Abstract
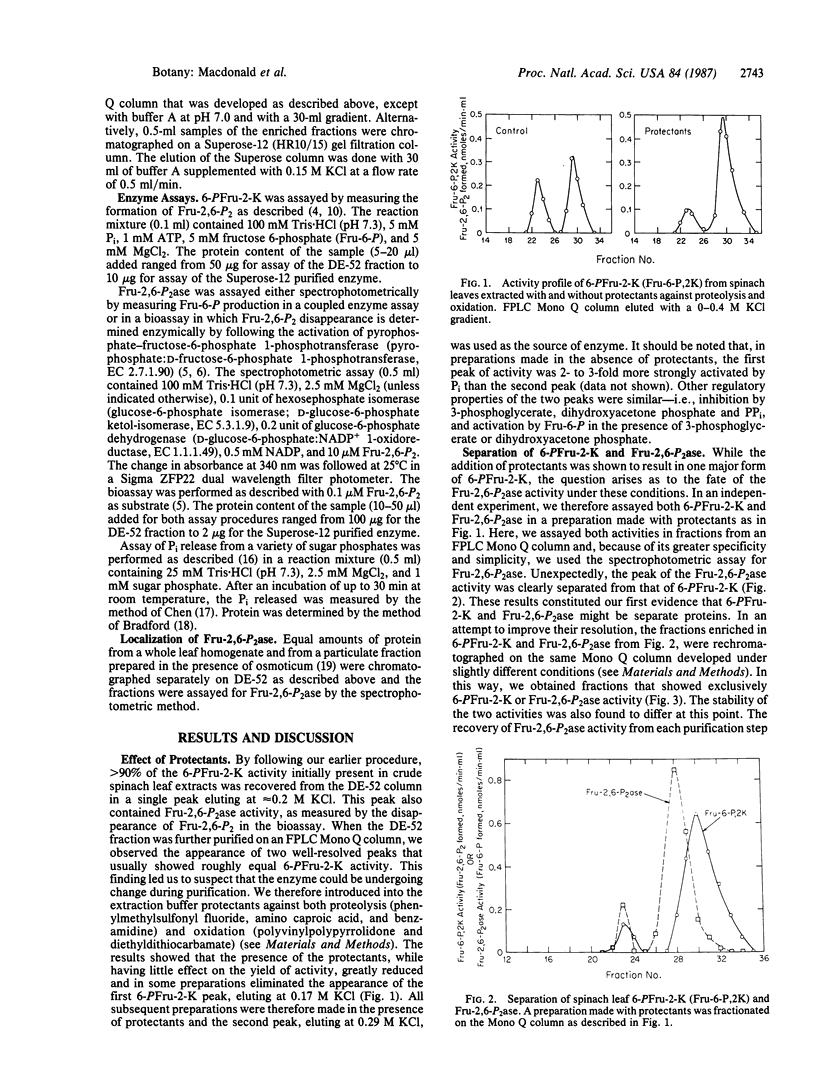
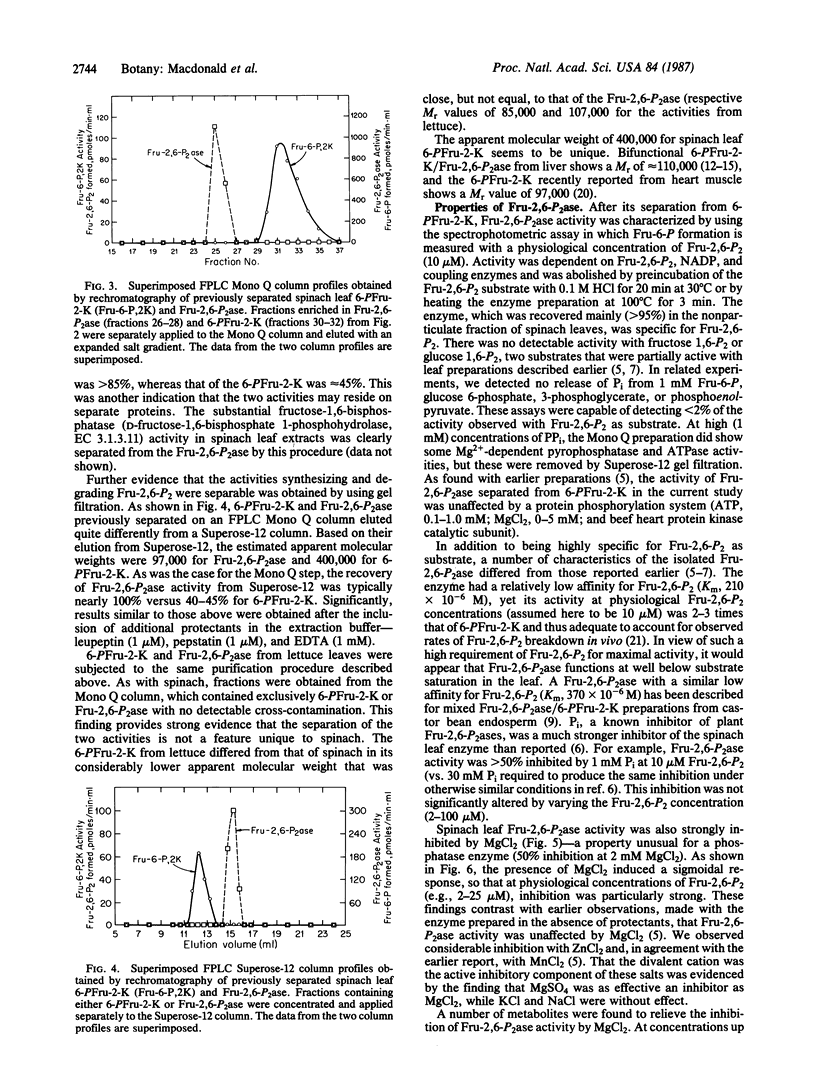
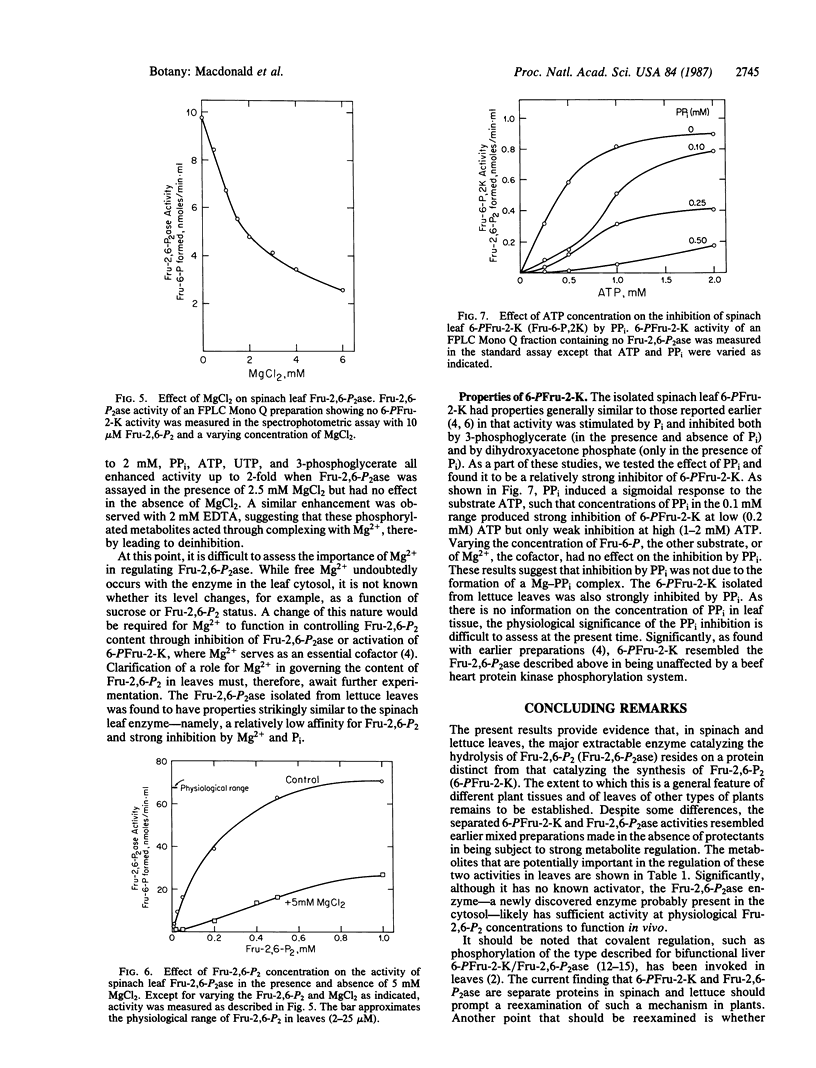
Activities catalyzing the synthesis and degradation of fructose 2,6-bisphosphate—6-phosphofructo-2-kinase (ATP:D-fructose-6-phosphate-2-phosphotransferase, EC 2.7.1.105) and fructose-2,6-bisphosphatase (D-fructose-2,6-bisphosphate 2-phosphohydrolase, EC 3.1.3.46)—were isolated from spinach leaves by an improved procedure and separated on the basis of both charge and molecular weight. The separated activities showed no detectable cross-contamination, indicating, in contrast to all previous data, that they are not present on a single bifunctional protein of the classical type in liver. The fructose-2,6-bisphosphatase—a newly discovered phosphatase enzyme—differed from previous mixed preparations by showing greater specificity but lower affinity for fructose 2,6-bisphosphate, greater sensitivity to inhibition by inorganic phosphate, and in being sensitive to inhibition by Mg2+. The 6-phosphofructo-2-kinase was found to be inhibited by low levels of inorganic pyrophosphate and, in addition, to be regulated by the metabolites described previously. Similar results were obtained with preparations from lettuce leaves. The results support the view that, through individual regulation of the activities catalyzing its synthesis and breakdown, cytosolic metabolites are key factors in controlling the fructose 2,6-bisphosphate content of leaves.
Keywords: fructose-2,6-bisphosphatase; 6-phosphofructo-2-kinase; sucrose synthesis; sucrose breakdown; enzyme regulation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baysdorfer C., Robinson J. M. Sucrose and Starch Synthesis in Spinach Plants Grown under Long and Short Photosynthetic Periods. Plant Physiol. 1985 Nov;79(3):838–842. doi: 10.1104/pp.79.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cséke C., Nishizawa A. N., Buchanan B. B. Modulation of chloroplast phosphofructokinase by NADPH : a mechanism for linking light to the regulation of glycolysis. Plant Physiol. 1982 Sep;70(3):658–661. doi: 10.1104/pp.70.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Maghrabi M. R., Claus T. H., Pilkis J., Fox E., Pilkis S. J. Regulation of rat liver fructose 2,6-bisphosphatase. J Biol Chem. 1982 Jul 10;257(13):7603–7607. [PubMed] [Google Scholar]
- Kitamura K., Uyeda K. The mechanism of activation of heart fructose 6-phosphate,2-kinase:fructose-2,6-bisphosphatase. J Biol Chem. 1987 Jan 15;262(2):679–681. [PubMed] [Google Scholar]
- Kruger N. J., Beevers H. Synthesis and degradation of fructose 2,6-bisphosphate in endosperm of castor bean seedlings. Plant Physiol. 1985 Feb;77(2):358–364. doi: 10.1104/pp.77.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larondelle Y., Mertens E., Van Schaftingen E., Hers H. G. Purification and properties of spinach leaf phosphofructokinase 2/fructose 2,6-bisphosphatase. Eur J Biochem. 1986 Dec 1;161(2):351–357. doi: 10.1111/j.1432-1033.1986.tb10454.x. [DOI] [PubMed] [Google Scholar]
- Rider M. H., Foret D., Hue L. Comparison of purified bovine heart and rat liver 6-phosphofructo-2-kinase. Evidence for distinct isoenzymes. Biochem J. 1985 Oct 1;231(1):193–196. doi: 10.1042/bj2310193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J., Wötzel C., Buchanan B. B. Enzyme regulation in c(4) photosynthesis : identification and localization of activities catalyzing the synthesis and hydrolysis of fructose-2,6-bisphosphate in corn leaves. Plant Physiol. 1985 Apr;77(4):999–1003. doi: 10.1104/pp.77.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Cseke C., Buchanan B. B. Regulation of fructose 2,6-bisphosphate concentration in spinach leaves. Eur J Biochem. 1984 Aug 15;143(1):89–93. doi: 10.1111/j.1432-1033.1984.tb08345.x. [DOI] [PubMed] [Google Scholar]
- Stitt M., Cséke C., Buchanan B. Ethylene-induced increase in fructose-2,6-bisphosphate in plant storage tissues. Plant Physiol. 1986 Jan;80(1):246–248. doi: 10.1104/pp.80.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Herzog B., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : I. Coordination of CO(2) Fixation and Sucrose Synthesis. Plant Physiol. 1984 Jul;75(3):548–553. doi: 10.1104/pp.75.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K., Furuya E., Richards C. S., Yokoyama M. Fructose-2,6-P2, chemistry and biological function. Mol Cell Biochem. 1982 Oct 18;48(2):97–120. doi: 10.1007/BF00227610. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Coulie P. G., Van Snick J., Hers H. G. Reaction of phosphofructokinase 2/fructose 2,6-bisphosphatase with monoclonal antibodies. A proof of the bifunctionality of the enzyme. Eur J Biochem. 1986 Sep 1;159(2):367–373. doi: 10.1111/j.1432-1033.1986.tb09877.x. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hers H. G. Purification and properties of phosphofructokinase 2/fructose 2,6-bisphosphatase from chicken liver and from pigeon muscle. Eur J Biochem. 1986 Sep 1;159(2):359–365. doi: 10.1111/j.1432-1033.1986.tb09876.x. [DOI] [PubMed] [Google Scholar]
- el-Maghrabi M. R., Correia J. J., Heil P. J., Pate T. M., Cobb C. E., Pilkis S. J. Tissue distribution, immunoreactivity, and physical properties of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5005–5009. doi: 10.1073/pnas.83.14.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]


