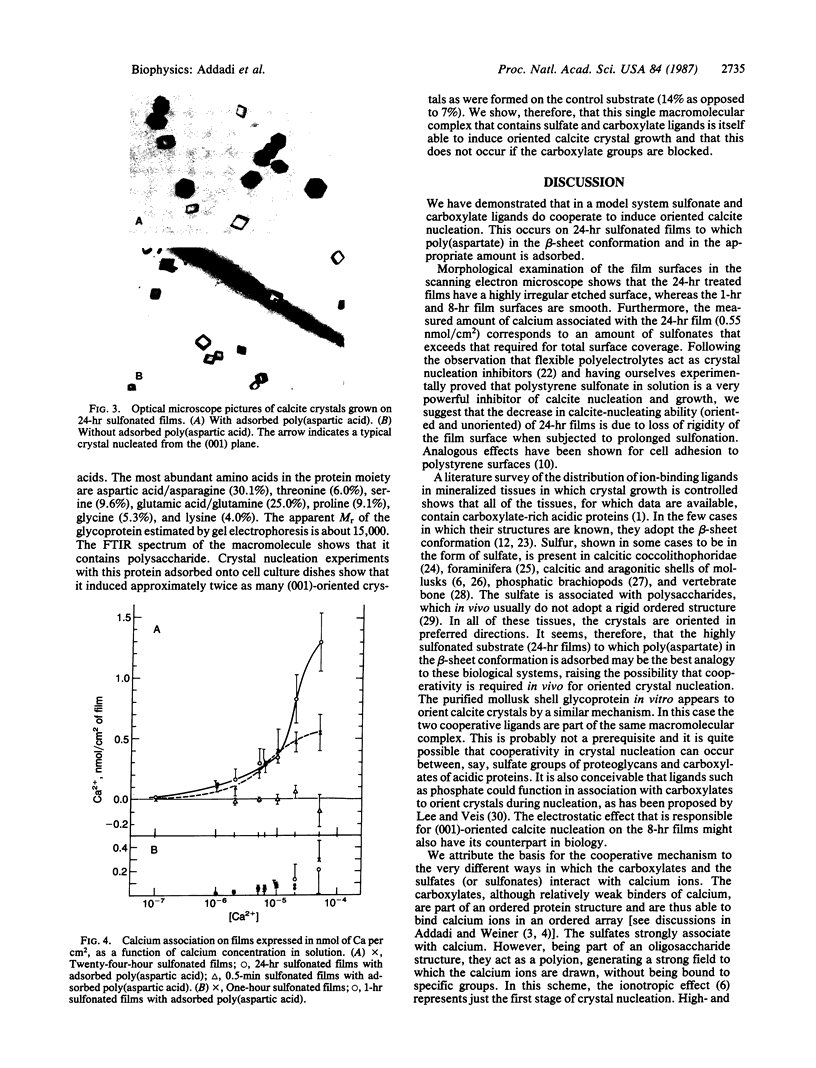
Abstract
Acidic matrix macromolecules involved in regulation of biological crystal growth often contain aspartic acid-rich domains and covalently bound sulfated polysaccharides. We propose that sulfates and β-sheet structured carboxylates cooperate in oriented calcite crystal nucleation. The sulfates concentrate calcium, creating the supersaturation necessary for nucleation on the structured carboxylate domains. An artificial model, composed of sulfonated polystyrene surfaces and adsorbed β-sheet poly(aspartate), demonstrates that the two components indeed act cooperatively with respect to two independent assays, both by induction of calcite nucleation off the (001) plane and by calcium association. Evidence is presented that a purified organic matrix acidic glycoprotein from mollusk shells may behave in vitro in a similar way.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addadi L., Weiner S. Interactions between acidic proteins and crystals: stereochemical requirements in biomineralization. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4110–4114. doi: 10.1073/pnas.82.12.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Boskey A. L. Mechanisms of proteoglycan inhibition of hydroxyapatite growth. Calcif Tissue Int. 1985 Jul;37(4):395–400. doi: 10.1007/BF02553709. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Dejter S. W., Jr, Whitson S. W., Yanagishita M., Kimura J. H., Hascall V. C., Kleinman H. K., Hassell J. R., Nilsson B. Proteoglycans of developing bone. J Biol Chem. 1983 May 25;258(10):6588–6594. [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Veis A. Cooperativity in calcium ion binding to repetitive, carboxylate-serylphosphate polypeptides and the relationship of this property to dentin mineralization. Int J Pept Protein Res. 1980 Sep;16(3):231–240. doi: 10.1111/j.1399-3011.1980.tb02957.x. [DOI] [PubMed] [Google Scholar]
- Maroudas N. G. Sulphonated polystyrene as an optimal substratum for the adhesion and spreading of mesenchymal cells in monovalent and divalent saline solutions. J Cell Physiol. 1977 Mar;90(3):511–519. doi: 10.1002/jcp.1040900314. [DOI] [PubMed] [Google Scholar]
- Nagasawa K., Inoue Y., Kamata T. Solvolytic desulfation of glycosaminoglycuronan sulfates with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1977 Sep;58(1):47–55. doi: 10.1016/s0008-6215(00)83402-3. [DOI] [PubMed] [Google Scholar]
- Simkiss K. The organic matrix of the oyster shell. Comp Biochem Physiol. 1965 Dec;16(4):427–435. doi: 10.1016/0010-406x(65)90307-5. [DOI] [PubMed] [Google Scholar]
- Thiele H., Awad A. Nucleation and oriented crystallization apatite in ionotropic gels. J Biomed Mater Res. 1969 Sep;3(3):431–441. doi: 10.1002/jbm.820030305. [DOI] [PubMed] [Google Scholar]
- Torchia D. A., Hasson M. A., Hascall V. C. Investigation of molecular motion of proteoglycans in cartilage by 13C magnetic resonance. J Biol Chem. 1977 Jun 10;252(11):3617–3625. [PubMed] [Google Scholar]
- Tsay T. G., Veis A. Preparation, detection, and characterization of an antibody to rat alpha-phosphophoryn. Biochemistry. 1985 Nov 5;24(23):6363–6369. doi: 10.1021/bi00344a007. [DOI] [PubMed] [Google Scholar]
- Weiner S. Organization of extracellularly mineralized tissues: a comparative study of biological crystal growth. CRC Crit Rev Biochem. 1986;20(4):365–408. doi: 10.3109/10409238609081998. [DOI] [PubMed] [Google Scholar]