Abstract
BACKGROUND
Greater body mass index (BMI) is associated with shorter time to prostate-specific antigen (PSA) failure following radical prostatectomy and radiation therapy (RT). Whether BMI is associated with prostate cancer-specific mortality (PCSM) was investigated in a large randomized trial of men treated with RT and androgen deprivation therapy (ADT) for locally advanced prostate cancer.
METHODS
Between 1987 and 1992, 945 eligible men with locally advanced prostate cancer were enrolled in a phase 3 trial (RTOG 85-31) and randomized to RT and immediate goserelin or RT alone followed by goserelin at recurrence. Height and weight data were available at baseline for 788 (83%) subjects. Cox regression analyses were performed to evaluate the relations between BMI and all-cause mortality, PCSM, and nonprostate cancer mortality. Covariates included age, race, treatment arm, history of prostatectomy, nodal involvement, Gleason score, clinical stage, and BMI.
RESULTS
The 5-year PCSM rate for men with BMI <25 kg/m2 was 6.5%, compared with 13.1% and 12.2% in men with BMI ≥25 to <30 and BMI ≥30, respectively (Gray's P = .005). In multivariate analyses, greater BMI was significantly associated with higher PCSM (for BMI ≥25 to <30, hazard ratio [HR] 1.52, 95% confidence interval [CI], 1.02–2.27, P = .04; for BMI ≥30, HR 1.64, 95% CI, 1.01–2.66, P = .04). BMI was not associated with nonprostate cancer or all-cause mortality.
CONCLUSIONS
Greater baseline BMI is independently associated with higher PCSM in men with locally advanced prostate cancer. Further studies are warranted to evaluate the mechanism(s) for increased cancer-specific mortality and to assess whether weight loss after prostate cancer diagnosis alters disease course.
Keywords: obesity, BMI, mortality, prostate cancer, hormonal therapy, radiation therapy
Obesity and prostate cancer are 2 important causes of morbidity and mortality afflicting men in the US.1–3 Approximately one-third of American men are obese1 and greater than 218,000 men are estimated to be diagnosed with prostate cancer in 2007.2 While certain types of cancer may occur more frequently and may be more likely to be fatal in obese patients,4,5 observational studies remain unclear as to the link between an elevated body mass index (BMI) and risk of prostate cancer development.4–10
Greater BMI, however, has been shown to be associated with more aggressive higher-grade prostate cancer11–13 and higher prostate-specific antigen (PSA) recurrence rates following radical prostatectomy (RP).11,14–16 The data following radiation therapy (RT) is limited. Two recent retrospective analyses suggested that BMI is a predictor of PSA failure among patients treated with external beam RT with or without androgen deprivation therapy (ADT),17,18 while another report suggested that this may not be the case following brachytherapy.19 Biochemical failure, however, only weakly correlates with risk of cancer-specific mortality.20,21 Survival after RP may not be affected by BMI22 and the effect after RT is unknown.
Several phase 3 randomized trials have demonstrated a survival benefit to adjuvant ADT for patients with locally advanced or high-grade prostate cancer.23–26 Based on evidence of improved survival, the use of hormonal therapy in addition to RT has increased markedly.27 Yet ADT exposes patients to a number of potential adverse effects, including weight gain and increased fat mass.28,29 Whether obesity influences overall or disease-specific outcomes in men treated with RT and ADT is unknown.
In this study we investigated the relations between BMI and prostate cancer-specific mortality (PCSM), noncancer mortality, and overall mortality using data from a large-scale randomized trial of men treated with RT and ADT for locally advanced prostate cancer.
MATERIALS AND METHODS
Radiation Therapy Oncology Group (RTOG) protocol 85-31 was a phase 3 trial designed to compare the effectiveness of adjuvant ADT with goserelin, a gonadotropin-releasing hormone (GnRH) agonist, given in addition to standard external beam RT versus the use of ADT therapeutically following RT at the time of recurrence in a population of patients with locally advanced prostate cancer.24
Patient Eligibility
All subjects had histologically confirmed adenocarcinoma of the prostate and either had grossly palpable tumor beyond the confines of the prostate (clinical stage T3) or documented involvement of the regional lymphatics. Patients with primary tumor confined to the prostate (clinical stage T1-2) were eligible if there was evidence of spread to the regional lymph nodes either radiographically or histologically. Patients with bulky primary lesions (product of palpable tumor dimensions ≥25 cm) were not eligible for this study, but were for a parallel study (RTOG 86-10). Exceptions were those with evidence of spread to lymphatics outside the pelvis (common iliac and/or paraaortic) who were eligible regardless of the primary tumor size. Patients who had undergone RP were eligible if penetration through the prostatic capsule to the resection margin and/or to the seminal vesicles was histologically documented. The Karnofsky performance status had to be >60%. All institutional state and federal guidelines had to be followed. All patients provided written informed consent before study enrollment.
Pretreatment Evaluation
Pretreatment evaluation included history and physical examination. Laboratory studies included serum acid phosphatase, complete blood cell count, serum testosterone determination, and, after July 1990, PSA measurement. PSA determination was not mandatory at study inception because it was not widely available. Radiographic evaluation included chest x-ray and bone scan. Lymph node assessment was mandatory by lymphangiography, computed tomography (CT), or lymphadenectomy.
Study Design
Patients were entered in the study by a telephone call to RTOG headquarters within the first week of RT. After confirmation of eligibility, patients were stratified by histologic differentiation (well-differentiated or Gleason score 2–5; moderately differentiated or Gleason score 6–7; and poorly differentiated or Gleason score 8–10), nodal status and extent of nodal involvement (none vs involvement below common iliacs vs common iliac involvement vs paraaortic involvement), acid phosphatase status (not elevated vs elevated), and prior RP (no vs yes). The randomization scheme described by Zelen30 was used to achieve balance in treatment assignment among institutions using the 4 stratification variables.24 Patients were randomized either to RT and adjuvant goserelin (Arm I) or to RT alone followed by observation and administration of goserelin at recurrence (Arm II). Among patients assigned to Arm I, ADT was to be started during the last week of RT and was to be continued indefinitely or until signs of progression. Among patients assigned to Arm II, ADT was to start as soon as recurrence (local and/or distant) was established.
Treatment
Radiation technique
All patients received RT on megavoltage units with a multiple field technique. The initial target volume (prostate plus draining lymph nodes) received a total dose of 44–46 Gy. The prostatic target volume was to receive a boost dose of 20–25 Gy, which brought the total prescribed dose to 65–70 Gy. Among postoperatively (ie, following RP) irradiated patients, the prostatic bed was to receive 60–65 Gy and irradiation of the regional lymphatics was not required if there was no histopathologic evidence of lymph node involvement. In all cases a boost target volume was designed to include the prostate with margins sufficiently wide to encompass all tumor extensions into surrounding tissues. The daily dose was 1.8–2.0 Gy per fraction, given 4 to 5 times weekly.
In designing the initial fields the inferior border was set at a projection point located 5–6 cm below the superior margin of the symphysis. Among patients with evidence of tumor spread to the pelvic lymphatics (obturator, external and internal iliac), the superior border of the initial target volume was placed at the L5-S1 interspace. If the common iliac chain was involved the superior border was raised to the level of the L2-L3 interspace, and if the paraaortic nodes were involved it was raised to encompass vertebral body T11. The lateral borders of the initial fields were placed 2 cm lateral to the pelvic brim. Although it was known that the amount of radiation selected for gross nodal disease was unlikely to provide control, the protocol was not written to include higher doses, since conformal techniques were not widely available during the study period.
Drug therapy
Subjects assigned to Arm I were treated with goserelin acetate (Zoladex, Zeneca Pharmaceutical, Wilmington, Del) (3.6 mg subcutaneously in the anterior abdominal wall monthly), started during the last week of RT. Subjects in Arm II were treated with goserelin at recurrence. In both arms goserelin was continued indefinitely or until sign of disease progression.
Data Collection and Analysis
Central review of radiation therapy delivered, calibration of all machines on which a patient was treated, and review of materials on which the diagnosis was based were performed for each case as per the usual RTOG/National Cancer Institute (NCI) requirements.24
Body mass index
BMI (weight in kilograms divided by height in meters squared [kg/m2]) was calculated using patient height and weight data as measured at baseline. BMI was categorized as per the National Institutes for Health classifications, with individuals with a BMI <25 kg/m2 considered normal, those with a BMI of 25–29.9 kg/m2 considered overweight, and those with a BMI ≥30 kg/m2 considered obese.31
Survival endpoints
Prostate cancer-specific mortality (PCSM) was defined as death from prostate cancer or protocol treatment. Non-PCSM was defined as death from any cause other than prostate cancer or protocol treatment. All-cause mortality (ACM) was defined as death from any cause. These endpoints were measured from the date of randomization to the date of death or most recent follow-up through 2005.
Statistical methods
Chi-square test statistics were used to compare pretreatment characteristics of patients at study entry. The cumulative incidence method32 was used to estimate times to PCSM and non-PCSM because it specifically adjusts for other competing causes of mortality. Gray's test statistic33 for comparing cumulative incidence rates was used. ACM was estimated according to the Kaplan-Meier method34 and comparisons were performed with the log-rank test.35 Univariate Cox proportional hazard regression analyses36 using the chi-square test were performed to evaluate the solitary effect of each variable on the various survival endpoints. To analyze whether BMI was independently associated with PCSM, non-PCSM, and ACM while adjusting for known prognostic factors, multivariate analyses were performed using a Cox proportional hazards regression model36 with the following categorical covariates: age (<70 [reference level] vs ≥70 years), race (black [reference level] vs white/other), centrally reviewed Gleason score (2–6 [reference level] vs 7–10), clinical stage (A/B [reference level] vs C), nodal involvement (no [reference level] vs yes), prostatectomy (no [reference level] vs yes), treatment (Arm II [reference level] vs Arm I), and BMI (<25 [reference level] vs ≥25–30 vs >30 kg/m2). For the categorical variables the cut-points selected were made before the data were examined and were based on established strata.24,31 BMI was also analyzed as a continuous variable. Unadjusted and adjusted hazard ratios (HRs) were calculated for all covariates using the Cox proportional hazards model with associated 95% confidence intervals (CIs) and P-values. All statistical comparisons were 2-sided and a P-value <.05 was considered statistically significant. Statistical Analysis System (SAS Institute, Cary, NC) was used for all statistical analyses.
RESULTS
Pretreatment Characteristics
Between February 1987 and April 1992, when the study was closed, a total of 977 patients were entered, 488 on Arm I and 489 on Arm II. Thirty-two patients were retrospectively classified as ineligible and excluded from the subsequent analysis, leaving 945 eligible patients, 477 on Arm I and 468 on Arm II. Height and weight data were available at baseline for 788 (83%) of these subjects and the current analyses are restricted to this subset. As shown in Table 1, pretreatment characteristics, including median BMI and BMI categorization, were similar according to the treatment arms. The median BMI was 26.6 kg/m2 (range, 14.7–47.9). In all, 241 (31%) of subjects were categorized as having normal weight, 402 (51%) as overweight, and 145 (18%) as obese.
TABLE 1.
Pretreatment Characteristics
| Arm I |
Arm II |
||||
|---|---|---|---|---|---|
| (n = 403) |
(n = 385) |
||||
| No. | % | No. | % | P | |
| Age, y | |||||
| <70 | 191 | 47 | 181 | 47 | .91 |
| ≥70 | 212 | 53 | 204 | 53 | |
| Race | |||||
| White | 361 | 90 | 347 | 90 | .80 |
| Black | 37 | 9 | 35 | 9 | |
| Other | 5 | 1 | 3 | 1 | |
| Prostatectomy | |||||
| No | 338 | 84 | 324 | 84 | .91 |
| Yes | 65 | 16 | 61 | 16 | |
| Nodal involvement | |||||
| No | 283 | 70 | 285 | 74 | .23 |
| Yes | 120 | 30 | 100 | 26 | |
| Gleason score (central) | |||||
| Score missing | 31 | 8 | 35 | 9 | |
| Score available | 372 | 92 | 350 | 91 | |
| 2–6 | 104 | 28 | 99 | 28 | .92 |
| 7–10 | 268 | 72 | 251 | 72 | |
| Clinical stage | |||||
| A/B | 122 | 30 | 106 | 28 | .40 |
| C | 281 | 70 | 279 | 72 | |
| BMI (kg/m2) | |||||
| BMI category | |||||
| <25 | 132 | 33 | 109 | 28 | .40 |
| ≥25 to <30 | 200 | 50 | 202 | 52 | |
| ≥30 | 71 | 18 | 74 | 19 | |
| BMI, median (kg/m2) | 26.6 | 26.6 | |||
| BMI, range (kg/m2) | 16.2–44.8 | 14.7–47.9 | |||
BMI indicates body mass index.
Main Study Outcomes
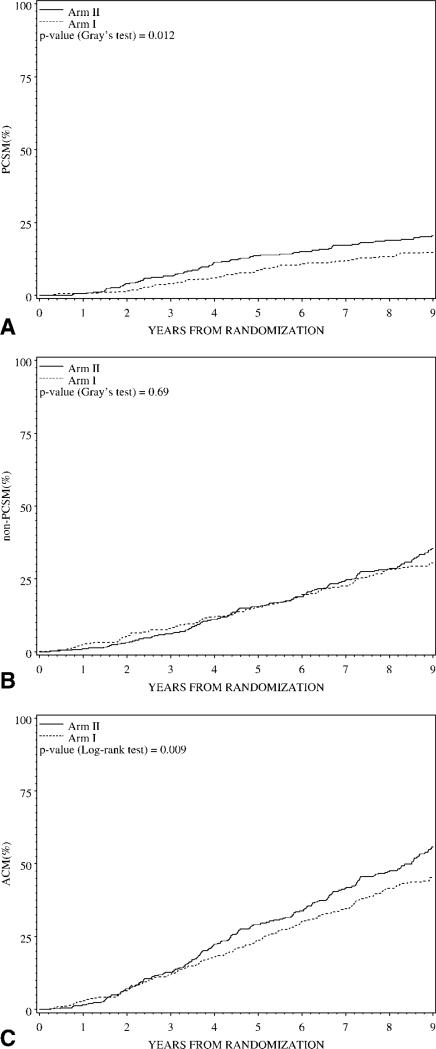
Figure 1 graphically displays the main outcomes of PCSM, non-PCSM, and ACM for the 788 subjects with available BMI. The median follow-up was 8.1 (range, 0.2–15.1) years overall. There were a total of 476 deaths, 169 of which were prostate cancer-related. As shown in Table 2, men treated with immediate ADT on Arm I were significantly less likely than men on Arm II to die of prostate cancer or of any cause. At 5 years, PCSM was 8.5% for Arm I versus 13.6% for Arm II (HR 0.65, 95% CI 0.48–0.88, P = .006) and ACM was 23.8% for Arm I versus 29.1% for Arm II (HR 0.79, 95% CI 0.66–0.94, P = .003).
FIGURE 1.
Time to (top) prostate cancer-specific mortality (PCSM); (middle) non-PCSM; and (bottom) all-cause mortality (ACM) by treatment arm for the 788 subjects with available body mass index (BMI).
TABLE 2.
Univariate Analyses of Survival Outcomes Stratified by (A) Treatment Arm and (B) BMI Category
| A: Outcome | Treatment arm | No. | No. events | 5-year failure rate (95% CI) | Unadjusted HR (95% CI) | P* |
|---|---|---|---|---|---|---|
| Prostate cancer-specific mortality | Arm II | 385 | 95 | 13.6 (10.1, 17.1) | – | |
| Arm I | 403 | 72 | 8.5 (5.7, 11.2) | 0.65 (0.48, 0.88) | .006 | |
| Nonprostate cancer-specific mortality | Arm II | 385 | 153 | 15.5 (11.8, 19.2) | – | |
| Arm I | 403 | 156 | 15.3 (11.8, 18.9) | 0.87 (0.70, 1.09) | .23 | |
| All-cause mortality | Arm II | 385 | 248 | 29.1 (24.5, 33.7) | – | |
| Arm I | 403 | 228 | 23.8 (19.6, 28.0) | 0.79 (0.66, 0.94) | .003 |
| B: Outcome | BMI Category | No. | No. events | 5-Year Failure Rate (95% CI) | Unadjusted HR (95% CI) | P* |
|---|---|---|---|---|---|---|
| Prostate cancer-specific mortality | <25 | 241 | 34 | 6.5 (3.3, 9.6) | – | |
| ≥25, <30 | 402 | 98 | 13.1 (9.8, 16.5) | 1.78 (1.20, 2.63) | .004 | |
| ≥30 | 145 | 37 | 12.2 (6.7, 17.6) | 1.79 (1.13, 2.86) | .014 | |
| Nonprostate cancer-specific mortality | <25 | 241 | 109 | 15.2 (10.6, 19.9) | – | |
| ≥25, <30 | 402 | 152 | 15.9 (12.3, 19.5) | 0.87 (0.68, 1.11) | .26 | |
| ≥30 | 145 | 48 | 14.4 (8.5, 20.3) | 0.71 (0.51, 1.00) | .052 | |
| All-cause mortality | <25 | 241 | 143 | 21.7 (16.4, 26.9) | – | |
| ≥25, <30 | 402 | 248 | 29.0 (24.6, 33.5) | 1.08 (0.88, 1.33) | .44 | |
| ≥30 | 145 | 85 | 26.6 (19.3, 33.8) | 0.97 (0.74, 1.27) | .82 |
BMI indicates body mass index; CI, confidence interval; HR, hazard ratio.
P-value from chi-square test using the Cox proportional hazards model.
Effect of BMI: Univariate Analysis
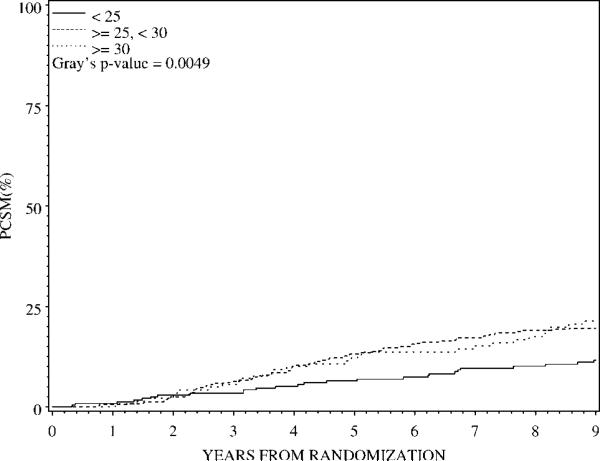
On univariate analysis, the 5-year PCSM rate for men with BMI <25 kg/m2 was 6.5%, compared with 13.1% in men with BMI ≥25 to <30 and 12.2% in men with BMI ≥30 (Gray's P = .005) (Table 2). Overweight and obese patients were approximately 1.8 times more likely to die of prostate cancer than those with normal weight (HR 1.78 [95% CI 1.20–2.63, P = .004] and HR 1.79 [95% CI 1.13–2.86, P = .014], respectively). Figure 2 graphically displays the time to PCSM by BMI category.
FIGURE 2.
Time to prostate cancer-specific mortality (PCSM) by body mass index (BMI) category.
Effect of BMI: Multivariate Analysis
Results of the multivariate analysis are shown in Table 3. After adjusting for age, race, treatment arm, history of prostatectomy, nodal involvement, Gleason score, and clinical stage, a greater BMI remained significantly associated with higher PCSM (for BMI ≥25- <30, adjusted HR 1.52, 95% CI 1.02–2.28, P = .04; for BMI ≥30, adjusted HR 1.64, 95% CI 1.01–2.66, P = .04). Results were similar when BMI was analyzed as a continuous variable (data not shown). Delayed ADT (P = .0004), no history of prostatectomy (P = .01), presence of nodal involvement (P = .0002), and Gleason 7–10 cancer (P < .0001) were also significantly associated with higher PCSM. BMI was not associated with non-PCSM or ACM.
TABLE 3.
Multivariate Analyses of Survival Outcomes
| Outcome | Covariate | Comparison | HR | (95% CI) | P |
|---|---|---|---|---|---|
| Prostate cancer-specific mortality | Age | <70 vs ≥70 | 1.21 | (0.86, 1.70) | .27 |
| Race | Black vs Other | 0.9 | (0.52, 1.56) | .72 | |
| Treatment arm | Arm II vs Arm I | 0.57 | (0.41, 0.78) | .0004 | |
| Prostatectomy | No vs Yes | 0.51 | (0.30, 0.87) | .013 | |
| Nodal involvement | No vs Yes | 2.22 | (1.46, 3.37) | .0002 | |
| Gleason score (Central review) | 2–6 vs 7–10 | 3.47 | (2.19, 5.49) | <.0001 | |
| Clinical stage | A-B vs C | 1.28 | (0.82, 2.02) | .28 | |
| BMI | <25 | — | |||
| ≥25, <30 | 1.52 | (1.02, 2.28) | .041 | ||
| ≥30 | 1.64 | (1.01, 2.66) | .043 | ||
| Nonprostate cancer-specific mortality | Age | <70 vs ≥70 | 2.12 | (1.62, 2.77) | <.0001 |
| Race | Black vs Other | 0.72 | (0.49, 1.07) | .11 | |
| Treatment arm | Arm II vs Arm I | 0.83 | (0.66, 1.05) | .12 | |
| Prostatectomy | No vs Yes | 0.58 | (0.36, 0.93) | .025 | |
| Nodal involvement | No vs Yes | 1.28 | (0.89, 1.84) | .19 | |
| Gleason score (Central review) | 2–6 vs 7–10 | 1.43 | (1.10, 1.85) | .008 | |
| Clinical stage | A-B vs C | 1.60 | (1.05, 2.43) | .029 | |
| BMI | <25 | — | |||
| ≥25, <30 | 0.95 | (0.73, 1.23) | .70 | ||
| ≥30 | 0.77 | (0.53, 1.11) | .16 | ||
| All-cause mortality | Age | <70 vs ≥70 | 1.72 | (1.40, 2.12) | <.0001 |
| Race | Black vs Other | 0.78 | (0.57, 1.08) | .13 | |
| Treatment arm | Arm II vs Arm I | 0.73 | (0.60, 0.88) | .0008 | |
| Prostatectomy | No vs Yes | 0.54 | (0.38, 0.78) | .0009 | |
| Nodal involvement | No vs Yes | 1.60 | (1.22, 2.10) | .0007 | |
| Gleason score (Central review) | 2–6 vs 7–10 | 1.84 | (1.48, 2.30) | <.0001 | |
| Clinical stage | A-B vs C | 1.46 | (1.07, 1.98) | .016 | |
| BMI | <25 | — | |||
| ≥25, <30 | 1.09 | (0.88, 1.36) | .42 | ||
| ≥30 | 1.00 | (0.75, 1.33) | 1.00 |
BMI indicates body mass index; HR, hazard ratio; CI, confidence interval.
DISCUSSION
Using data from a large, multicenter, randomized controlled trial with long follow-up, we found that a greater baseline BMI is independently associated with higher cancer-specific mortality in men with locally advanced prostate cancer. Compared with men with normal BMI, overweight and obese men had an approximately 2-fold greater risk of prostate cancer-related death. Specifically, at 5 years the PCSM rate for men with normal BMI was 6.5%, compared with 13.1% for overweight men and 12.2% for obese men. To the best of our knowledge, this is the first large study using prospective data to evaluate the relationship between obesity and mortality in men treated for locally advanced prostate cancer.
Our findings are consistent with a recent population-based case-control study37 and epidemiologic studies.5,6,38,39 In a prospective study of 135,000 Swedish construction workers with more than 18 years of follow-up, obesity was associated with about a 40% increased risk of PCSM than a normal BMI.6 A study of 6763 Seventh-day Adventists followed between 1960 and 1980 reported that the risk of fatal prostate cancer was 2.5 times higher in overweight compared with normal weight men, and even higher in those who heavily consumed animal products.39 In 2 large prospective cohorts known as the Cancer Prevention Study (CPS) I and II, the American Cancer Society followed 816,268 men enrolled in 1959 and again in 1982, respectively, among whom there were 5212 prostate cancer deaths.38 Both CPS I and II reported that obese men (BMI ≥30 kg/m2) had significantly higher PCSM rates than normal weight men, with a 27% and 21% increased risk of death, respectively. In a more recent update of CPS II with 16 years of follow-up,5 severely obese men (BMI >35 kg/m2) were at an even greater risk (34%) of prostate cancer death relative to normal weight men.
Several mechanisms may account for the shorter cancer-specific survival among obese men. Obesity is associated with higher estradiol, lower testosterone, and lower sex hormone-binding globulin levels and this microenvironment may predispose to more aggressive disease.40–42 Low baseline serum testosterone levels are associated with a higher incidence of extracapsular disease in men undergoing RP for early-stage prostate cancer40 and shorter overall survival in men with metastatic prostate cancer.43 Obesity is linked to insulin resistance and diabetes.44 Insulin and insulin-like growth factors (IGFs) may promote prostate cancer progression.45,46 In addition, elevated leptin and lower adiponectin levels among obese men have been implicated in prostate cancer aggressiveness.47–49
Clinical understaging of the extent of disease may also contribute to increased PCSM in obese men. Obese men tend to have larger prostate glands22,50 and their body habitus may interfere with digital rectal examination. In prostatectomy series that control for adverse pathologic features such as Gleason sum, stage, extracapsular extension, seminal vesicle invasion, margin status, and lymph node metastases, however, there remains an association between increased BMI and risk of biochemical progression.14,15 Obese patients may have lower serum PSA values51 due to lower testosterone and higher estradiol levels but increased pretreatment PSA velocity.52 Notably, we cannot comment on this effect given that RTOG 85-31 was conducted before PSA screening was widely available.
Decreased effectiveness of local therapy may also contribute to shorter cancer-specific survival in overweight and obese men. Obese men have greater risk of positive surgical margins following RP.11,14,22 Similarly, greater organ motion and set-up error may interfere with accurate delivery of RT to obese men.53,54 Notably, these technical problems may be of even greater concern in the very obese.
Hormone therapy may also be less effective in obese men. Despite lower pretreatment serum testosterone levels, obese men have significantly higher testosterone levels during treatment with gonadotropin-releasing hormone (GnRH) agonists than men with normal BMI.55 The substantially smaller relative decline in testosterone levels after GnRH agonist treatment may contribute to greater cancer-specific mortality in obese men. Additional research is needed to further delineate the relationships between obesity, sex steroid levels, and survival in men receiving ADT.
Obesity is associated with greater ACM in the general population. The relative increase in mortality associated with obesity is modest, however, and has required very large population-based studies with long follow-up. For example, in a 12-year prospective cohort study of over 1 million Koreans,56 overweight and obese men and women had higher rates of death than those of normal weight. In other prospective cohort studies of over 500,000 US adults57 and approximately 170,000 Chinese men and women,58 obesity was associated with increased mortality. In another study from the National Health and Nutritional Examination Surveys (NHANES) I-III,3 obesity (and particularly higher levels of obesity), but not overweight, was associated with excess deaths relative to the normal weight category. Given the number of subjects in our study, it is thus not surprising that we did not observe a significant association between BMI and non-PCSM or ACM. Moreover, our locally advanced patient population was at a high risk for PCSM.
Potential limitations of this study need to be considered. BMI data were collected prospectively but not originally to understand the association between obesity and PCSM. We lack information on lifestyle factors, such as diet and physical activity, which may mediate some of the effect of obesity on cancer-specific mortality. Our analyses were restricted to baseline BMI. Further studies are warranted to assess the impact of obesity earlier in life, weight changes over time, and the impact of weight loss on the clinical course of disease. Since ADT itself is known to cause weight gain and increase fasting insulin levels, as well as decrease insulin sensitivity,28,29,59 it will be important to investigate whether such adverse effects of therapy have an independent effect on outcomes.
In conclusion, we found that a greater baseline BMI is independently associated with higher cancer-specific mortality in men with locally advanced prostate cancer. Further studies are warranted to evaluate the mechanisms for this increased mortality among obese men and to assess the impact of BMI on survival following other management strategies and in clinically localized disease. Whether weight loss after prostate cancer diagnosis can alter the disease course remains to be determined.
Acknowledgments
Supported by RTOG U10 CA21661, CCOP U10 CA37422, and Stat U10 CA32115 grants from the NCI.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 4.Rapp K, Schroeder J, Klenk J, et al. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93:1062–1067. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Andersson SO, Wolk A, Bergstrom R, et al. Body size and prostate cancer: a 20-year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst. 1997;89:385–389. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Rimm EB, Liu Y, et al. Body mass index and risk of prostate cancer in U.S. health professionals. J Natl Cancer Inst. 2003;95:1240–1244. doi: 10.1093/jnci/djg009. [DOI] [PubMed] [Google Scholar]
- 8.Engeland A, Tretli S, Bjorge T. Height, body mass index, and prostate cancer: a follow-up of 950000 Norwegian men. Br J Cancer. 2003;89:1237–1242. doi: 10.1038/sj.bjc.6601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Presti JC, Jr, Lee U, Brooks JD, Terris MK. Lower body mass index is associated with a higher prostate cancer detection rate and less favorable pathological features in a biopsy population. J Urol. 2004;171(6 Pt 1):2199–2202. doi: 10.1097/01.ju.0000124847.82541.60. [DOI] [PubMed] [Google Scholar]
- 10.Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol. 2000;151:541–549. doi: 10.1093/oxfordjournals.aje.a010241. [DOI] [PubMed] [Google Scholar]
- 11.Amling CL, Riffenburgh RH, Sun L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22:439–445. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 12.Kane CJ, Bassett WW, Sadetsky N, et al. Obesity and prostate cancer clinical risk factors at presentation: data from CaPSURE. J Urol. 2005;173:732–736. doi: 10.1097/01.ju.0000152408.25738.23. [DOI] [PubMed] [Google Scholar]
- 13.Gong Z, Neuhouser ML, Goodman PJ, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1977–1983. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 14.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 15.Freedland SJ, Grubb KA, Yiu SK, et al. Obesity and risk of biochemical progression following radical prostatectomy at a tertiary care referral center. J Urol. 2005;174:919–922. doi: 10.1097/01.ju.0000169459.78982.d7. [DOI] [PubMed] [Google Scholar]
- 16.Strom SS, Wang X, Pettaway CA, et al. Obesity, weight gain, and risk of biochemical failure among prostate cancer patients following prostatectomy. Clin Cancer Res. 2005;11(19 Pt 1):6889–6894. doi: 10.1158/1078-0432.CCR-04-1977. [DOI] [PubMed] [Google Scholar]
- 17.Strom SS, Kamat AM, Gruschkus SK, et al. Influence of obesity on biochemical and clinical failure after external-beam radiotherapy for localized prostate cancer. Cancer. 2006;107:631–639. doi: 10.1002/cncr.22025. [DOI] [PubMed] [Google Scholar]
- 18.Efstathiou JA, Chen MH, Renshaw AA, Loffredo MJ, D'Amico AV. Influence of body mass index on prostate-specific antigen failure after androgen suppression and radiation therapy for localized prostate cancer. Cancer. 2007;109:1493–1498. doi: 10.1002/cncr.22564. [DOI] [PubMed] [Google Scholar]
- 19.Merrick GS, Butler WM, Wallner KE, et al. Influence of body mass index on biochemical outcome after permanent prostate brachytherapy. Urology. 2005;65:95–100. doi: 10.1016/j.urology.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 20.Jhaveri FM, Zippe CD, Klein EA, Kupelian PA. Biochemical failure does not predict overall survival after radical prostatectomy for localized prostate cancer: 10-year results. Urology. 1999;54:884–890. doi: 10.1016/s0090-4295(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 21.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 22.Siddiqui SA, Inman BA, Sengupta S, et al. Obesity and survival after radical prostatectomy: a 10-year prospective cohort study. Cancer. 2006;107:521–529. doi: 10.1002/cncr.22030. [DOI] [PubMed] [Google Scholar]
- 23.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 24.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 25.Denham JW, Steigler A, Lamb DS, et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 2005;6:841–850. doi: 10.1016/S1470-2045(05)70348-X. [DOI] [PubMed] [Google Scholar]
- 26.D'Amico AV, Manola J, Loffredo M, Renshaw AA, Della-Croce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 27.Barry MJ, Delorenzo MA, Walker-Corkery ES, Lucas FL, Wennberg DC. The rising prevalence of androgen deprivation among older American men since the advent of prostate-specific antigen testing: a population-based cohort study. BJU Int. 2006;98:973–978. doi: 10.1111/j.1464-410X.2006.06416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 29.Smith MR. Changes in body composition during hormonal therapy for prostate cancer. Clin Prostate Cancer. 2003;2:18–21. doi: 10.3816/cgc.2003.n.008. [DOI] [PubMed] [Google Scholar]
- 30.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 31.National Institutes of Health Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 32.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. John Wiley & Sons; New York: 1980. [Google Scholar]
- 33.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 34.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 35.Mantel N. Evaluation of survival data and 2 new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 36.Cox DR. Regression models and life tables. J R Stat Soc Series B. 1972;34:187–229. [Google Scholar]
- 37.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109:1192–1202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in 2 large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10:345–353. [PubMed] [Google Scholar]
- 39.Snowdon DA, Phillips RL, Choi W. Diet, obesity, and risk of fatal prostate cancer. Am J Epidemiol. 1984;120:244–250. doi: 10.1093/oxfordjournals.aje.a113886. [DOI] [PubMed] [Google Scholar]
- 40.Massengill JC, Sun L, Moul JW, et al. Pretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomy. J Urol. 2003;169:1670–1675. doi: 10.1097/01.ju.0000062674.43964.d0. [DOI] [PubMed] [Google Scholar]
- 41.Orwoll E, Lambert LC, Marshall LM, et al. Testosterone and estradiol among older men. J Clin Endocrinol Metab. 2006;91:1336–1344. doi: 10.1210/jc.2005-1830. [DOI] [PubMed] [Google Scholar]
- 42.Pasquali R, Casimirri F, Cantobelli S, et al. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism. 1991;40:101–104. doi: 10.1016/0026-0495(91)90199-7. [DOI] [PubMed] [Google Scholar]
- 43.Chodak GW, Vogelzang NJ, Caplan RJ, Soloway M, Smith JA. Independent prognostic factors in patients with meta-static (stage D2) prostate cancer. The Zoladex Study Group. JAMA. 1991;265:618–621. [PubMed] [Google Scholar]
- 44.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 45.Chan JM, Stampfer MJ, Ma J, et al. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst. 2002;94:1099–1106. doi: 10.1093/jnci/94.14.1099. [DOI] [PubMed] [Google Scholar]
- 46.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 47.Considine RV, Sinha MK, Heiman ML, et al. Serum immuno-reactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 48.Saglam K, Aydur E, Yilmaz M, Goktas S. Leptin influences cellular differentiation and progression in prostate cancer. J Urol. 2003;169:1308–1311. doi: 10.1097/01.ju.0000055903.18400.25. [DOI] [PubMed] [Google Scholar]
- 49.Freedland SJ, Sokoll LJ, Platz EA, et al. Association between serum adiponectin, and pathological stage and grade in men undergoing radical prostatectomy. J Urol. 2005;174(4 Pt 1):1266–1270. doi: 10.1097/01.ju.0000173093.89897.97. [DOI] [PubMed] [Google Scholar]
- 50.Freedland SJ, Platz EA, Presti JC, Jr, et al. Obesity, serum prostate specific antigen and prostate size: implications for prostate cancer detection. J Urol. 2006;175:500–504. doi: 10.1016/S0022-5347(05)00162-X. discussion 504. [DOI] [PubMed] [Google Scholar]
- 51.Barqawi AB, Golden BK, O'Donnell C, Brawer MK, Crawford ED. Observed effect of age and body mass index on total and complexed PSA: analysis from a national screening program. Urology. 2005;65:708–712. doi: 10.1016/j.urology.2004.10.074. [DOI] [PubMed] [Google Scholar]
- 52.Loeb S, Yu X, Nadler RB, et al. Does body mass index affect preoperative prostate specific antigen velocity or pathological outcomes after radical prostatectomy? J Urol. 2007;177:102–106. doi: 10.1016/j.juro.2006.08.097. discussion 106. [DOI] [PubMed] [Google Scholar]
- 53.Millender LE, Aubin M, Pouliot J, Shinohara K, Roach M., 3rd Daily electronic portal imaging for morbidly obese men undergoing radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59:6–10. doi: 10.1016/j.ijrobp.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 54.Song PY, Washington M, Vaida F, et al. A comparison of 4 patient immobilization devices in the treatment of prostate cancer patients with 3 dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34:213–219. doi: 10.1016/0360-3016(95)02094-2. [DOI] [PubMed] [Google Scholar]
- 55.Smith MR. Obesity and sex steroids during gonadotropin-releasing hormone agonist treatment for prostate cancer. Clin Cancer Res. 2007;13:241–245. doi: 10.1158/1078-0432.CCR-06-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 57.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 58.Gu D, He J, Duan X, et al. Body weight and mortality among men and women in China. JAMA. 2006;295:776–783. doi: 10.1001/jama.295.7.776. [DOI] [PubMed] [Google Scholar]
- 59.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]