Abstract
Abstract: Real-time histology or virtual biopsy for the diagnosis of colonic cancer is of great medical significance. In this work, we show that label-free multiphoton imaging is feasible and effective in monitoring colonic cancer progression by providing cellular and subcellular details in fresh, unfixed, unstained colonic specimens. Our results also demonstrate the capability of using tissue quantitative analysis of the redox ratio for quantifying colonic cancer progression. These results suggest that multiphoton microscopy has potential to become an in situ histological tool, which is free from the labeling requirement of conventional methods, for the early diagnosis and detection of malignant lesions in the colon.
OCIS codes: (180.4315) Nonlinear microscopy; (170.3880) Medical and biomedical imaging; (170.6510) Spectroscopy, tissue diagnostics
1. Introduction
Colonic cancer is one of the leading worldwide cancers with high mortality [1]. In general, it is necessary to perform mucosal biopsy followed by the histopathological examinations of biopsied tissues for the diagnosis of colonic cancer. However, the procedure is limited by sampling error, costs, risks to the patient, and the delay in getting results. Hence, the development of new and noninvasive imaging modality for enabling real-time histology or virtual biopsy at the time of endoscopic examination is of great medical significance.
Recently, confocal endomicroscopy has shown the ability to actually see histological details during ongoing endoscopy, but the usefulness of this imaging modality in patients is limited because it needs the administration of fluorescence dyes [2,3]. Multiphoton microscopy (MPM) is well suited for the observation of unstained samples based on intrinsic sources of nonlinear signals [4,5]. MPM provides several advantages over confocal microscopy, such as being label-free, inherent three-dimensional resolution, near-infrared excitation for superior optical penetration, lower photodamage, and capable of providing quantitative information [6–11]. With the advent of the multiphoton-based endoscopy [12,13], the multiphoton technique will be adapted for use in clinics in the near future. However, prior to performing in vivo endoscopic investigation for clinical application, it is critical to image and characterize optical features for colonic cancer.
It is well known that the development of colonic cancer is a multi-stage process, including normal case, precancer, and cancer [14,15]. In other words, the monitoring of colonic cancer progression can improve the early diagnosis and detection of malignant lesions in the colon. In this work, we attempted to monitor colonic cancer progression by MPM microscopy.
2. Materials and methods
2.1. Multiphoton microscopy
Multiphoton microscopy was achieved using a nonlinear optical system which has been described previously [16,17]. In short, multiphoton images were acquired using a commercial laser scanning microscopic imaging system (Zeiss LSM 510 META, Jena, Germany) coupled to a femtosecond Ti:sapphire laser (Coherent Mira 900-F) operating at 800 nm. The polarization direction of the laser light is the horizontal polarization. An oil immersion objective (×63 and NA = 1.4) was employed for focusing the excitation beam into tissue samples (average power less than 10 mW) and was also used to collect the backscattered intrinsic two-photon excited fluorescence (TPEF) signals. A fine focusing stage (HRZ 200 stage, Carl Zeiss) is used to translate the samples after x-y scan of the samples for obtaining a large-area image, and to change the focus position for imaging at various depths. In this work, the depth of 0 μm refers to the tissue surface where the multiphoton signal of reflection from the interface between the tissue and the glass coverslip reaches to maximum. This system has many channels and each channel can selectively be set to detect emission signals within the random range from 377 to 716 nm to achieve imaging. It is known that the intrinsic fluorescence of epithelium is determined mainly by the signals of nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD), the enzymes carrying information of cellular metabolism [5,7]. The previous works show NADH and FAD have the peaks at around 470 nm and 530 nm, respectively [4,5,7,11]. Moreover, the ratio of NADH over FAD fluorescence, called the redox ratio, is a good indicator of the cellular metabolic state [5,7,11]. Thus, in this work two channels were used: two channels (430-490 nm, green color-coded; 500-560 nm, red color-coded) were used to collect NADH and FAD fluorescence signals under identical condition, respectively. Selection of the excitation wavelength and two channels is suitable for the simultaneous collection of intrinsic NADH and FAD fluorescence [18]. The images were obtained at 2.56 μs per pixel. For the 800 nm, theoretical resolution of 0.69 μm (lateral) and 1.63 μm (axial) can be expected [19].
2.2. Colonic biopsy specimens
A total of 19 fresh colonic biopsy specimens with a size of 0.3-0.9 mm in thickness were obtained from seven patients underwent endoscopic biopsy. All patients signed an informed consent before study participation, and this study was approved by Institutional Review Board of Fujian Provincial Tumor Hospital. The biopsy specimen was placed in the Glass Bottom Dish (MatTek, coverglass: 0.085-0.13 mm) for multiphoton imaging. In this work, the specimen preparation and multiphoton imaging were completed within 1 hour after endoscopic biopsy. Following imaging, the tissue specimens were placed in buffered formalin and submitted for histopathology, showing that 5 examined specimens were normal, 6 specimens exhibited precancer (3 adenomatous polyps, 3 adenoma), and 8 specimens showed cancer.
3. Results and Discussions
Large-area and high-resolution multiphoton imaging was performed, and the representative images from normal, precancerous, and cancerous colonic tissues are respectively shown in Figs. 1 (a) , 1(b), and 1(c). As can be seen, large morphological differences are shown with colonic cancer progression. In normal case, a honeycomb arrangement of circular crypts with uniform size is observed, goblet cells and epithelial nuclei can be identified (red arrows), and the crypts are separated by a dense interstitial space. Moreover, a sparse cellular infiltrate also is seen within the interstitial matrix, with peak fluorescence at a longer wavelength range; this most likely represents interstitial lymphocytes (white arrows) [20]. In precancer, the distorted, elongated crypts are observed, there are a reduced amount of goblet cells and a loss of cellular junctions, the imaged cells have elliptical-shaped nuclei, the crypts are separated by much sparser and less regular interstitial space, and little to no cellular infiltrate in the interstitium. In cancer, the crypts found in normal case are missing, there is a total loss of goblet cells, the imaged cells are featured by irregular size and shape, enlarged nuclei, and increased nuclear-to-cytoplasmic ratio, and the intercellular space between individual cells are not readily discerned. Moreover, some cells undergoing mitosis (white arrow) can be identified, indicating the rapid growth of the cancer. Together, these results suggest that label-free, qualitative multiphoton imaging is feasible and effective in monitoring colonic cancer progression by providing cellular and subcellular details in fresh, unfixed, unstained colonic specimens.
Fig. 1.
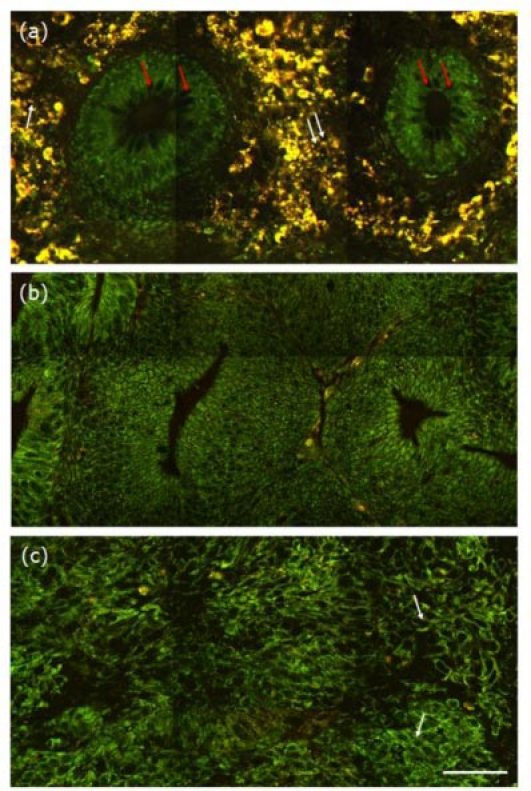
Representative multiphoton images from the normal (a), precancerous (b), and cancerous (c) colonic tissues at depth of 0 μm. The excitation wavelength λex was 800 nm. Scale bar = 50 μm.
In addition to comparing morphological differences, the redox ratio was also analyzed to quantitatively monitor colonic cancer progression. Since cancer cells generate more energy by glycolysis followed by lactic acid in the cytosol, the difference in metabolic activity with cancer progression enables the use of redox ratio analysis for quantitatively monitoring cancer progression [11]. The quantitative analysis of the redox ratio was performed using the following method. The intensity of NADH spectral band and the intensity of FAD spectral band were defined as A and B, respectively. Then the redox ratio was calculated by using the following formula: A/B. In this work, we selected regions where the cells can be identified and performed the redox ratio analysis. Furthermore, considering the variation with depth, three optical sections at various depths (0, 10, 30 μm) were analyzed for each biopsy. As presented in Fig. 2 , the redox ratio increases with colonic cancer progression. To be specific, the redox ratio in normal case is 1.82 ± 0.12 (n = 15 sections of 5 biopsies), in precancer is 2.57 ± 0.19 (n = 18 sections of 6 biopsies), and in cancer is 3.26 ± 0.28 (n = 24 sections of 8 biopsies). This observation supports the notion that there is an increase in metabolic activity with colonic cancer progression. This result is consistent with the previous reports [7,11,21]. Thus, the redox ratio may provide a useful indicator to quantitatively monitor the development of colonic cancer.
Fig. 2.
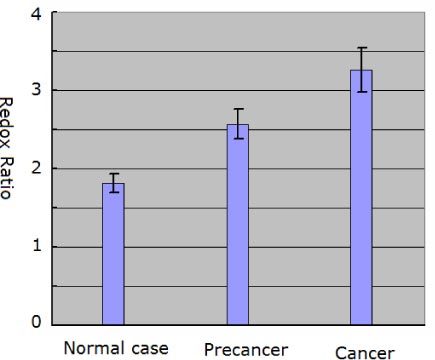
Redox ratio obtained from the normal, precancerous, and cancerous colonic tissues. Error bars indicate calculated standard deviations.
4. Conclusion
We have quantitatively monitored colonic cancer progression by both qualitative, label-free multiphoton imaging and quantitative redox analysis. We found that multiphoton imaging allows the visualization of cellular and subcellular details in colonic cancer progression; it is the quantitative analysis of the redox ratio that can provide a useful indicator to quantitatively monitor colonic cancer progression. The capability of label-freely monitoring colonic cancer progression renders multiphoton microscopy an in situ histological tool that is free from the labeling requirement of conventional methods. With the advent of the multiphoton-based endoscopy, the multiphoton technique will open new horizons for immediate diagnosis and management during endoscopy, but also for clinical science in coloproctology. The present study will provide the groundwork for this exploration into the application of multiphoton-based endoscopy in a clinical setting.
Acknowledgments
The project was supported by the National Natural Science Foundation of China (No. 60908043 and No. 30970783), the Program for New Century Excellent Talents in University (NCET-07-0191), the Natural Science Funds for Distinguished Young Scholar in Fujian Province (2009J06031), and the Natural Science Foundation of Fujian Province (2010J01136).
References and links
- 1.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M. J., “Cancer statistics, 2009,” CA Cancer J. Clin. 59(4), 225–249 (2009). 10.3322/caac.20006 [DOI] [PubMed] [Google Scholar]
- 2.Goetz M., Kiesslich R., “Advances of endomicroscopy for gastrointestinal physiology and diseases,” Am. J. Physiol. Gastrointest. Liver Physiol. 298(6), G797–G806 (2010). 10.1152/ajpgi.00027.2010 [DOI] [PubMed] [Google Scholar]
- 3.Kiesslich R., Burg J., Vieth M., Gnaendiger J., Enders M., Delaney P., Polglase A., McLaren W., Janell D., Thomas S., Nafe B., Galle P. R., Neurath M. F., “Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo,” Gastroenterology 127(3), 706–713 (2004). 10.1053/j.gastro.2004.06.050 [DOI] [PubMed] [Google Scholar]
- 4.Zipfel W. R., Williams R. M., Christie R., Nikitin A. Y., Hyman B. T., Webb W. W., “Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation,” Proc. Natl. Acad. Sci. U.S.A. 100(12), 7075–7080 (2003). 10.1073/pnas.0832308100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skala M. C., Riching K. M., Gendron-Fitzpatrick A., Eickhoff J., Eliceiri K. W., White J. G., Ramanujam N., “In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia,” Proc. Natl. Acad. Sci. U.S.A. 104(49), 19494–19499 (2007). 10.1073/pnas.0708425104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.So P. T. C., Dong C. Y., Masters B. R., Berland K. M., “Two-photon excitation fluorescence microscopy,” Annu. Rev. Biomed. Eng. 2(1), 399–429 (2000). 10.1146/annurev.bioeng.2.1.399 [DOI] [PubMed] [Google Scholar]
- 7.Zhuo S. M., Zheng L. Q., Chen J. X., Xie S. S., Zhu X. Q., Jiang X. S., “Depth-cumulated epithelial redox ratio and stromal collagen quantity as quantitative intrinsic indicators for differentiating normal, inflammatory, and dysplastic epithelial tissues,” Appl. Phys. Lett. 97(17), 173701 (2010). 10.1063/1.3505762 [DOI] [Google Scholar]
- 8.Campagnola P. J., Loew L. M., “Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms,” Nat. Biotechnol. 21(11), 1356–1360 (2003). 10.1038/nbt894 [DOI] [PubMed] [Google Scholar]
- 9.Hovhannisyan V. A., Su P.-J., Lin S.-J., Dong C. Y., “Quantifying thermodynamics of collagen thermal denaturation by second harmonic generation imaging,” Appl. Phys. Lett. 94(23), 233902 (2009). 10.1063/1.3142864 [DOI] [Google Scholar]
- 10.Zhuo S. M., Chen J. X., Wu G. Z., Xie S. S., Zheng L. Q., Jiang X. S., Zhu X. Q., “Quantitatively linking collagen alteration and epithelial tumor progression by second harmonic generation microscopy,” Appl. Phys. Lett. 96(21), 213704 (2010). 10.1063/1.3441337 [DOI] [Google Scholar]
- 11.Cicchi R., Crisci A., Cosci A., Nesi G., Kapsokalyvas D., Giancane S., Carini M., Pavone F. S., “Time- and Spectral-resolved two-photon imaging of healthy bladder mucosa and carcinoma in situ,” Opt. Express 18(4), 3840–3849 (2010). 10.1364/OE.18.003840 [DOI] [PubMed] [Google Scholar]
- 12.König K., Ehlers A., Riemann I., Schenkl S., Bückle R., Kaatz M., “Clinical two-photon microendoscopy,” Microsc. Res. Tech. 70(5), 398–402 (2007). 10.1002/jemt.20445 [DOI] [PubMed] [Google Scholar]
- 13.Gu M., Bao H. C., Li J. L., “Cancer-cell microsurgery using nonlinear optical endomicroscopy,” J. Biomed. Opt. 15(5), 050502 (2010). 10.1117/1.3502566 [DOI] [PubMed] [Google Scholar]
- 14.Jass J. R., Whitehall V. L., Young J., Leggett B. A., “Emerging concepts in colorectal neoplasia,” Gastroenterology 123(3), 862–876 (2002). 10.1053/gast.2002.35392 [DOI] [PubMed] [Google Scholar]
- 15.Leslie A., Carey F. A., Pratt N. R., Steele R. J., “The colorectal adenoma-carcinoma sequence,” Br. J. Surg. 89(7), 845–860 (2002). 10.1046/j.1365-2168.2002.02120.x [DOI] [PubMed] [Google Scholar]
- 16.Zhuo S., Chen J., Luo T., Zou D., Zhao J., “Multimode nonlinear optical imaging of the dermis in ex vivo human skin based on the combination of multichannel mode and Lambda mode,” Opt. Express 14(17), 7810–7820 (2006). 10.1364/OE.14.007810 [DOI] [PubMed] [Google Scholar]
- 17.Zhuo S. M., Chen J. X., Xie S. S., Hong Z. B., Jiang X. S., “Extracting diagnostic stromal organization features based on intrinsic two-photon excited fluorescence and second-harmonic generation signals,” J. Biomed. Opt. 14(2), 020503 (2009). 10.1117/1.3088029 [DOI] [PubMed] [Google Scholar]
- 18.Huang S., Heikal A. A., Webb W. W., “Two-photon fluorescence spectroscopy and microscopy of NAD(P)H and flavoprotein,” Biophys. J. 82(5), 2811–2825 (2002). 10.1016/S0006-3495(02)75621-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong C. Y., So P. T. C., Buehler C., Gratton E., “Spatial resolution in pump-probe microscopy,” Optik (Stuttg.) 106, 7–14 (1997). [Google Scholar]
- 20.Rogart J. N., Nagata J., Loeser C. S., Roorda R. D., Aslanian H., Robert M. E., Zipfel W. R., Nathanson M. H., “Multiphoton imaging can be used for microscopic examination of intact human gastrointestinal mucosa ex vivo,” Clin. Gastroenterol. Hepatol. 6(1), 95–101 (2008). 10.1016/j.cgh.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skala M. C., Squirrell J. M., Vrotsos K. M., Eickhoff J. C., Gendron-Fitzpatrick A., Eliceiri K. W., Ramanujam N., “Multiphoton microscopy of endogenous fluorescence differentiates normal, precancerous, and cancerous squamous epithelial tissues,” Cancer Res. 65(4), 1180–1186 (2005). 10.1158/0008-5472.CAN-04-3031 [DOI] [PMC free article] [PubMed] [Google Scholar]


